Human iPS Cell-Derived Germ Cells: Current Status and Clinical Potential
Abstract
:1. Introduction
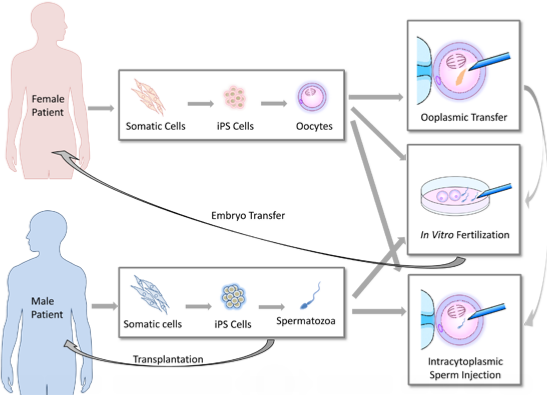
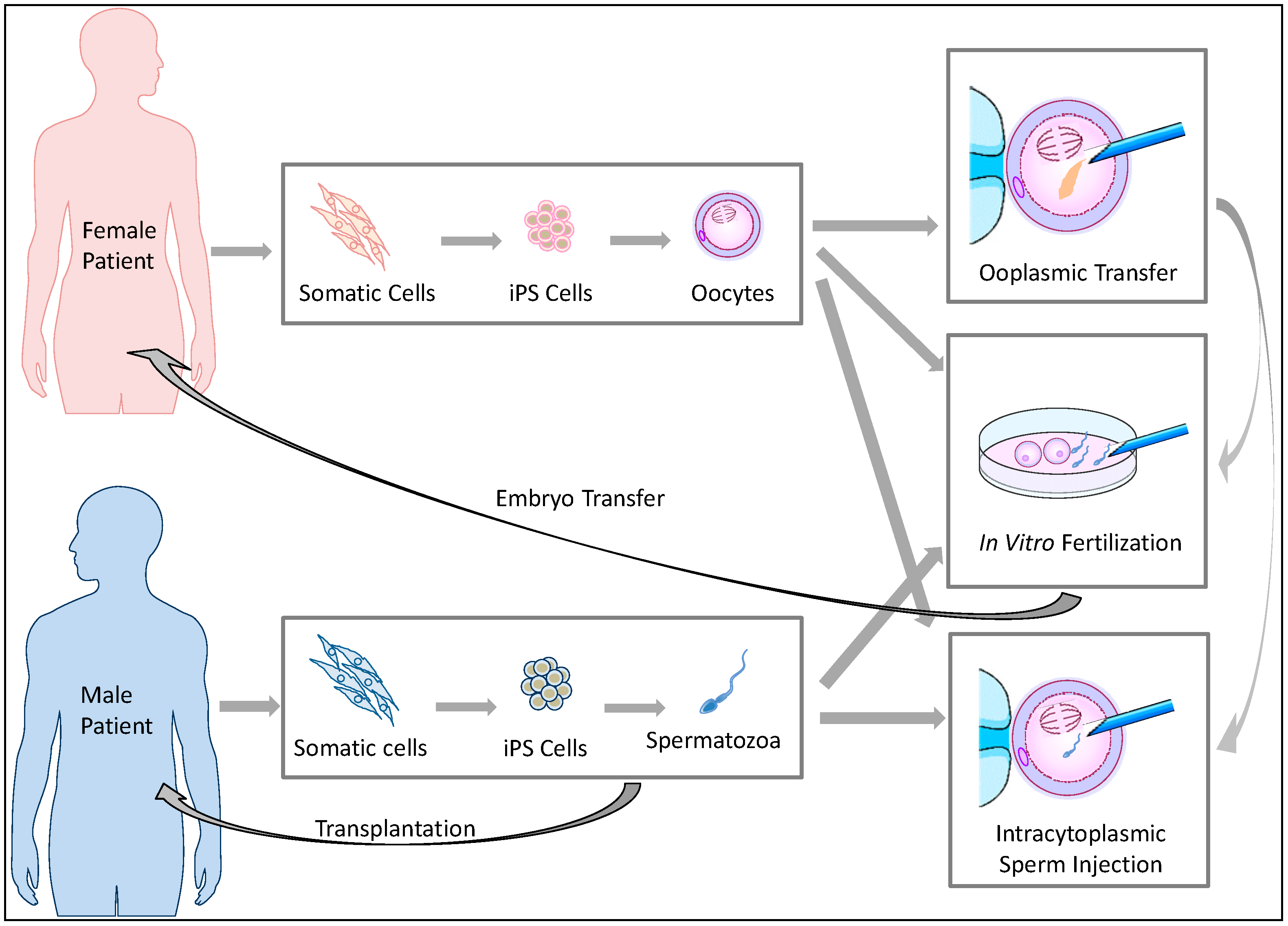
2. Clinical Implications of Germ Cell Induction in Vitro
3. The Induction of Germ Cells from iPS Cells
| Differentiation Method | Cell Lines Used | Differentiation Stage | Remarks | References | ||||
|---|---|---|---|---|---|---|---|---|
| iPS Cells | ES Cells | PGCs | Meiotic Cells | Haploid Cells | ||||
| EB formation | - | HSF-6(XX) HSF-1(XY) H9(XX) | - | - | - | Germ cell-like cells expressing VASA, SCP1, SCP3, BOULE, TEKT1, and GDF3 were observed. | Clark et al., 2004 [3] | |
| EB formation | - | NTU1(XX) NTU2(XX) NTU3(N.D.) | - | - | - | Germ cell-like cells expressing cKit, STELLA, VASA, and GDF9 were observed. | Chen et al., 2006 [4] | |
| Making colonies of fewer than 50 cells | - | HSF-6(XX) H9(XX) | Yes | - | - | Sertoli-like cells were simultaneously generated in this process. | Bucay et al., 2008 [7] | |
| Monolayer differentiation and FACS enrichment of SSEA1-positive cells | - | H9(XX) hES-NCL1(XX) | Yes | - | - | PGCs with removal of parental imprints and chromatin modification changes were generated. | Tilgner et al., 2008 [6] | |
| Differentiation on primary human fetal gonadal stromal cells, and isolation of a triple biomarker (cKIT, SSEA1, VASA)—positive cells | hIPS2(XY) hIPS1(XY) | HSF-6(XX) HSF-1(XY) H9(XX) | Yes | - | - | PGCs derived from human iPS cells did not initiate imprint erasure as efficiently. | Park et al., 2009 [8] | |
| Overexpression of DAZL, DAZ and BOULE following induction by BMPs | - | HSF-1(XY) HSF-6(XX) H1(XY) H9(XX) | Yes | Yes | Yes | DAZL functions in PGC formation, whereas DAZ and BOULE promote later stages of meiosis and development of haploid gametes. | Kee et al., 2010 [5] | |
| Overexpression of DAZ, DAZL, and BOULE following induction by BMPs | iPS(IMR90) (XX) iHUF4(XY) | H9(XX) HSF-1(XY) | Yes | Yes | Yes | Fetal-derived iPS cell line iPS (IMR90) and adult-derived iPS cell line iHUF4 were generated by lentiviral transfection with OCT3/4, SOX2, KLF4 and c-MYC. | Panula et al., 2011 [10] | |
| Overexpression of VASA and/or DAZL following differentiation on matrigel-coated plates | iPS(IMR90)(XX) iHUF4(XY) | iHUF3(XX) iHUF4(XY) | Yes | Yes | Yes | The same iPS cell lines described in Panula et al. were used. | Medrano et al., 2011 [9] | |
| Two step protocol: Culture in bFGF-depleted ES cell media, subsequently, RA added; Sorted cells are cultured with FRSK, rLIF, bFGF, and R115866 | KiPS1(XY) KiPS2(XY) KiPS3(XY) KiPS4(XX) CBiPS1(XY) CBiPS2(XY) CBiPS3(XX) CBiPS4(XY) CBiPS5(XX) | HS306(XX) ES[6](XY) | - | Yes | Yes | iPS cells of different origin (keratinocytes and cord blood) were generated with a different number (2–4) of transcription factors. | Eguizabal et al., 2011 [13] | |
| Direct differentiation using mouse SSC culture conditions | H1(XY) | HFF1(XY) | - | Yes | Yes | iPS cells derived from male foreskin fibroblasts were used. | Easley et al., 2012 [12] | |
| 1. Differentiation into PGCs with BMPs, RA, and hrLIF. 2. Induction of gonocytes by transplanting iPS cells directly into murine seminiferous tubules in vivo | iAZF1(XY) iAZF2(XY) iAZFΔbc(XY) iAZFΔc(XY) iAZFΔa(XY) | H1(XY) | Yes | - | - | iPS cells derived from dermal fibroblasts of males with intact Y chromosome (iAZF) and Y chromosome deletions (iAZFΔ) were used. Gonocytes expressing VASA, STELLA, UTF1, PLZF, and DAZ were induced. | Ramanthal et al., 2014 [11] | |
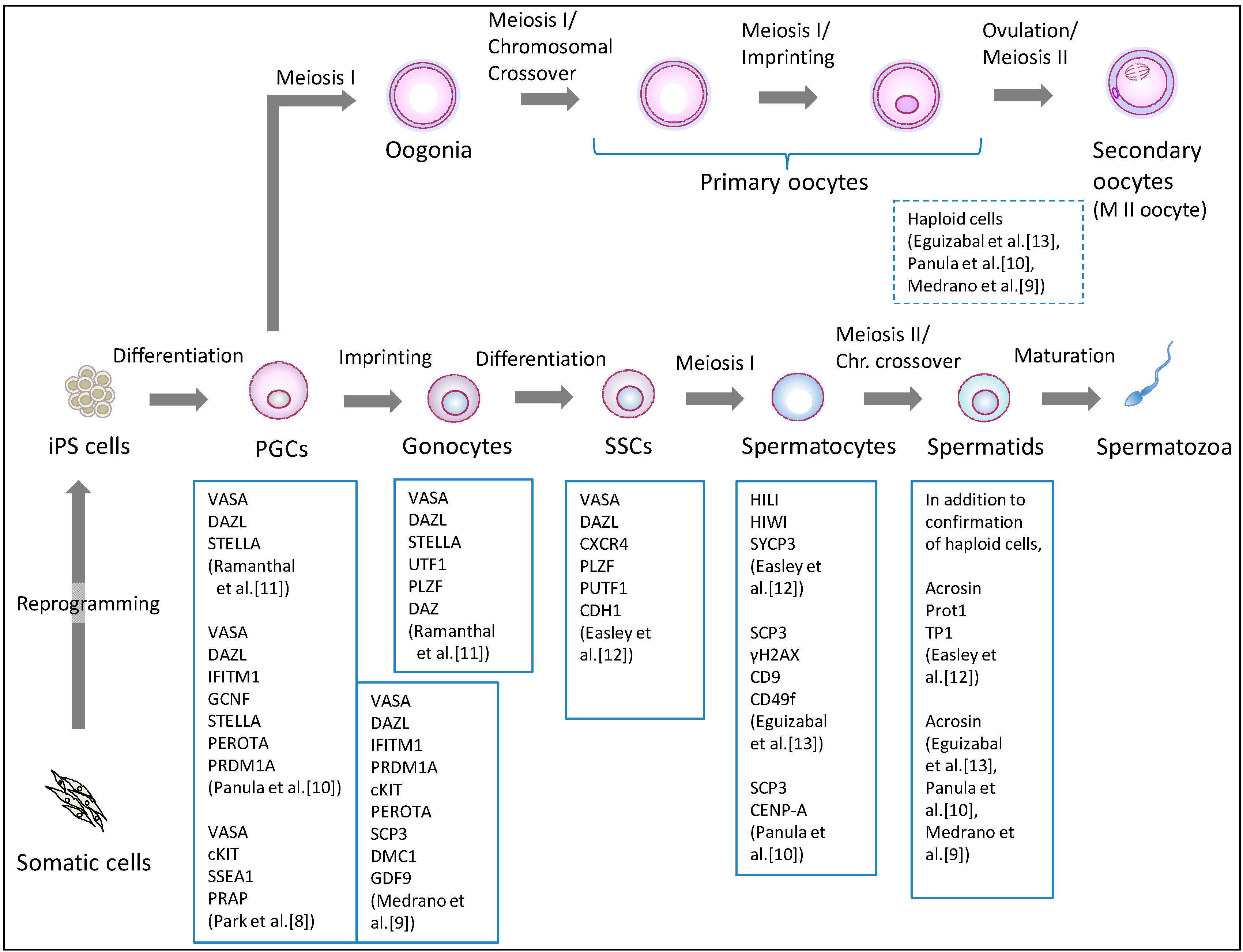
3.1. Induction of the Male Germline
3.2. The Induction of Female Germline
4. Future Directions
4.1. Genetic and Epigenetic Stability of Human iPS Cells
4.2. The Pluripotency State of Human iPS Cells
4.3. Spatio-Temporal Factors in Gametogenesis
4.4. Assessing the Developmental Potential of Induced Germ Cells
5. Conclusions
Acknowledgments
Appendix
Assisted Reproductive Technology (ART)
Intrauterine Insemination (IUI)
In Vitro Fertilization (IVF)
Intracytoplasmic Sperm Injection (ICSI)
Ooplasmic Transfer [23,27,28]
Conflicts of Interest
References
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef]
- Clark, A.T.; Bodnar, M.S.; Fox, M.; Rodriquez, R.T.; Abeyta, M.J.; Firpo, M.T.; Pera, R.A. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum. Mol. Genet. 2004, 13, 727–739. [Google Scholar] [CrossRef]
- Chen, H.F.; Kuo, H.C.; Chien, C.L.; Shun, C.T.; Yao, Y.L.; Ip, P.L.; Chuang, C.Y.; Wang, C.C.; Yang, Y.S.; Ho, H.N. Derivation, characterization and differentiation of human embryonic stem cells: Comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum. Reprod. 2007, 22, 567–577. [Google Scholar] [CrossRef]
- Kee, K.; Gonsalves, J.M.; Clark, A.T.; Pera, R.A. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006, 15, 831–837. [Google Scholar] [CrossRef]
- Tilgner, K.; Atkinson, S.P.; Golebiewska, A.; Stojkovic, M.; Lako, M.; Armstrong, L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells 2008, 26, 3075–3085. [Google Scholar] [CrossRef]
- Bucay, N.; Yebra, M.; Cirulli, V.; Afrikanova, I.; Kaido, T.; Hayek, A.; Montgomery, A.M. A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells 2009, 27, 68–77. [Google Scholar] [CrossRef]
- Park, T.S.; Galic, Z.; Conway, A.E.; Lindgren, A.; van Handel, B.J.; Magnusson, M.; Richter, L.; Teitell, M.A.; Mikkola, H.K.; Lowry, W.E.; et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells 2009, 27, 783–795. [Google Scholar] [CrossRef]
- Medrano, J.V.; Ramathal, C.; Nguyen, H.N.; Simon, C.; Reijo Pera, R.A. Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells 2012, 30, 441–451. [Google Scholar] [CrossRef]
- Panula, S.; Medrano, J.V.; Kee, K.; Bergström, R.; Nguyen, H.N.; Byers, B.; Wilson, K.D.; Wu, J.C.; Simon, C.; Hovatta, O.; et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum. Mol. Genet. 2011, 20, 752–762. [Google Scholar] [CrossRef]
- Ramathal, C.; Durruthy-Durruthy, J.; Sukhwani, M.; Arakaki, J.E.; Turek, P.J.; Orwig, K.E.; Reijo Pera, R.A. Fate of iPSCs Derived from Azoospermic and Fertile Men following Xenotransplantation to Murine Seminiferous Tubules. Cell Rep. 2014, 7, 1284–1297. [Google Scholar] [CrossRef]
- Easley, C.A., IV; Phillips, B.T.; McGuire, M.M.; Barringer, J.M.; Valli, H.; Hermann, B.P.; Simerly, C.R.; Rajkovic, A.; Miki, T.; Orwig, K.E.; et al. Direct Differentiation of Human Pluripotent Stem Cells into Haploid Spermatogenic Cells. Cell Rep. 2012, 2, 440–446. [Google Scholar] [CrossRef]
- Eguizabal, C.; Montserrat, N.; Vassena, R.; Barragan, M.; Garreta, E.; Garcia-Quevedo, L.; Vidal, F.; Giorgetti, A.; Veiga, A.; Izpisua Belmonte, J.C. Complete meiosis from human induced pluripotent stem cells. Stem Cells 2011, 29, 1186–1195. [Google Scholar] [CrossRef]
- Weismann, A.; Newton Parker, W.; Rönnfeldt, H. Germ-Plasm, a Theory of Heredity; Scribner’s: New York, NY, USA, 1893. [Google Scholar]
- Ishii, T.; Pera, R.A.; Greely, H.T. Ethical and legal issues arising in research on inducing human germ cells from pluripotent stem cells. Cell Stem Cell 2013, 13, 145–148. [Google Scholar] [CrossRef]
- Saha, K.; Jaenisch, R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell 2009, 5, 584–595. [Google Scholar] [CrossRef]
- Lee, J.; Kanatsu-Shinohara, M.; Morimoto, H.; Kazuki, Y.; Takashima, S.; Oshimura, M.; Toyokuni, S.; Shinohara, T. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell 2009, 5, 76–86. [Google Scholar] [CrossRef]
- Becker, G.; Butler, A.; Nachtigall, R.D. Resemblance talk: A challenge for parents whose children were conceived with donor gametes in the US. Soc. Sci. Med. 2005, 61, 1300–1309. [Google Scholar] [CrossRef]
- De Melo-Martín, I. The ethics of anonymous gamete donation: is there a right to know one’s genetic origins? Hastings Cent Rep. 2014, 44, 28–35. [Google Scholar]
- Meistrich, M.L. Male gonadal toxicity. Pediatr. Blood Cancer 2009, 53, 261–266. [Google Scholar] [CrossRef]
- Howell, S.J.; Shalet, S.M. Spermatogenesis after cancer treatment: Damage and recovery. J. Natl. Cancer Inst. Monogr. 2005, 34, 12–17. [Google Scholar] [CrossRef]
- Smit, M.; van Casteren, N.J.; Wildhagen, M.F.; Romijn, J.C.; Dohle, G.R. Sperm DNA integrity in cancer patients before and after cytotoxic treatment. Hum. Reprod. 2010, 25, 1877–1883. [Google Scholar] [CrossRef]
- Bentov, Y.; Casper, R.F. The aging oocyte—Can mitochondrial function be improved? Fertil. Steril. 2013, 99, 18–22. [Google Scholar] [CrossRef]
- Nagano, M.; McCarrey, J.R.; Brinster, R.L. Primate spermatogonial stem cells colonize mouse testes. Biol. Reprod. 2001, 64, 1409–1416. [Google Scholar] [CrossRef]
- Nagano, M.; Patrizio, P.; Brinster, R.L. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil. Steril. 2002, 78, 1225–1233. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Hansel, M.C.; Orwig, K.E. Spermatogonial stem cells in higher primates: Are there differences from those in rodents? Reproduction 2010, 139, 479–493. [Google Scholar] [CrossRef]
- Levy, R.; Elder, K.; Menezo, Y. Cytoplasmic transfer in oocytes: Biochemical aspects. Hum. Reprod. Update 2004, 10, 241–250. [Google Scholar] [CrossRef]
- Callaway, E. Reproductive medicine: The power of three. Nature 2014, 509, 414–417. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Li, J.; Song, W.; Pan, G.; Zhou, J. Advances in understanding the cell types and approaches used for generating induced pluripotent stem cells. J. Hematol. Oncol. 2014, 7, 50. [Google Scholar] [CrossRef]
- Teramura, T.; Takehara, T.; Kawata, N.; Fujinami, N.; Mitani, T.; Takenoshita, M.; Matsumoto, K.; Saeki, K.; Iritani, A.; Sagawa, N.; et al. Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning Stem Cells 2007, 9, 144–156. [Google Scholar] [CrossRef]
- Toyooka, Y.; Tsunekawa, N.; Akasu, R.; Noce, T. Embryonic stem cells can form germ cells in vitro. Proc. Natl. Acad. Sci. USA 2003, 100, 11457–11462. [Google Scholar] [CrossRef]
- Geijsen, N.; Horoschak, M.; Kim, K.; Gribnau, J.; Eggan, K.; Daley, G.Q. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 2004, 427, 148–154. [Google Scholar] [CrossRef]
- Nayernia, K.; Nolte, J.; Michelmann, H.W.; Lee, J.H.; Rathsack, K.; Drusenheimer, N.; Dev, A.; Wulf, G.; Ehrmann, I.E.; Elliott, D.J.; et al. In vitro—Differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev. Cell 2006, 11, 125–132. [Google Scholar] [CrossRef]
- Eguizabal, C.; Shovlin, T.C.; Durcova-Hills, G.; Surani, A.; McLaren, A. Generation of primordial germ cells from pluripotent stem cells. Differentiation 2009, 78, 116–123. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Miki, H.; Ogura, A.; Toyokuni, S.; Shinohara, T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003, 69, 612–616. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Winkler, F.; Pascarella, J.N.; Peters, K.A.; Sheng, Y.; Valli, H.; Rodriguez, M.; Ezzelarab, M.; Dargo, G.; et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012, 11, 715–726. [Google Scholar] [CrossRef]
- Amariglio, N.; Hirshberg, A.; Scheithauer, B.W.; Cohen, Y.; Loewenthal, R.; Trakhtenbrot, L.; Paz, N.; Koren-Michowitz, M.; Waldman, D.; Leider-Trejo, L.; et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009, 6, e1000029. [Google Scholar] [CrossRef]
- Antinori, S.; Versaci, C.; Dani, G.; Antinori, M.; Pozza, D.; Selman, H.A. Fertilization with human testicular spermatids: Four successful pregnancies. Hum. Reprod. 1997, 12, 286–291. [Google Scholar] [CrossRef]
- Sofikitis, N.V.; Yamamoto, Y.; Miyagawa, I.; Mekras, G.; Mio, Y.; Toda, T.; Antypas, S.; Kawamura, H.; Kanakas, N.; Antoniou, N.; et al. Ooplasmic injection of elongating spermatids for the treatment of non-obstructive azoospermia. Hum. Reprod. 1998, 13, 709–714. [Google Scholar] [CrossRef]
- Yamanaka, K.; Sofikitis, N.V.; Miyagawa, I.; Yamamoto, Y.; Toda, T.; Antypas, S.; Dimitriadis, D.; Takenaka, M.; Taniguchi, K.; Takahashi, K.; et al. Ooplasmic round spermatid nuclear injection procedures as an experimental treatment for nonobstructive azoospermia. J. Assist. Reprod. Genet. 1997, 14, 55–62. [Google Scholar] [CrossRef]
- Levran, D.; Nahum, H.; Farhi, J.; Weissman, A. Poor outcome with round spermatid injection in azoospermic patients with maturation arrest. Fertil. Steril. 2000, 74, 443–449. [Google Scholar] [CrossRef]
- Vicdan, K.; Isik, A.Z.; Delilbasi, L. Development of blastocyst-stage embryos after round spermatid injection in patients with complete spermiogenesis failure. J. Assist. Reprod. Genet. 2001, 18, 78–86. [Google Scholar] [CrossRef]
- Hübner, K.; Fuhrmann, G.; Christenson, L.K.; Kehler, J.; Reinbold, R.; de La Fuente, R.; Wood, J.; Strauss, J.F., III; Boiani, M.; Schöler, H.R. Derivation of oocytes from mouse embryonic stem cells. Science 2003, 300, 1251–1256. [Google Scholar] [CrossRef]
- Novak, I.; Lightfoot, D.A.; Wang, H.; Eriksson, A.; Mahdy, E.; Höög, C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells 2006, 24, 1931–1936. [Google Scholar] [CrossRef]
- Lacham-Kaplan, O.; Chy, H.; Trounson, A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells 2006, 24, 266–273. [Google Scholar] [CrossRef]
- Qing, T.; Shi, Y.; Qin, H.; Ye, X.; Wei, W.; Liu, H.; Ding, M.; Deng, H. Induction of oocyte-like cells from mouse embryonic stem cells by co-culture with ovarian granulosa cells. Differentiation 2007, 75, 902–911. [Google Scholar]
- Nicholas, C.R.; Haston, K.M.; Grewall, A.K.; Longacre, T.A.; Reijo Pera, R.A. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum. Mol. Genet. 2009, 18, 4376–4389. [Google Scholar] [CrossRef]
- Felici, M.D. Origin, Migration, and Proliferation of Human Primordial Germ Cells. In Oogenesis; Springer: London, UK, 2013. [Google Scholar]
- Sadri-Ardekani, H.; Mizrak, S.C.; van Daalen, S.K.; Korver, C.M.; Roepers-Gajadien, H.L.; Koruji, M.; Hovingh, S.; de Reijke, T.M.; de la Rosette, J.J.; van der Veen, F.; et al. Propagation of human spermatogonial stem cells in vitro. JAMA 2009, 302, 2127–2134. [Google Scholar] [CrossRef]
- Zuckerman, S. The number of oocytes in the mature ovary. Recent Prog. Horm. Res. 1951, 6, 63–109. [Google Scholar]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef]
- Johnson, J.; Bagley, J.; Skaznik-Wikiel, M.; Lee, H.J.; Adams, G.B.; Niikura, Y.; Tschudy, K.S.; Tilly, J.C.; Cortes, M.L.; Forkert, R.; et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 2005, 122, 303–315. [Google Scholar] [CrossRef]
- Zou, K.; Yuan, Z.; Yang, Z.; Luo, H.; Sun, K.; Zhou, L.; Xiang, J.; Shi, L.; Yu, Q.; Zhang, Y.; et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009, 11, 631–636. [Google Scholar] [CrossRef]
- White, Y.A.; Woods, D.C.; Takai, Y.; Ishihara, O.; Seki, H.; Tilly, J.L. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012, 18, 413–421. [Google Scholar] [CrossRef]
- Eggan, K.; Jurga, S.; Gosden, R.; Min, I.M.; Wagers, A.J. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature 2006, 441, 1109–1114. [Google Scholar] [CrossRef]
- Begum, S.; Papaioannou, V.E.; Gosden, R.G. The oocyte population is not renewed in transplanted or irradiated adult ovaries. Hum. Reprod. 2008, 23, 2326–2330. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, W.; Shen, Y.; Adhikari, D.; Ueno, H.; Liu, K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc. Natl. Acad. Sci. USA 2012, 109, 12580–12585. [Google Scholar] [CrossRef]
- Taapken, S.M.; Nisler, B.S.; Newton, M.A.; Sampsell-Barron, T.L.; Leonhard, K.A.; McIntire, E.M.; Montgomery, K.D. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat. Biotechnol. 2011, 29, 313–314. [Google Scholar] [CrossRef]
- Mayshar, Y.; Ben-David, U.; Lavon, N.; Biancotti, J.C.; Yakir, B.; Clark, A.T.; Plath, K.; Lowry, W.E.; Benvenisty, N. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell 2010, 7, 521–531. [Google Scholar] [CrossRef]
- Amps, K.; Andrews, P.W.; Anyfantis, G.; Armstrong, L.; Avery, S.; Baharvand, H.; Baker, J.; Baker, D.; Munoz, M.B.; Beil, S.; et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011, 29, 1132–1144. [Google Scholar] [CrossRef]
- Martins-Taylor, K.; Nisler, B.S.; Taapken, S.M.; Compton, T.; Crandall, L.; Montgomery, K.D.; Lalande, M.; Xu, R.H. Recurrent copy number variations in human induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 488–491. [Google Scholar] [CrossRef]
- Laurent, L.C.; Ulitsky, I.; Slavin, I.; Tran, H.; Schork, A.; Morey, R.; Lynch, C.; Harness, J.V.; Lee, S.; Barrero, M.J.; et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 2011, 8, 106–118. [Google Scholar] [CrossRef]
- Hussein, S.M.; Batada, N.N.; Vuoristo, S.; Ching, R.W.; Autio, R.; Närvä, E.; Ng, S.; Sourour, M.; Hämäläinen, R.; Olsson, C.; et al. Copy number variation and selection during reprogramming to pluripotency. Nature 2011, 471, 58–62. [Google Scholar] [CrossRef]
- Song, G.J.; Lewis, V. Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil. Steril. 2008, 90, 2238–2244. [Google Scholar] [CrossRef]
- Reynier, P.; May-Panloup, P.; Chrétien, M.F.; Morgan, C.J.; Jean, M.; Savagner, F.; Barrière, P.; Malthièry, Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol. Hum. Reprod. 2001, 7, 425–429. [Google Scholar] [CrossRef]
- Barritt, J.A.; Kokot, M.; Cohen, J.; Steuerwald, N.; Brenner, C.A. Quantification of human ooplasmic mitochondria. Reprod. Biomed. Online 2002, 4, 243–247. [Google Scholar] [CrossRef]
- May-Panloup, P.; Chrétien, M.F.; Jacques, C.; Vasseur, C.; Malthièry, Y.; Reynier, P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum. Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef]
- Steuerwald, N.; Barritt, J.A.; Adler, R.; Malter, H.; Schimmel, T.; Cohen, J.; Brenner, C.A. Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote 2000, 8, 209–215. [Google Scholar] [CrossRef]
- Prigione, A.; Lichtner, B.; Kuhl, H.; Struys, E.A.; Wamelink, M.; Lehrach, H.; Ralser, M.; Timmermann, B.; Adjaye, J. Human induced pluripotent stem cells harbor homoplasmic and heteroplasmic mitochondrial DNA mutations while maintaining human embryonic stem cell-like metabolic reprogramming. Stem Cells 2011, 29, 1338–1348. [Google Scholar]
- Van Haute, L.; Spits, C.; Geens, M.; Seneca, S.; Sermon, K. Human embryonic stem cells commonly display large mitochondrial DNA deletions. Nat. Biotechnol. 2013, 31, 20–23. [Google Scholar]
- Wahlestedt, M.; Ameur, A.; Moraghebi, R.; Norddahl, G.L.; Sten, G.; Woods, N.B.; Bryder, D. Somatic cells with a heavy mitochondrial DNA mutational load render induced pluripotent stem cells with distinct differentiation defects. Stem Cells 2014, 32, 1173–1182. [Google Scholar] [CrossRef]
- Nazor, K.L.; Altun, G.; Lynch, C.; Tran, H.; Harness, J.V.; Slavin, I.; Garitaonandia, I.; Müller, F.J.; Wang, Y.C.; Boscolo, F.S.; et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell 2012, 10, 620–634. [Google Scholar] [CrossRef]
- Lister, R.; Pelizzola, M.; Kida, Y.S.; Hawkins, R.D.; Nery, J.R.; Hon, G.; Antosiewicz-Bourget, J.; O’Malley, R.; Castanon, R.; Klugman, S.; et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011, 471, 68–73. [Google Scholar] [CrossRef]
- Ohi, Y.; Qin, H.; Hong, C.; Blouin, L.; Polo, J.M.; Guo, T.; Qi, Z.; Downey, S.L.; Manos, P.D.; Rossi, D.J.; et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat. Cell Biol. 2011, 13, 541–549. [Google Scholar] [CrossRef]
- Tesar, P.J.; Chenoweth, J.G.; Brook, F.A.; Davies, T.J.; Evans, E.P.; Mack, D.L.; Gardner, R.L.; McKay, R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007, 448, 196–199. [Google Scholar] [CrossRef]
- Brons, I.G.; Smithers, L.E.; Trotter, M.W.; Rugg-Gunn, P.; Sun, B.; Chuva de Sousa Lopes, S.M.; Howlett, S.K.; Clarkson, A.; Ahrlund-Richter, L.; Pedersen, R.A.; et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007, 448, 191–195. [Google Scholar] [CrossRef]
- Marks, H.; Kalkan, T.; Menafra, R.; Denissov, S.; Jones, K.; Hofemeister, H.; Nichols, J.; Kranz, A.; Stewart, A.F.; Smith, A.; et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 2012, 149, 590–604. [Google Scholar] [CrossRef]
- Gafni, O.; Weinberger, L.; Mansour, A.A.; Manor, Y.S.; Chomsky, E.; Ben-Yosef, D.; Kalma, Y.; Viukov, S.; Maza, I.; Zviran, A.; et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286. [Google Scholar] [CrossRef]
- Chan, Y.S.; Göke, J.; Ng, J.H.; Lu, X.; Gonzales, K.A.; Tan, C.P.; Tng, W.Q.; Hong, Z.Z.; Lim, Y.S.; Ng, H.H. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 2013, 13, 663–675. [Google Scholar] [CrossRef]
- Theunissen, T.W.; Powell, B.E.; Wang, H.; Mitalipova, M.; Faddah, D.A.; Reddy, J.; Fan, Z.P.; Maetzel, D.; Ganz, K.; Shi, L.; et al. Systematic Identification of Culture Conditions for Induction and Maintenance of Naive Human Pluripotency. Cell Stem Cell 2014. [Google Scholar] [CrossRef]
- Li, P.; Hu, H.; Yang, S.; Tian, R.; Zhang, Z.; Zhang, W.; Ma, M.; Zhu, Y.; Guo, X.; Huang, Y.; et al. Differentiation of induced pluripotent stem cells into male germ cells in vitro through embryoid body formation and retinoic acid or testosterone induction. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Svechnikov, K.; Landreh, L.; Weisser, L.; Izzo, G.; Colón, E.; Svechnikova, I.; Söder, O. Origin, Development and Regulation of Human Leydig Cells. Horm. Res. Paediatr. 2010, 73, 93–101. [Google Scholar] [CrossRef]
- Rebourcet, D.; O’Shaughnessy, P.J.; Pitetti, J.L.; Monteiro, A.; O’Hara, L.; Milne, L.; Tsai, Y.T.; Cruickshanks, L.; Riethmacher, D.; Guillou, F.; et al. Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development 2014, 141, 2139–2149. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, T. Human iPS Cell-Derived Germ Cells: Current Status and Clinical Potential. J. Clin. Med. 2014, 3, 1064-1083. https://doi.org/10.3390/jcm3041064
Ishii T. Human iPS Cell-Derived Germ Cells: Current Status and Clinical Potential. Journal of Clinical Medicine. 2014; 3(4):1064-1083. https://doi.org/10.3390/jcm3041064
Chicago/Turabian StyleIshii, Tetsuya. 2014. "Human iPS Cell-Derived Germ Cells: Current Status and Clinical Potential" Journal of Clinical Medicine 3, no. 4: 1064-1083. https://doi.org/10.3390/jcm3041064
APA StyleIshii, T. (2014). Human iPS Cell-Derived Germ Cells: Current Status and Clinical Potential. Journal of Clinical Medicine, 3(4), 1064-1083. https://doi.org/10.3390/jcm3041064