1. Introduction
Tricuspid regurgitation (TR) in patients with cardiac devices is a complex clinical scenario, particularly as transcatheter tricuspid valve interventions (TTVI) have become more common, with 12% to 36% of patients evaluated for TTVI having a transvalvular pacemaker or defibrillator lead present [
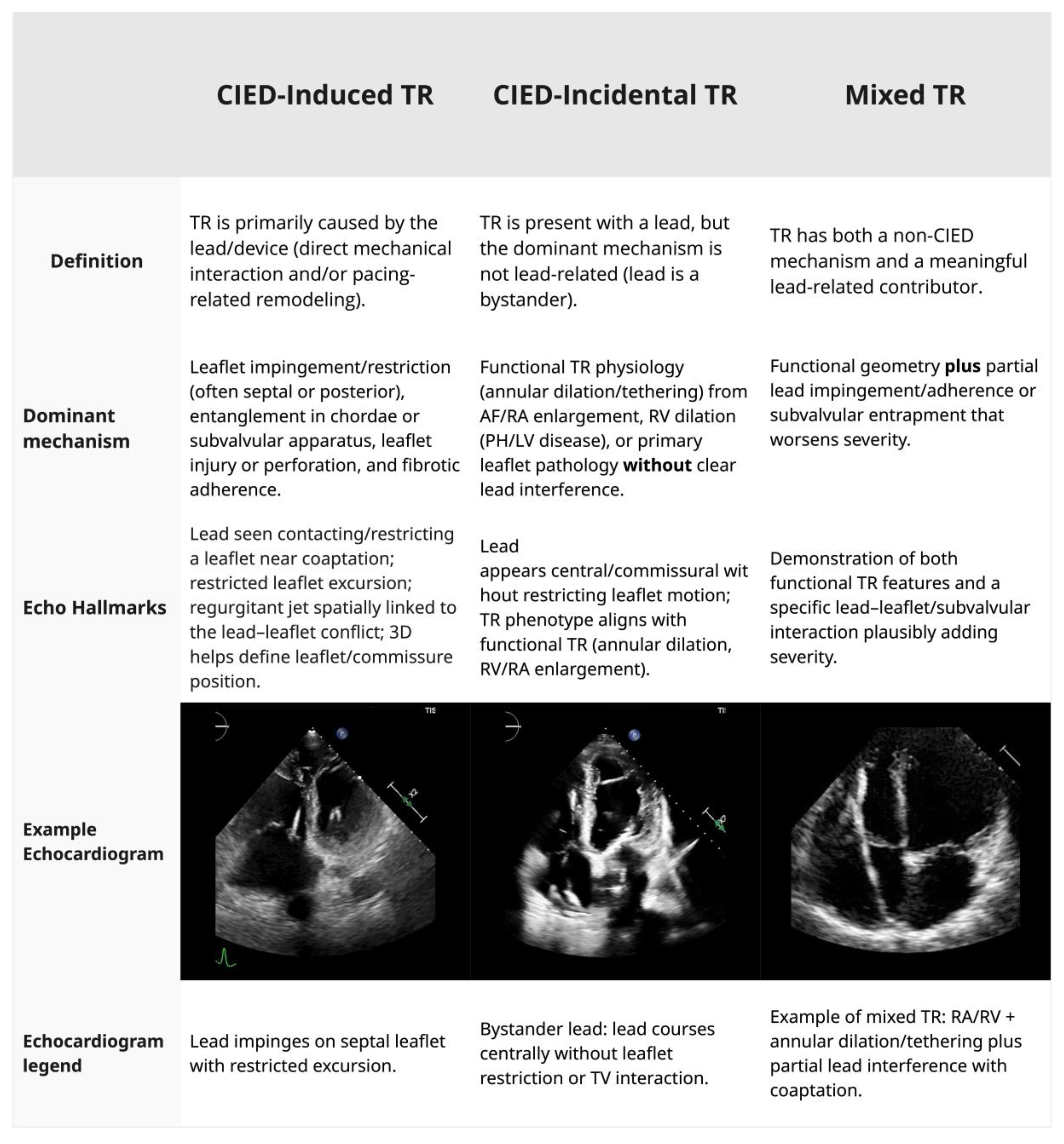
1]. The presence of a CIED lead across the tricuspid valve can be causally related to TR (CIED-induced TR) or a bystander (CIED-incidental TR), with the lead lying across the valve without impeding leaflet coaptation (
Figure 1). The mechanisms of CIED-induced TR are: (1) mechanical interference from the lead, (2) leaflet perforation, (3) chordal damage, (4) pacing-induced RV remodeling, (5) device-related infections resulting in valve destruction from endocarditis, and (6) fibrosis involving the lead and TV [
2,
3,
4]. True CIED-induced TR occurs in 5–7% of patients with clinically significant TR [
5]. The presence of CIED leads can result in the progression of TR in 7.2% to 44.7% of cases [
6,
7,
8,
9]. Distinguishing passive coexistence from CIED-induced TR is critical, as it has implications for management. Moderate or severe TR in the presence of CIED confers a markedly worse prognosis, with a 1.6- to 2.5-times increased risk of death [
10,
11].
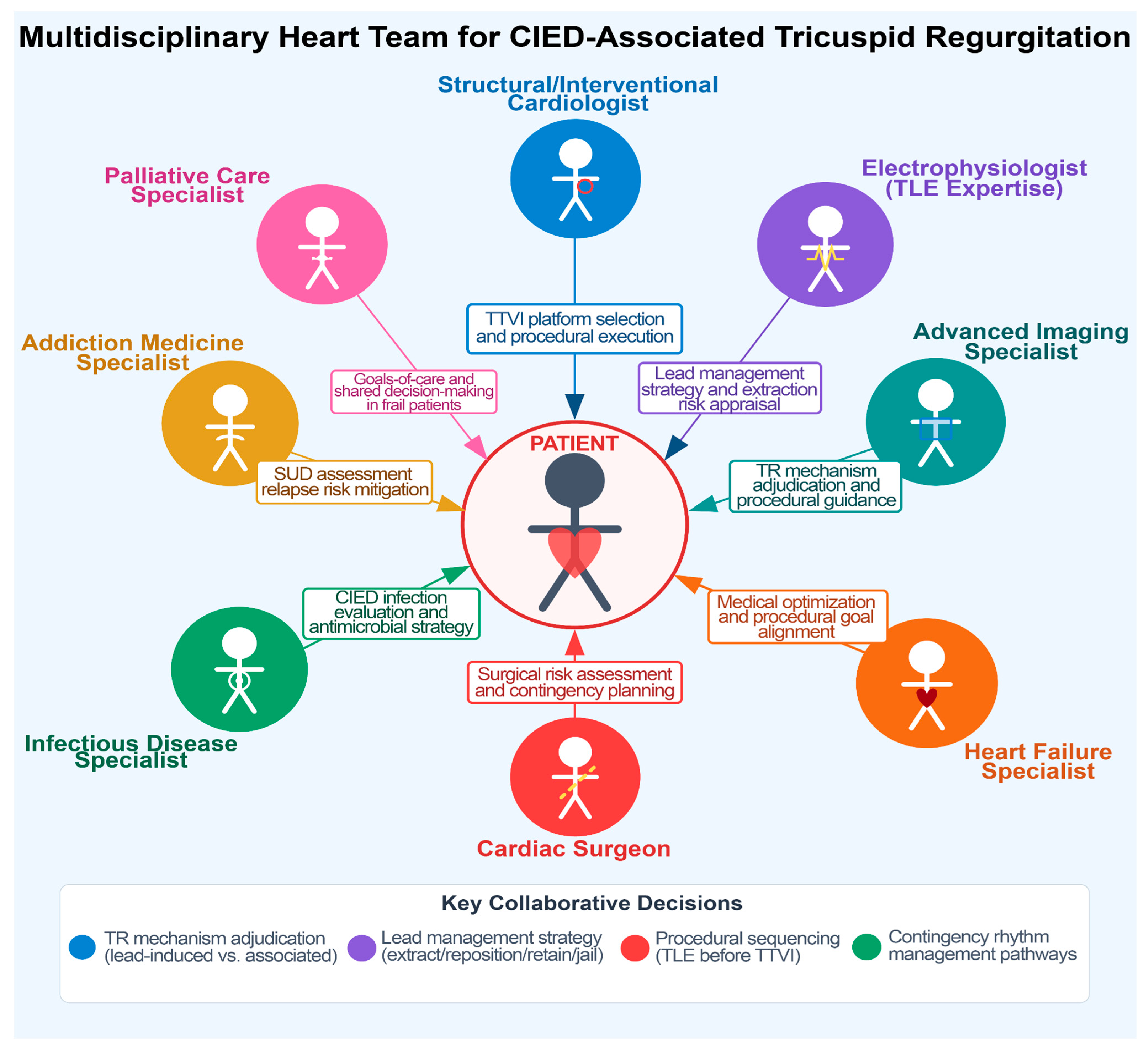
The management of TR in the presence of transvalvular CIED poses unique challenges. The presence of a lead across the valve interferes with transcatheter therapies, raising additional procedural considerations: (1) jailing of a lead between a prosthetic valve or annular device and the native anatomy may damage the lead or impede the valve intervention; (2) entanglement or dislodgement of the lead may occur during catheter manipulation; (3) extracting a chronic pacing lead to avoid jailing prior to TTVI carries procedural risks such as lead fracture, embolization, and need for emergency surgery; and (4) careful decision-making is required to decide if, and what type of, CIED that spares TV needs to be implanted. The complexity of these decisions is ideally addressed by multidisciplinary Heart Teams (
Figure 2,
Table 1) that carefully weigh the trade-offs of each individual decision [
12,
13].
This review synthesizes the available evidence from registries, observational studies, and recent expert consensus statements to provide a practical framework for Heart Teams managing this increasingly common complex clinical scenario. We highlight the critical knowledge gaps and propose directions for future investigations. Currently, no randomized clinical trials (RCTs) have compared management strategies in this population, and clinicians must navigate complex trade-offs, such as lead extraction versus jailing, repair versus replacement, and alternative pacing strategies, with limited long-term outcome data available. This interdependence of rhythm-management and valve-therapy strategies represents the “two-device problem,” wherein decisions about CIED management are inseparably linked to the feasibility, safety, and durability of tricuspid valve interventions, creating an urgent need for consolidated guidance.
2. Methodology
We conducted a narrative review to provide a comprehensive synthesis of the existing literature on CIED-related TR and its management in the context of transcatheter tricuspid valve interventions. A targeted literature search was performed in PubMed/MEDLINE and Embase supplemented by manual review of references from key articles and relevant society documents to identify clinical studies, registries, device reports, and consensus statements addressing TR in CIED populations and/or lead management in the context of TTVI. Search terms included combinations of tricuspid regurgitation, pacemaker, implantable cardioverter-defibrillator, cardiac implantable electronic device, transcatheter tricuspid, tricuspid edge-to-edge repair, TriClip, PASCAL, transcatheter tricuspid valve replacement, EVOQUE, annuloplasty, Cardioband, caval valve implantation, valve-in-valve, lead extraction, and lead jailing.
We prioritized (i) randomized trials when available; (ii) prospective and retrospective observational studies and registries; (iii) device-specific reports relevant to lead–TTVI interactions; and (iv) major professional society guidelines/consensus statements addressing TTVI, CIED lead management/extraction, and valve-sparing pacing/defibrillation options. We focused on adult human data and included studies in English. We excluded studies not pertinent to transvalvular lead–tricuspid valve interaction and reports where outcomes could not be reasonably attributed to the CIED–tricuspid interface.
3. Mechanisms of Tricuspid Regurgitation in Patients with CIEDs and Diagnostic Evaluation
The mechanisms by which CIED leads contribute to TR are categorized as (1) direct mechanical interference, including leaflet impingement, perforation, chordal entanglement, and fibrotic adhesion, and (2) functional TR, from RV pacing-induced dyssynchrony and/or remodeling, leading to annular dilation and papillary muscle displacement. Understanding these mechanisms is essential for distinguishing CIED-induced TR from CIED-incidental TR (
Figure 1), a distinction that directly informs management strategies.
3.1. Mechanical Interference
The mechanical interaction between CIED leads and the TV is the most common mechanism of CIED-induced TR. The septal leaflet is most frequently affected [
3]. CIED-induced injury may occur at the time of implantation or evolve chronically over the years. Acute TR is rare and occurs due to leaflet perforation, damage to the chordae or papillary muscles, resulting in flail leaflet physiology and rapid clinical deterioration [
14,
15]. Lead impingement can prevent adequate leaflet coaptation, resulting in eccentric regurgitant jets that are often underestimated on standard two-dimensional imaging. Over time, repetitive motion and chronic inflammation may lead to fibrous encapsulation of the lead, tethering of the leaflet or subvalvular apparatus, and further exacerbation of TR [
16].
3.2. Functional TR
CIED leads can be causally related to TR through pacing-induced structural remodeling and alterations of RV function. Chronic RV pacing alters the normal sequence of ventricular activation, leading to mechanical dyssynchrony and ultimately promoting RV dilatation. This, in turn, results in TV annular enlargement, apical displacement of RV papillary muscles, and tethering of the TV chords, causing functional TR even in the absence of direct lead–leaflet interaction [
17]. Although there is no consensus on the RV pacing threshold that increases the risk of developing functional TR, a higher RV pacing burden is associated with a higher risk of developing RV dysfunction and functional TR. Pacing-induced left ventricular systolic dysfunction may further worsen TR severity by increasing pulmonary pressure and RV afterload [
17,
18].
3.3. Diagnostic Evaluation: Multimodality Imaging
Accurate characterization of the TR mechanism in patients with CIEDs relies on a multimodality imaging approach.
3.3.1. Echocardiography
Two-dimensional and three-dimensional transthoracic echocardiography (TTE) are the primary diagnostic modalities for evaluating TR severity, mechanism, and right-sided chamber remodeling [
19,
20]. Three-dimensional echocardiography is particularly valuable for defining the spatial relationship between the lead and the TV apparatus, allowing visualization of leaflet impingement, restricted motion, or commissural versus central lead position [
21]. Leads positioned near the commissures or the middle of the annulus are generally associated with less leaflet interference than those coursing directly over the leaflet bodies [
22].
Transesophageal echocardiography (TEE) provides higher spatial and temporal resolution and is often required for definitive mechanism classification, particularly when planning transcatheter intervention or transvenous lead extraction (TLE). TEE enables a detailed assessment of leaflet perforation, adhesions, or flail segments and plays a central role in intraprocedural guidance during TTVI [
22,
23,
24]. Standardized TR quantification, integrating vena contracta width, effective regurgitant orifice area, hepatic vein flow, and three-dimensional jet assessment, is essential to avoid underestimation of the severity of CIED-induced TR [
19,
20].
3.3.2. Computed Tomography
Cardiac CT offers superior spatial resolution and is increasingly used for procedural planning in patients with CIEDs undergoing TTVI or transvenous lead extraction (TLE). CT allows precise assessment of lead–leaflet interaction, tricuspid annular dimensions, and proximity to adjacent structures such as the right coronary artery, which is critical for transcatheter valve replacement planning. CT characterizes central venous anatomy, which is relevant in patients being considered for extraction or CIED implants that do not include transvalvular leads [
25].
4. Transcatheter Tricuspid Valve Interventions and CIED Lead Considerations
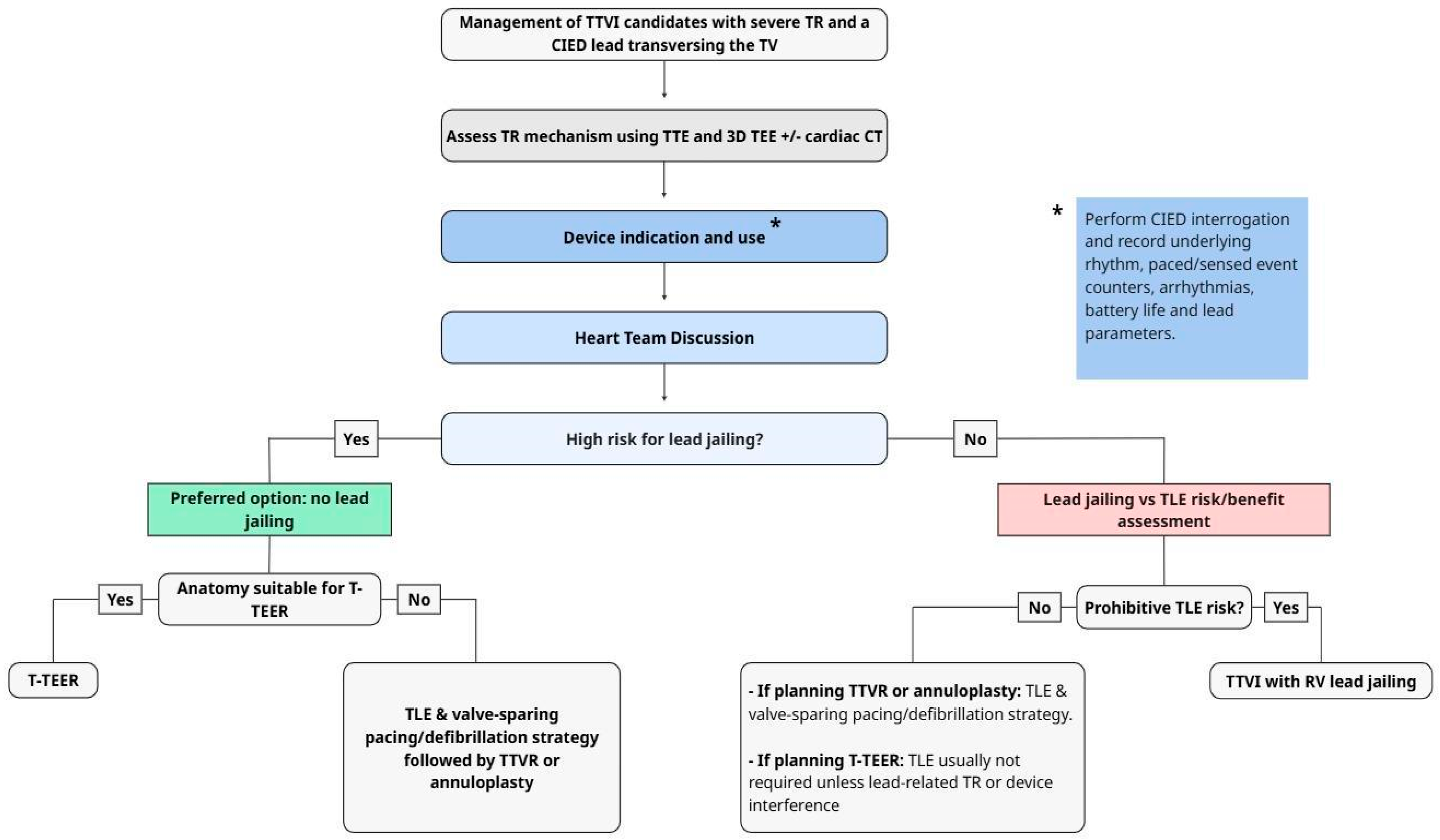
Current TTVI modalities include (1) transcatheter edge-to-edge repair (T-TEER) using devices such as TriClip and PASCAL; (2) transcatheter tricuspid valve replacement (TTVR) with systems such as EVOQUE; (3) direct percutaneous annuloplasty (Cardioband); (4) heterotopic caval valve implantation (CAVI); and (5) tricuspid valve-in-valve (TTVIV) procedures for failed surgical bioprostheses. Understanding how each TTVI modality interacts with existing CIED leads is essential for procedural planning and lead management. This section reviews the current TTVI strategies and the lead-related risks associated with each approach (
Table 2,
Figure 3).
4.1. Transcatheter Tricuspid Edge-to-Edge Repair (T-TEER)
T-TEER is the most widely adopted TTVI modality due to its favorable safety profile and the ability to maintain future therapeutic options. T-TEER approximates the tricuspid leaflets to reduce the regurgitant orifice area, mimicking the surgical Alfieri stitch. Currently available T-TEER devices are the TriClip [
26] (Abbott Structural Heart) and PASCAL [
27] (Edwards Lifesciences, CE-marked in the EU, but not FDA-approved yet). In the TRILUMINATE trial, T-TEER was superior to medical therapy alone in reducing heart failure hospitalizations and improving quality of life and patient-reported health status [
28].
T-TEER is the preferred first-line TTVIs strategy for patients with CIED. This allows for the preservation of lead mobility and does not mandate the fixation of the lead against annular tissue. T-TEER preserves future lead-management options. T-TEER does not jail the lead in a manner that precludes future TLE, and standard TLE tools can be advanced alongside the clips. Furthermore, the presence of a transvalvular lead was not associated with any differences in the safety and effectiveness of T-TEER, the degree of residual TR, and or the one-year mortality in the TRIUMINATE trial and the post-approval TriValve Registry [
28,
29].
A critical decision that needs to be made during the planning of T-TEER in a patient with TR and CIED leads is whether the patient has CIED-induced TR or if the leads are innocent bystanders. In most cases suitable for T-TEER, the lead is an “innocent bystander.” The lead typically lies in a commissural position or sits freely within the regurgitant orifice without tethering the leaflets [
30]. In these cases, T-TEER is feasible, and the clips are deployed at a distance from the lead, ensuring that the grasping elements do not capture the lead body. Conversely, in CIED-induced TR, the presence of leaflet impingement, chordal entanglement, or fibrous adhesions makes T-TEER technically more challenging. In these patients, device deployment close to or on the lead might be necessary, in which case there is a risk of damaging the lead or causing lead jailing, requiring a multi-disciplinary approach [
12,
30].
With the use of intraprocedural 3D TEE, the risk of T-TEER and the risk of grasping a transvalvular lead are exceedingly low (<1%). If, however, a lead is inadvertently captured, the clipping elements can penetrate the lead insulation or crush the internal conductors, causing lead failure [
31].
4.2. Transcatheter Tricuspid Valve Replacement (TTVR)
TTVR corrects TR through orthotopic implantation of a bioprosthetic valve. The only currently approved device is EVOQUE (Edwards Lifesciences). Both rely on radial force and varying anchoring mechanisms to be secured within the native annulus. TTVR offers superior efficacy in correcting TR compared to T-TEER, with near-complete elimination of TR [
32]. In the TRISCEND II RCT, the EVOQUE valve was associated with a significant improvement in 1-year all-cause mortality, functional status, and quality of life. However, given the proximity of the conduction system to the tricuspid annulus and the radial force exerted by the self-expanding frame of the EVOQUE valve, complete heart block requiring pacemaker implantation can occur in up to one-third of the implants [
33,
34]. This significant risk is a critical consideration for patient selection, particularly in those with RV dysfunction, where dyssynchronous pacing can be deleterious [
17].
In TTVR, lead jailing is an unavoidable procedural consequence in any patient with a pre-existing transvenous lead [
1]. Historically, “metal on tissue jailing” was hypothesized to be relatively benign for jailed leads [
35]. However, emerging data challenge the perceived safety of metal-on-tissue jailing. In a retrospective analysis of 32 patients undergoing EVOQUE implantation with a jailed lead, 31% developed lead malfunction during a median follow-up of 210 days, including a decline in R-wave sensing and insulation breach (13% of participants). While only one patient required immediate lead revision in this series, the long-term implications of jailing remain unknown [
36]. As a result, it is common practice for the multidisciplinary TTVR team discussion to involve electrophysiology for consideration of non-transvalvular pacing options discussed in
Section 4.5. Last, the most profound implication of TTVR for CIED leads is the permanent preclusion of transvenous lead extraction. Once a lead is jailed behind a TTVR device, it cannot be safely extracted using standard mechanical or laser tools without the risk of avulsing the entire valve complex or tearing the annulus. Careful multidisciplinary evaluation and shared decision-making to proceed with jailing or with TLE prior to TTVR is critical and will be described in
Section 4.
4.3. Direct Percutaneous Annuloplasty
Direct percutaneous annuloplasty is a reconstructive approach that replicates surgical suture annuloplasty. The Cardioband system (Edwards Lifesciences) is the only clinically available device. It has CE-mark approval in Europe but has not received FDA approval in the United States, where use remains investigational in clinical studies [
37]. The system consists of a Dacron band anchored along the tricuspid annulus using a series of stainless-steel screws and cinched to reduce annular dimensions. In limited RCTs, the Cardioband system results in significant improvement in TR and patient functional status [
38].
The interaction profile of the Cardioband system and CIED leads differs from that of T-TEER and TTVR. The interaction mechanism is a direct mechanical collision between the device anchors and transvalvular lead. Transvenous leads typically cross the tricuspid valve in the septal region, often settling in the posteroseptal or anteroseptal commissures. This overlaps with the anchoring zone of the Cardioband along the anterior and posterior annulus. There is a theoretical risk that a Cardioband system anchor penetrates the lead body, causing insulation failure and/or conductor fracture [
39]. Even in the absence of direct lead injury from the anchoring system, as the band is cinched to reduce the annular circumference, the lead can be pulled medially or compressed against the septal wall, forming a fulcrum point on the lead, potentially accelerating mechanical failure. While Cardioband does not create a circumferential cage like TTVR, it can effectively jail a lead if the band is cinched tightly against it. However, unlike TTVR, lead extraction remains theoretically feasible, provided that the lead has not been skewered by an anchor.
4.4. Caval Valve Implantation (CAVI)
CAVI is a palliative strategy that involves the implantation of bioprosthetic valves into the superior vena cava (SVC) and inferior vena cava (IVC). Among dedicated CAVI platforms, the TricValve system (P&F Products and Features) is CE-marked and available in Europe, whereas U.S. use remains investigational under studies. In addition, balloon-expandable transcatheter heart valves (e.g., Edwards SAPIEN XT/3) have been used for intracaval implantation, typically after creation of a landing zone with a large stent, but this is off-label/investigational repurposing rather than an approved CAVI indication for SAPIEN. CAVI prevents systolic backflow and alleviates systemic venous congestion. CAVI is reserved for patients with severe TR, massive annular dilation (>65–70 mm), who are not candidates for transcatheter repair or replacement, and are at prohibitive surgical risk. CAVI improves ascites, peripheral edema, functional class, and, in limited studies, reverses the remodeling of the right ventricle [
40,
41].
CAVI presents a unique challenge from a lead interaction perspective: the entrapment of the lead in the superior vena cava. The implantation of a stent valve in the SVC jails all transvenous leads behind the stent mesh along the entire length of the SVC. Lead dislocation during CAVI has been previously described [
42]. The superior extent of the SVC valve often reaches or covers the brachiocephalic vein confluence. This creates a mechanical barrier to future implantations. Extracting a lead that is jailed behind an SVC stent is high-risk because the extraction sheath must traverse the stent, with a high risk of stent dislodgment, SVC laceration, or lead fracture. The decision to proceed with CAVI in a patient with transvenous leads should be made after careful multidisciplinary Heart Team assessment and shared decision-making, similar to TTVR.
4.5. Tricuspid Valve-in-Valve (TTVIV)
TTVIV procedures use transcatheter valves to address degenerated surgical bioprosthetic valves. Most commonly used platforms are the Medtronic Melody and Edwards Sapien. TTVIV is an alternative to high-risk redo surgery for bioprosthetic TV failure, with high procedural success and low mortality [
43]. Importantly, although these transcatheter valves are commercially available in the United States and Europe for their labeled indications (SAPIEN for aortic position, Melody for pulmonic position), use in the tricuspid position is typically off-label and performed under Heart Tteam and institutional governance rather than a tricuspid-specific regulatory indication. TTVIV has the same implications for transvalvular CIED leads with TTVR, with the addition that in TTVIV, there is a more hostile mechanical environment for the lead due to the presence of a rigid surgical frame as opposed to native annular tissue. This results in a “Metal-on-Metal” jailing mechanism. Leads jailed between these two valves or between a valve and a ring are subjected to extreme focal compression and shearing forces that can rapidly sever lead insulation and/or fracture the conductors. In the VIVID registry, the lead failure rate was 10.7% over a median follow-up of only 15.2 months in patients with jailed leads in the setting of TTVIV [
44,
45]. This failure rate is significantly higher than that observed in native valve TTVR or T-TEER [
43,
44]. We suggest that the presence of a functional lead is a strong relative contraindication to TTVIV. TLE and replacement in favor of a new leadless or epicardial system should be strongly considered in such cases.
5. CIED Lead Management Strategies
5.1. Patient Assessment
5.1.1. Assessment of Transvenous Lead Extraction Risk
The risk associated with TLE is integral to extraction–preservation decision-making. Risk assessment for TLE should distinguish (i) predictors of major procedure-related complications/procedural death from (ii) predictors of short-term mortality and long-term prognosis after an otherwise successful extraction. Patient factors such as low body mass index, anemia, coagulopathy, and frailty more consistently track with procedural vulnerability/bleeding reserve, whereas comorbidity burden (e.g., renal dysfunction, reduced LVEF, multimorbidity) more strongly influences early and late mortality rather than the mechanical complication risk of extraction itself [
46,
47,
48]. Lead-related factors include prolonged dwell time (typically >10–15 years for pacemaker leads and >5–10 years for ICD leads), multiple transvenous leads, dual-coil ICD leads, fixation mechanism, and insulation characteristics [
47,
48,
49]. Contemporary data from high-volume centers report major complication rates of approximately 1.7% and mortality around 0.5%, with outcomes strongly correlated with operator experience and institutional volume [
47,
48]. To put this risk into perspective, the complication rates and mortality risks at 30 days from TAVR are 9–12% and 2.2–2.4%, respectively [
50]. Thus, it is a common misconception that the risk of TLE is extremely high. We support inclusion of the electrophysiology team as part of the Heart Team evaluating patients with CIED leads undergoing TTVI evaluation at centers with high-volume experience in TLE. Importantly, contemporary analyses show that advanced age alone is not an independent predictor of major TLE complications when procedures are performed in experienced centers. Conversely, some datasets have reported higher major complication risk in younger patients/younger age at first implant, likely reflecting longer cumulative dwell time and more complex lead biology, reinforcing that chronological age should not be used as a reflex disqualifier [
51].
5.1.2. Assessment of Lead Jailing Risk
The risks of lead jailing are categorized as (1) risks related to the lead and (2) risks related to the prosthetic transcatheter valve. The risks related to the lead are as follows: (1) pacemaker dependency, as lead failure in a pacemaker-dependent patient could have catastrophic consequences; (2) high infectious risk (see
Section 5.1.3), as TLE of a jailed infected lead would be of extremely high risk and even not possible; and (3) history of appropriate ICD therapies, as this selects a higher risk population for the development of life-threatening arrhythmias, in which case a failing lead will be ineffective in delivering successful cardioversion/defibrillation. Risks related to the valve include (1) multiple transvenous leads crossing the tricuspid valve, (2) high lead tension with minimal slack, and (3) established leaflet impingement, particularly in the context of direct annuloplasty systems [
35,
44]. In the presence of these features, lead jailing may irreversibly compromise life-sustaining device therapy and preclude future extraction, and is therefore discouraged in favor of TLE [
12,
44].
5.1.3. Assessment of CIED Infection Risk
Risk stratification for CIED infection is a critical adjunct to lead management decisions, particularly when lead preservation or jailing is considered. Factors associated with increased CIED risk factors are summarized in
Table 3 [
46,
47]. In patients with high infection risk, the consequences of lead jailing are significant, as standard management of CIED infection requires complete system removal, which may be prohibitively risky and even technically not possible in jailed leads [
35,
46].
5.2. Transvenous Lead Extraction as a Standalone Strategy
Transvenous lead extraction (TLE) can improve tricuspid valve function in a subset of patients with CIED-induced TR. Improvement in tricuspid valve function after extraction was observed in approximately one-third of cases [
52]. TLE alone does not cure TR, specifically if TV annular dilatation has occurred or if the TV leaflet has adhered to the annulus. TLE as a standalone intervention for TR can be considered in cases where there is (1) unequivocal imaging data that show that the lead–leaflet interaction is the primary driver of TR, (2) absence of significant annular dilatation, and (3) absence of valve adhesions to the annulus or RV that limit mobility. Even in these cases, shared decision-making should include the roughly 1/3 chance of meaningful TR improvement with TLE alone and the possibility of worsening TR requiring urgent valvular intervention (percutaneous or surgical). However, TLE should be avoided when procedural risk clearly outweighs potential benefit, such as in patients with extreme frailty or prohibitive comorbidities, in the presence of hostile lead anatomy (severe calcification, long dwell times, multiple ICD leads, or abandoned leads), when surgical backup is limited, or when patient preferences favor a conservative strategy.
Rigorous risk stratification is essential for TLE shared decision-making, requiring distinction between factors predicting procedural complications versus long-term mortality. High-risk extraction features that most consistently predict major procedure-related complications include hostile lead/anatomic factors (e.g., long dwell time, multiple/abandoned leads, ICD leads, severe fibrosis/calcification, venous occlusion) and limited institutional readiness for rescue. In contrast, advanced age, renal dysfunction, reduced LVEF, and multimorbidity primarily predict early/late mortality after TLE and should be integrated into overall benefit–risk and goals-of-care discussions rather than treated as direct surrogates of extraction mechanical hazard [
46].
5.3. TLE and Lead Preservation/Jailing in Conjunction with Percutaneous Tricuspid Valve Intervention
TLE followed by TTVI can be considered in three settings: (1) TTVI procedures that will result in CIED lead jailing, (2) TTVI procedures that do not result in jailing, and (3) TTVI procedures as “rescue” procedures for worsening TR after TLE. For TTVI that will result in CIED jailing, the authors strongly recommend TLE over jailing the lead and jailing only if there are absolute contraindications or extreme technical difficulties to TLE. Elective TLE mitigates the permanent problem of an entrapped lead segment that is difficult or impossible to extract later, including in the event of device infection [
46,
50]. Although there is concern that TLE could cause traumatic tricuspid apparatus injury (e.g., leaflet avulsion/flail) that might compromise feasibility of subsequent T-TEER or TTVI, contemporary consensus data suggest this is uncommon in experienced programs: the 2018 EHRA consensus reports flail tricuspid leaflet requiring intervention in ~0.03% of cases, with worsening tricuspid valve function in ~0.02–0.59% [
53]. For repair strategies, the advantage of extraction is less uniform and should be decided on a case-by-case basis. In certain scenarios, TLE has an additive benefit, such as when it is performed to eliminate leaflet restriction that would impair coaptation after TEER [
46]. Of note, TTVI are typically planned elective procedures for chronic symptomatic TR, timing of TLE is flexible: the valve intervention can be postponed to allow (i) referral to an experienced extraction center when TLE risk/expertise warrants and/or (ii) a staged strategy in which TLE is performed days–weeks before (rather than during) transcatheter intervention when clinically appropriate. We recommend that jailing is strongly avoided and is performed only when there is a strong justification based on a multidisciplinary risk–benefit analysis (
Figure 3). Jailing allows near-term procedural simplicity but introduces lifetime formidable challenges in lead management, particularly in pacing-dependent patients, those with prior appropriate ICD therapy, or those at high risk for infection [
46].
When lead jailing is contemplated, the following need to be strongly considered and presented to the patient in a shared-decision-making framework: (1) Jailed leads are at risk for lead failure due to insulation breach and conductor fracture, resulting in loss of defibrillation/pacing reliability [
35,
44,
45]. These risks are incompletely quantified because follow-up is limited and cohorts remain small; however, worsening of electrical parameters after TTVR has been reported, including the need for lead revision [
35,
45]. (2) Future TLE may be higher risk and even impossible once a lead is entrapped. If any pocket, device-related, or endovascular infection occurs, the standard of care is complete system removal, and the patient may not have access to a life-saving procedure.
5.4. Pacing and Defibrillation Strategies in Patients with TTVIs After Extraction or Jailing
After TTVI is completed, regardless of whether the existing leads are extracted or jailed, a decision should be made regarding the new pacing or defibrillator system that needs to be implanted. First, it is critical to reassess the need for a pacemaker or ICD. In a large observational cohort, ~84% of patients with implanted pacemakers were not pacemaker-dependent on follow-up (i.e., 16% pacing-dependent) [
54]. Approximately 25% of patients with an ICD for primary prevention no longer meet the indications for primary prevention ICDs [
55]. In these patients, device reimplantation may be deferred. Conversely, following orthotopic TTVR, patients may develop worsening or de novo conduction disease necessitating pacemaker implantation. In TRISCEND II (Evoque), 17.4% of patients required a new permanent pacemaker.
Second, for patients who need a pacemaker or ICD, the risk of having a pacemaker or ICD lead across the newly implanted device needs to be conceptualized. There is limited and conflicting evidence on whether having a lead across a prosthetic tricuspid valve is deleterious to valve function [
52,
56,
57]. Third, for patients who need CIED reimplantation and a transvalvular lead is not desired, the optimal non-transvalvular system needs to be selected, weighing the risks and benefits of each option, together with the patient’s preferences.
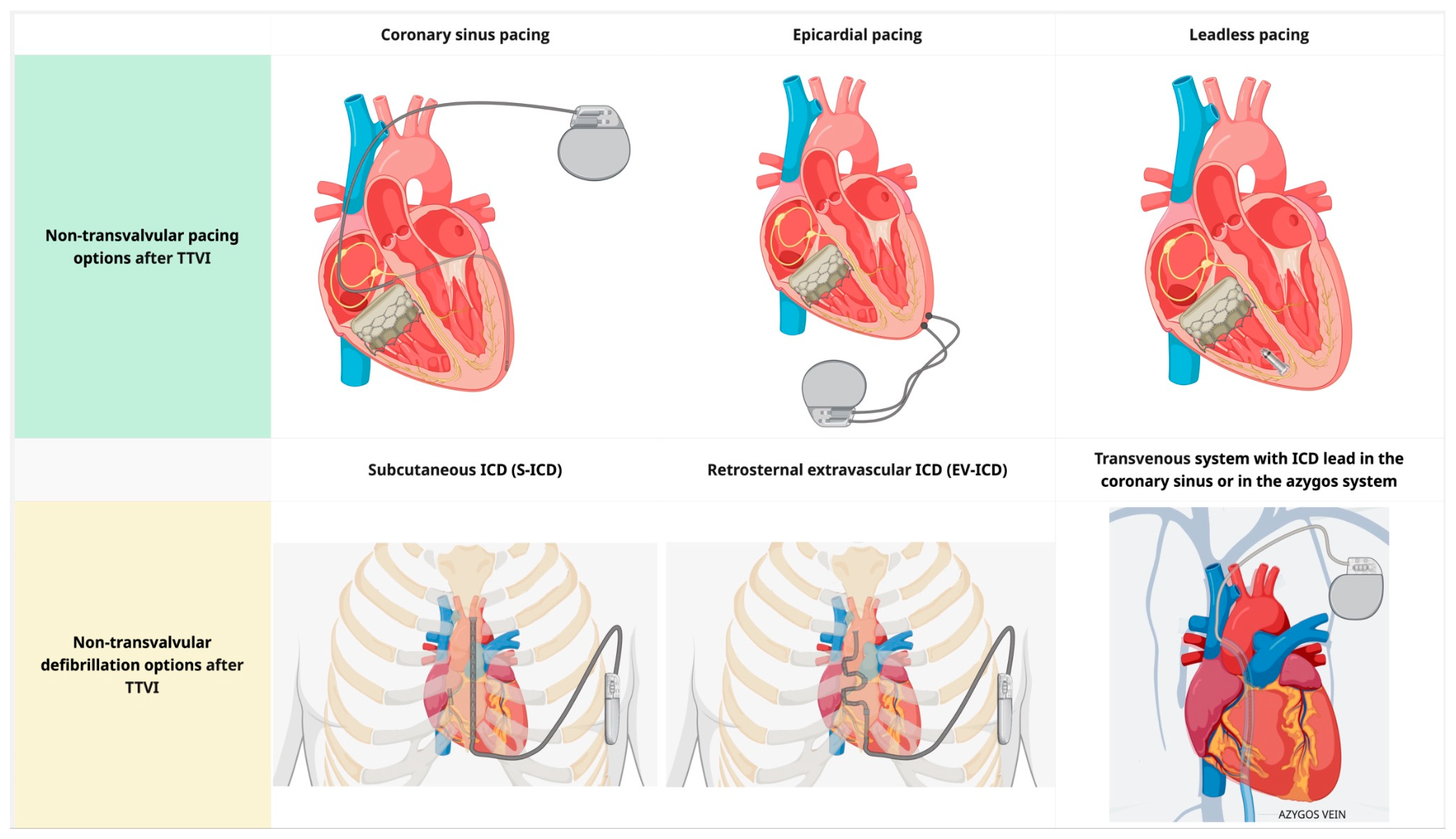
For patients who require right ventricular pacing, non-transvalvular pacing options include (1) coronary sinus (CS) pacing, (2) a leadless pacemaker (MICRA or AVEIR), and (3) epicardial pacing (
Figure 4). CS pacing is an option in patients in whom crossing the tricuspid valve is undesirable or impossible, with acceptable long-term lead performance [
58,
59]. CS-only ventricular pacing can be limited by higher capture thresholds, phrenic nerve stimulation, lead instability, a higher risk of acute dislodgment compared to RV leads, and challenges in achieving reliable sensing/capture in anatomically constrained venous targets. Leadless pacemakers can overcome some limitations of CS-only ventricular pacing. Implantation of an RV leadless pacemaker requires crossing the prosthetic valve with a large-caliber delivery system at the time of implantation, but leaves no lead across the tricuspid valve. The implantation of leadless pacemakers can be safely performed in patients with prosthetic tricuspid valves. The Micra system is smaller than AVEIR and may be preferred to reduce device–catheter interaction during valve deployment; however, Micra AV provides accelerometer-based atrial mechanical sensing without atrial pacing, and therefore does not offer ‘true’ AV synchrony. Leadless pacemakers should be strongly preferred in the setting of high infection risk or limited vascular access [
60]. Long-term real-world follow-up of leadless pacemakers demonstrates low rates of major complications and system revisions, with an extremely low incidence of infection [
61]. Epicardial pacing systems for single-site ventricular pacing should be considered in the extremely rare setting where the other options presented here are not feasible.
For patients who need ICD, non-transvalvular pacing options are: (1) subcutaneous ICD (S-ICD), (2) retrosternal extravascular ICD (EV-ICD), and (3) transvenous system with ICD lead in the coronary sinus or in the azygos vein (
Figure 4). The latter is associated with a high risk of extraction and should be used judiciously [
62]. Both S-ICD and EV-ICD are non-inferior to transvenous systems in providing adequate cardioversion/defibrillation [
63,
64]. The main advantages of the EV-ICD over the S-ICD are the smaller generator size, longer battery longevity, and ability to deliver ATP [
65]. This comes with the trade-off of a more complex implantation procedure and a higher risk of over- or undersensing [
65]. Prior sternotomy is a relative contraindication for EV-ICD implantation. Neither S-ICD nor EV-ICD can deliver pacing for bradycardia indications; if pacing is also required, a hybrid approach with implantation of a leadless device (as described in the previous paragraph) can be combined with the S-ICD and EV-ICD. Caution should be exercised to prevent device–device interactions that result in inappropriate sensing. The presence of atrial pacing is a contraindication for EV-ICD due to the risk of inappropriate sensing, resulting in inappropriate shock [
63]. A modular leadless pacemaker that can be combined with the S-ICD is currently available in limited market release (EMPOWER leadless pacemaker, Boston Scientific) [
66].
For patients who require cardiac resynchronization therapy, the only option that spares the tricuspid valve is total epicardial or hybrid epicardial (RV) combined with transvenous (RA and CS) pacing systems. Epicardial systems are also a salvage approach for patients without vascular access. Placement of an epicardial system has its own technical challenges and surgery-related morbidity [
67,
68]. Epicardial leads have a significantly higher risk of lead failure and worsening lead parameters over time than endocardial leads [
69,
70]. These need to be weighed carefully against the controversial risk of having a single lead across a bioprosthetic tricuspid valve. In our institutions, we prefer an endovascular CRT system over an epicardial system for patients with TTVI who require CRT, provided that the risk of infection is low.
6. Management of Jailed CIED Leads
6.1. Follow-Up of Patients with CIED and Jailed Leads
Given the potential for accelerated lead failure in the setting of a jailed CIED lead, structured post-procedural surveillance is mandatory [
35,
44]. Remote monitoring should be activated whenever available, with alert pathways configured for impedance deviations, threshold rises, sensing drops, noise/oversensing, mode switches, and ICD therapy events [
71,
72].
In-person follow-up frequency after jailing is not supported by high-quality comparative trials and therefore should be framed explicitly as expert-consensus practice [
35,
44,
73]. For patients without remote monitoring capability, in-office evaluations every 3 months during the first year and every 3–6 months thereafter are reasonable, with closer evaluations in pacemaker-dependent patients or those with prior appropriate ICD therapy [
44,
73]. Any new device alert, abrupt parameter change, recurrent oversensing/noise, inappropriate therapies, or symptomatic deterioration should trigger expedited assessment [
46]. If lead failure is confirmed, management decisions should occur within a Heart Team framework, given the added complexity conferred by a jailed lead [
44,
46].
6.2. Management of Lead Malfunction in Jailed Leads
Lead management in failing jailed leads follows the same principles as non-jailed leads, with special considerations that (1) TLE is of considerably higher risk and complexity and might not even be feasible; (2) crossing the prosthetic valve has a theoretical risk of causing valve dysfunction, although data for the latter are limited and conflicting; and (3) the presence of a transvalvular lead might compromise future valve re-interventions [
48,
52,
56]. If a lead malfunction is detected in a CIED with a jailed lead, it must first be determined whether the patient still needs the function of the failing lead [
54,
55]. If the lead function is still needed, first-line management should be conservative with device reprogramming (output/sensitivity adjustment, optimization of noise discrimination algorithms). In the setting of an ICD, defibrillation threshold or margin testing can be considered to assess the ability of the ICD to cardiovert patients in real life. It is critical to assess the impact of programming changes on battery longevity, considering patients’ survival expectancy, as multiple generator changes exponentially increase the risk of infection. If reprogramming cannot restore meaningful lead function with reasonable battery longevity, then the addition of a new pacing or ICD system with abandonment is generally preferred, given the increased risk associated with TLE of jailed leads, even in experienced centers, and the optional nature of the latter [
74]. When a new pacing or ICD system is pursued, valve-sparing options described in
Section 4.5 are preferred, due to concerns about the need for future valve reinterventions [
45,
74,
75].
6.3. Management of CIED and Endovascular Infections in the Setting of Jailed Leads
Infection management in patients with jailed leads is complex. First, the desired scope of treatment (curative vs. palliative) should be decided based on patient goals of care, frailty, comorbidity, and operative candidacy [
76,
77,
78]. Second, the type of infection should be determined: (1) isolated pocket infection, (2) bloodstream infection without definite tricuspid valve device involvement, or (3) lead or valve endocarditis with tricuspid involvement [
76,
78]. If the scope of treatment is curative, in the absence of TV involvement, complete device removal should be pursued; however, with jailed leads, a TLE may be considered only in carefully selected patients at experienced centers after Heart Team review [
49,
78]. TLE in this setting is technically challenging and, in some cases, impossible because traction and powered sheaths transmit force to the prosthesis and annulus, increasing the risk of valve damage, paravalvular leak, cardiac perforation, and lead breakage [
44]. TLE has been described in jailed leads in case reports [
79]. When infection involves the prosthetic tricuspid valve, the curative approach is surgical explant of the tricuspid device plus complete CIED removal [
79].
If the scope of treatment is palliative, long-term suppressive antibiotic therapy can be used for endovascular infections. The prognosis of chronic suppressive antibiotics is less favorable if complete source control is not achieved [
80]. For pocket infections, local ultrahigh-dose antibiotic administration remains investigational and should be framed as a salvage strategy for selected localized pocket infections in patients unsuitable or unwilling to undergo extraction [
81].
7. Heart Team Approach and Workflow
The unique “two-device” problem created by transvalvular leads in patients undergoing TTVI necessitates a formal multidisciplinary Heart Team assessment integrating valve disease decision-making with lead management expertise [
12,
44]. This multidisciplinary approach is essential because procedural strategy selection (percutaneous vs. surgical and type of TTVI) directly influences current and future rhythm-management options. Decisions regarding lead extraction, repositioning, retention, or jailing have durable implications for pacing dependency, defibrillation strategy, infection risk, and venous access complications. The Heart Team should include a structural valve specialist, cardiac electrophysiologist, advanced imaging specialist (with intraprocedural experience), heart failure clinician, and cardiac surgeon [
12]. Infectious disease, addiction medicine, and/or palliative care specialists can be included when indicated (
Table 1,
Figure 2). Key decisions include (1) accurate characterization of the TR mechanism (CIED-induced vs. CIED-associated), (2) optimal TTVI modality, (3) extraction vs. lead preservation risk appraisal, (4) procedural planning, especially if concomitant TLE and TTVI procedures are included, and (5) pre-specification of contingency CIED pathways that avoid chronic transvalvular hardware, when feasible.
8. Current Guidelines
8.1. EHRA/EAPCI 2025 Scientific Statement on Management of Patients with Transvalvular Right Ventricular Leads Undergoing Transcatheter Tricuspid Valve Interventions
The 2025 EHRA/EAPCI 2025 statement on the management of patients with transvalvular right ventricular leads undergoing transcatheter tricuspid valve interventions is the only currently available scientific statement that focuses explicitly on the management of patients with transvalvular CIED leads undergoing TTVI [
44]. This document highlights the cross-disciplinary scope of the problem and the need for multifaceted assessment and procedure planning. The proposed central algorithm for approaching patients with CIED leads requiring TTVI is summarized in
Figure 3. Key recommendations include obtaining complete details of the implanted system prior to any intervention, including assessment of pacemaker dependency and, for ICDs, the type and frequency of tachyarrhythmia therapies delivered. This statement emphasizes a thorough evaluation of TR etiology and TTVI suitability, with particular attention to potential mechanical interactions between CIED leads and transcatheter valve hardware. Central to procedural planning is the assessment of lead jailing risk, for which the statement defines specific “red flags”: pacemaker dependency, ICD with prior delivered therapy, multiple leads crossing the tricuspid valve, previous CIED infection, and high lead tension. Although TLE is recognized as a viable option—particularly in fragile, often elderly patients—it carries a low but non-negligible risk of major complications (1.7%) and death (0.5%) and may paradoxically worsen TR severity in 3.5–15% of cases due to adhesions between leads and the tricuspid apparatus [
47,
82]. For patients in whom lead jailing is pursued, frequent monitoring is essential, especially for those who are pacemaker-dependent or have an ICD for secondary prevention. Alternative pacing strategies have been proposed to mitigate CIED-induced TR and minimize interactions with implanted tricuspid devices, including coronary sinus ventricular pacing, epicardial pacing, and leadless pacemakers, whereas subcutaneous or extravascular ICDs are recommended for patients requiring defibrillator therapy. Given the limited scientific evidence currently available, the statement underscores that case-by-case Heart Team discussions and patient engagement are essential and calls for prospective systematic data collection and long-term follow-up to strengthen future recommendations [
44].
8.2. Other Valve or CIED Guidelines Discussing Management of CIED Relevant to Tricuspid Valve Interventions
Beyond the 2025 EHRA/EAPCI scientific statement, several complementary guidelines and expert documents have addressed the intersection of CIEDs and tricuspid valve disease. These are summarized in
Table 4.
The 2021 ESC Guidelines on Cardiac Pacing and CRT recognize tricuspid regurgitation as a clinically relevant complication of transvenous RV lead implantation and provide the following recommendations: (1) when pacing is required at the time of tricuspid valve surgery, epicardial ventricular leads should be considered (Class IIa, Level C) to prevent prosthesis interference and future lead–valve conflict; (2) for patients with tricuspid valve prostheses (particularly mechanical valves) in whom transvalvular access is contraindicated, coronary sinus–based or epicardial pacing strategies are acknowledged as valve-sparing alternatives; and (3) structured follow-up pathways, including remote monitoring, are supported to detect device- or lead-related issues early [
83].
The 2017 HRS Expert Consensus Statement on CIED Lead Management and Extraction, though predating contemporary TTVI technologies, remains foundational by (1) codifying definitions, procedural endpoints, and outcomes reporting standards; (2) establishing a safety-oriented framework emphasizing that extraction outcomes are tightly coupled to program infrastructure and operator expertise; and (3) mandating institutional preparedness for catastrophic complications—principles that align with the multidisciplinary Heart Team model now advocated for CIED–tricuspid cases [
46].
The 2025 ACC/AHA/ASE/HFSA/HRS/SCAI/SCCT/SCMR Appropriate Use Criteria (1) explicitly address the scenario of ventricular pacing indication in patients with prior tricuspid valve surgery and (2) rate leadless pacemaker implantation as “May Be Appropriate” in this setting [
84]. This is complemented by the 2022 EHRA/HRS/LAHRS/APHRS Position Paper on Leadless and Extravascular CIEDs, which (1) provides a clinician-facing framework for candidacy and procedural planning, (2) positions leadless pacing as a particularly attractive valve-sparing solution when transvalvular leads are undesirable or infeasible, and (3) outlines considerations for subcutaneous and extravascular ICD systems in patients requiring defibrillator therapy [
85].
Collectively, these documents reflect an evolving paradigm in which CIED and tricuspid valve management are no longer considered in isolation but rather as interdependent components of a comprehensive, patient-centered lifetime strategy.
9. Future Directions and Research Priorities
Several research priorities must be addressed to advance this field (
Table 5). First, multicenter randomized trials or rigorously designed prospective registries with prespecified treatment algorithms are needed to compare extraction versus lead preservation strategies. These studies should have clinically meaningful endpoints, including functional status, heart failure hospitalization, mortality, and device-specific outcomes, such as lead performance, infection, and feasibility of future extraction. Second, the standardization of criteria is essential: unified definitions distinguishing CIED-induced TR from CIED-incidental TR and mixed TR will reduce misclassification and improve cross-study comparability. Third, validated predictive tools are required to identify patients likely to benefit from extraction, stratify procedural risk from pre-procedural imaging, and guide individualized decision-making. Fourth, a dedicated data infrastructure, including international registries with harmonized imaging protocols and jailed-lead cohorts followed for >2 years, is necessary to generate the evidence base required for guideline-level recommendations.
However, a true paradigm shift would be to reimagine the relationship between devices and valves at the earliest stages of patient management, perform CIED implants in such a way that TR is prevented, and develop technologies and techniques that allow for percutaneous lead repair without compromising the leads. TR prevention should be a primary objective, beginning with implantation strategies using real-time 3D echocardiographic guidance to avoid leaflet-interfering trajectories and extending to device selection frameworks that proactively consider lifetime tricuspid valve health. The rapid maturation of leadless and extravascular platforms offers a compelling valve-sparing paradigm: modular systems combining leadless pacemakers with subcutaneous or extravascular ICDs can deliver pacing and defibrillation without transvalvular hardware, whereas emerging leadless conduction system pacing and fully leadless CRT configurations may provide physiologic activation without tricuspid traversal [
86]. Next-generation TTVI devices may incorporate lead-accommodating features, such as commissural alignment strategies or dedicated lead channels. Sensor-equipped “smart” leads could detect early valve interaction before clinical TR develops, and artificial intelligence may enable personalized decision-making based on integrated clinical, imaging, and device variables. Ultimately, the goal is a future in which device therapy and tricuspid valve health are managed not as competing considerations but as integrated components of a unified, patient-centered, lifetime strategy.
10. Limitations
This review has several limitations inherent to its narrative design. First, although we used a structured search approach, study identification and selection are subject to selection and publication bias. Second, the evidence base informing management of transvalvular leads in the TTVI era remains dominated by observational studies, registries, small series, and expert consensus, with a paucity of randomized comparisons—particularly for extract-versus-jail pathways and long-term outcomes after lead jailing. Last, substantial heterogeneity exists (qualitatively) across studies in TR phenotyping, imaging protocols, device platforms, endpoints, and follow-up duration.
11. Conclusions
The management of patients with transvalvular CIED leads undergoing TTVI represents one of the most complex aspects of contemporary cardiovascular medicine. It requires a coordinated Heart Team framework that includes expertise in electrophysiology, structural heart disease, imaging, heart failure, and cardiac surgery. Currently, there is a paucity of randomized evidence to guide management decisions, such as TLE versus lead preservation strategies, limited long-term data on jailed leads, and an incomplete understanding of which patients derive benefit from device modification prior to TTVI. However, this challenge also presents an unprecedented opportunity: the convergence of advancing leadless and extravascular technologies, maturing transcatheter tricuspid platforms, and evolving imaging capabilities creates a path toward a future in which CIEDs and TV disease are managed as integrated components of a unified, patient-centered lifetime strategy rather than competing clinical imperatives. Realizing this vision requires dedicated prospective registries, standardized definitions distinguishing CIED-induced, CIED-incidental, and mixed TR, and collaborative industry–academic partnerships focused on valve-sparing device innovations. As the population of patients with CIEDs and TR continues to grow, so does the urgency—and the promise—of transforming this clinical frontier into an exemplar of precision cardiovascular care.
Author Contributions
Conceptualization, A.R.K. and K.N.A.; Methodology, M.H.K. and K.N.A.; Writing—original draft preparation, M.H.K.; Writing—review and editing, M.H.K., E.M.P., F.R., A.R.K., and K.N.A.; Visualization, M.H.K.; Supervision, K.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
AI statement: Portions of this manuscript were edited for grammar and clarity using ChatGPT (5.2). The authors reviewed and approved all content and take full responsibility for the accuracy and integrity of the work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 3D | Three-dimensional |
| ACC/AHA/ASE/HFSA/HRS/SCAI/SCCT/SCMR | American College of Cardiology/American Heart Association/American Society of Echocardiography/Heart Failure Society of America/Heart Rhythm Society/Society for Cardiovascular Angiography and Interventions/Society of Cardiovascular Computed Tomography/Society for Cardiovascular Magnetic Resonance |
| AF | Atrial fibrillation |
| AI | Artificial intelligence |
| ATP | Antitachycardia pacing |
| AV | Atrioventricular |
| CAVI | Caval valve implantation (heterotopic caval valve implantation) |
| CE | Conformité Européenne (CE marking) |
| CIED | Cardiac implantable electronic device |
| CRT | Cardiac resynchronization therapy |
| CRT-D | CRT with defibrillator |
| CS | Coronary sinus |
| CT | Computed tomography |
| ECAVI/EHRA | European Association of Cardiovascular Imaging/European Heart Rhythm Association (commonly abbreviated EACVI/EHRA; appears as ECAVI/EHRA in your manuscript) |
| EHRA | European Heart Rhythm Association |
| EHRA/EAPCI | European Heart Rhythm Association/European Association of Percutaneous Cardiovascular Interventions |
| EHRA/HRS/LAHRS/APHRS | European Heart Rhythm Association/Heart Rhythm Society/Latin American Heart Rhythm Society/Asia Pacific Heart Rhythm Society |
| ESC | European Society of Cardiology |
| EU | European Union |
| EV-ICD | Extravascular ICD |
| FDA | U.S. Food and Drug Administration |
| HRS | Heart Rhythm Society |
| ICD | Implantable cardioverter-defibrillator |
| ICE | Intracardiac echocardiography |
| IVC | Inferior vena cava |
| LV | Left ventricle |
| PH | Pulmonary hypertension |
| PM | Pacemaker |
| RA | Right atrium |
| RCT | Randomized controlled trial |
| RV | Right ventricle |
| S-ICD | Subcutaneous ICD |
| SVC | Superior vena cava |
| T-TEER | Transcatheter tricuspid edge-to-edge repair |
| TAVR | Transcatheter aortic valve replacement |
| TEE | Transesophageal echocardiography |
| TEER | Transcatheter edge-to-edge repair |
| TLE | Transvenous lead extraction |
| TR | Tricuspid regurgitation |
| TRILUMINATE | TRILUMINATE trial |
| TRISCEND | TRISCEND trial |
| TRIUMINATE | TRIUMINATE study/trial |
| TTE | Transthoracic echocardiography |
| TTVI | Transcatheter tricuspid valve intervention |
| T-VIV | Tricuspid valve-in-valve |
| TTVR | Transcatheter tricuspid valve replacement |
| TV | Tricuspid valve |
| VHD | Valvular heart disease |
| VIVID | Valve-in-Valve International Data (VIVID) registry |
References
- Sannino, A.; Ilardi, F.; Hahn, R.T.; Lancellotti, P.; Lurz, P.; Smith, R.L.; Esposito, G.; Grayburn, P.A. Clinical and echocardiographic outcomes of transcatheter tricuspid valve interventions: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 919395. [Google Scholar] [CrossRef]
- Trankle, C.R.; Gertz, Z.M.; Koneru, J.N.; Kasirajan, V.; Nicolato, P.; Bhardwaj, H.L.; Ellenbogen, K.A.; Kalahasty, G. Severe tricuspid regurgitation due to interactions with right ventricular permanent pacemaker or defibrillator leads. Pacing Clin. Electrophysiol. 2018, 41, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Abutaleb, A.; Jeevanandam, V.; Smith, H.; Belkin, M.; Husain, A.; Pinney, S.; Ota, T.; Lang, R.M.; Addetia, K. Anatomic description of tricuspid apparatus interference from implantable intracardiac devices. Cardiovasc. Imaging 2022, 15, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Addetia, K.; Harb, S.C.; Hahn, R.T.; Kapadia, S.; Lang, R.M. Cardiac implantable electronic device lead-induced tricuspid regurgitation. JACC Cardiovasc. Imaging 2019, 12, 622–636. [Google Scholar] [CrossRef]
- Van De Heyning, C.M.; Elbarasi, E.; Masiero, S.; Brambatti, M.; Ghazal, S.; Al-Maashani, S.; Capucci, A.; Leong, D.; Shivalkar, B.; Saenen, J.B. Prospective study of tricuspid regurgitation associated with permanent leads after cardiac rhythm device implantation. Can. J. Cardiol. 2019, 35, 389–395. [Google Scholar] [CrossRef]
- Höke, U.; Auger, D.; Thijssen, J.; Wolterbeek, R.; van der Velde, E.T.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart 2014, 100, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Fanari, Z.; Hammami, S.; Hammami, M.B.; Hammami, S.; Shuraih, M. The effects of right ventricular apical pacing with transvenous pacemaker and implantable cardioverter defibrillator on mitral and tricuspid regurgitation. J. Electrocardiol. 2015, 48, 791–797. [Google Scholar] [CrossRef]
- Seo, J.; Kim, D.-Y.; Cho, I.; Hong, G.-R.; Ha, J.-W.; Shim, C.Y. Prevalence, predictors, and prognosis of tricuspid regurgitation following permanent pacemaker implantation. PLoS ONE 2020, 15, e0235230. [Google Scholar] [CrossRef]
- Lee, R.C.; Friedman, S.E.; Kono, A.T.; Greenberg, M.L.; Palac, R.T. Tricuspid regurgitation following implantation of endocardial leads: Incidence and predictors. Pacing Clin. Electrophysiol. 2015, 38, 1267–1274. [Google Scholar] [CrossRef]
- Delling, F.N.; Hassan, Z.K.; Piatkowski, G.; Tsao, C.W.; Rajabali, A.; Markson, L.J.; Zimetbaum, P.J.; Manning, W.J.; Chang, J.D.; Mukamal, K.J. Tricuspid regurgitation and mortality in patients with transvenous permanent pacemaker leads. Am. J. Cardiol. 2016, 117, 988–992. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Wei, M.; Xiang, R.; Lu, Y.-M.; Zhang, L.; Li, Y.-D.; Zhang, J.-H.; Xing, Q.; Tu-Erhong, Z.K.; Tang, B.-P. Incidence, risk factors, and prognosis of tricuspid regurgitation after cardiac implantable electronic device implantation: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1741–1755. [Google Scholar] [CrossRef]
- Gabriels, J.K.; Schaller, R.D.; Koss, E.; Rutkin, B.J.; Carrillo, R.G.; Epstein, L.M. Lead management in patients undergoing percutaneous tricuspid valve replacement or repair: A ‘heart team’approach. Europace 2023, 25, euad300. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Wilkoff, B.L.; Kodali, S.; Birgersdotter-Green, U.M.; Ailawadi, G.; Addetia, K.; Andreas, M.; Auricchio, A.; Ehlert, F.; George, I. Managing implanted cardiac electronic devices in patients with severe tricuspid regurgitation: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2024, 83, 2002–2014. [Google Scholar] [CrossRef]
- Kim, J.B.; Spevack, D.M.; Tunick, P.A.; Bullinga, J.R.; Kronzon, I.; Chinitz, L.A.; Reynolds, H.R. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: An observational study. J. Am. Soc. Echocardiogr. 2008, 21, 284–287. [Google Scholar] [CrossRef]
- Lin, G.; Nishimura, R.A.; Connolly, H.M.; Dearani, J.A.; Sundt, T.M.; Hayes, D.L. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J. Am. Coll. Cardiol. 2005, 45, 1672–1675. [Google Scholar] [CrossRef]
- Al-Bawardy, R.; Krishnaswamy, A.; Rajeswaran, J.; Bhargava, M.; Wazni, O.; Wilkoff, B.; Tuzcu, E.M.; Martin, D.; Thomas, J.; Blackstone, E. Tricuspid regurgitation and implantable devices. Pacing Clin. Electrophysiol. 2015, 38, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm 2016, 13, 2272–2278. [Google Scholar] [CrossRef]
- Khurshid, S.; Epstein, A.E.; Verdino, R.J.; Lin, D.; Goldberg, L.R.; Marchlinski, F.E.; Frankel, D.S. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm 2014, 11, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Jone, P.-N.; Chamsi-Pasha, M.A.; Chen, T.; Collins, K.A.; Desai, M.Y.; Grayburn, P.; Groves, D.W.; Hahn, R.T.; Little, S.H.; et al. Guidelines for the Evaluation of Prosthetic Valve Function With Cardiovascular Imaging: A Report From the American Society of Echocardiography Developed in Collaboration With the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2024, 37, 2–63. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Hahn, R.T.; Nabauer, M.; Zuber, M.; Nazif, T.M.; Hausleiter, J.; Taramasso, M.; Pozzoli, A.; George, I.; Kodali, S.; Bapat, V. Intraprocedural imaging of transcatheter tricuspid valve interventions. JACC Cardiovasc. Imaging 2019, 12, 532–553. [Google Scholar] [CrossRef]
- Khalique, O.K.; Hahn, R.T. Role of echocardiography in transcatheter valvular heart disease interventions. Curr. Cardiol. Rep. 2017, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Oestreich, B.A.; Ahlgren, B.; Seres, T.; Zipse, M.M.; Tompkins, C.; Varosy, P.D.; Aleong, R.G. Use of transesophageal echocardiography to improve the safety of transvenous lead extraction. JACC Clin. Electrophysiol. 2015, 1, 442–448. [Google Scholar] [CrossRef]
- Peterson, G.E.; Brickner, M.E.; Reimold, S.C. Transesophageal echocardiography: Clinical indications and applications. Circulation 2003, 107, 2398–2402. [Google Scholar] [CrossRef] [PubMed]
- van Rosendael, P.J.; Kamperidis, V.; Kong, W.K.; van Rosendael, A.R.; van der Kley, F.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Computed tomography for planning transcatheter tricuspid valve therapy. Eur. Heart J. 2017, 38, 665–674. [Google Scholar] [CrossRef]
- Drogy, M.S.; Whiteson, H.Z.; Frishman, W.H. Percutaneous tricuspid valve repair: The triclip. Cardiol. Rev. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Fam, N.P.; Ho, E.C.; Zahrani, M.; Samargandy, S.; Connelly, K.A. Transcatheter tricuspid valve repair with the PASCAL system. JACC Cardiovasc. Interv. 2018, 11, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Makkar, R.R.; Whisenant, B.K.; Hamid, N.; Naik, H.; Tadros, P.; Price, M.J.; Singh, G.; Schwartz, J.G.; Kapadia, S. Two-Year Outcomes of Transcatheter Edge-to-Edge Repair for Severe Tricuspid Regurgitation: The TRILUMINATE Pivotal Randomized Controlled Trial. Circulation 2025, 151, 1630–1638. [Google Scholar] [CrossRef]
- Mehr, M.; Taramasso, M.; Besler, C.; Ruf, T.; Connelly, K.A.; Weber, M.; Yzeiraj, E.; Schiavi, D.; Mangieri, A.; Vaskelyte, L. 1-Year outcomes after edge-to-edge valve repair for symptomatic tricuspid regurgitation: Results from the TriValve registry. JACC Cardiovasc. Interv. 2019, 12, 1451–1461. [Google Scholar] [CrossRef]
- Schaller, R.D.; El-Chami, M.F. Cardiac implantable electronic device placement in the era of transcatheter tricuspid replacement: Approaches and challenges. J. Innov. Card. Rhythm Manag. 2025, 16, 6478. [Google Scholar]
- Von Stein, J.; Von Stein, P.; Pfister, R.; Kresoja, K.-P.; Fortmeier, V.; Koell, B.; Rottbauer, W.; Kassar, M.; Goebel, B.; Denti, P. Tricuspid valve transcatheter edge-to-edge repair in patients with cardiac implantable electronic devices: Insights from EuroTR. JACC Cardiovasc. Interv. 2025, 18, 2878–2891. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Kodali, S.K.; Hahn, R.T.; Lurz, P.; Thourani, V.H.; Kozorovitsky, E.R.; Gilmore, S.Y.; Vinekar, C.; Zhang, B.; Boulware, K. TRISCEND II: Novel randomized trial design for transcatheter tricuspid valve replacement. Am. J. Cardiol. 2024, 225, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Hahn, R.T.; Makkar, R.; Makar, M.; Davidson, C.J.; Puthumana, J.J.; Zahr, F.; Chadderdon, S.; Fam, N.; Ong, G. Transfemoral tricuspid valve replacement and one-year outcomes: The TRISCEND study. Eur. Heart J. 2023, 44, 4862–4873. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, D.; Mattig, I.; Samim, D.; Goebel, B.; Jantsch, C.; Rubinic, B.; Ruf, T.; Geisler, T.; Kessler, M.; Adam, M. Early Outcomes of real-world transcatheter tricuspid valve replacement. JACC Cardiovasc. Interv. 2025, 18, 1896–1909. [Google Scholar] [CrossRef]
- Abbasi, M.; Killu, A.M.; Van Niekerk, C.; Deshmukh, A.; Madhavan, M.; Cha, Y.-M.; Mulpuru, S.K.; Friedman, P.A.; Holst, K.; Rihal, C.S. Device-lead abnormalities and function after transcatheter tricuspid valve replacement. Europace 2025, 27, euaf219. [Google Scholar] [CrossRef]
- Defaye, P.; Biffi, M.; El-Chami, M.; Boveda, S.; Glikson, M.; Piccini, J.; Vitolo, M. Cardiac pacing and lead devices management: 25 years of research at EP Europace journal. Europace 2023, 25, euad202. [Google Scholar] [CrossRef]
- Gray, W.A.; Abramson, S.V.; Lim, S.; Fowler, D.; Smith, R.L.; Grayburn, P.A.; Kodali, S.K.; Hahn, R.T.; Kipperman, R.M.; Koulogiannis, K.P. 1-year outcomes of cardioband tricuspid valve reconstruction system early feasibility study. Cardiovasc. Interv. 2022, 15, 1921–1932. [Google Scholar] [CrossRef]
- Nickenig, G.; Weber, M.; Schüler, R.; Hausleiter, J.; Nabauer, M.; von Bardeleben, R.S.; Sotiriou, E.; Schäfer, U.; Deuschl, F.; Alessandrini, H.; et al. Tricuspid valve repair with the Cardioband system: Two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention 2021, 16, e1264–e1271. [Google Scholar] [CrossRef]
- Miller, M.; Thourani, V.H.; Whisenant, B. The Cardioband transcatheter annular reduction system. Ann. Cardiothorac. Surg. 2018, 7, 741. [Google Scholar] [CrossRef]
- Bozbaş, H.; Barçın, C.; Asfour, M.; Çelebi, S.A.; Çam, E.; İlkay, E. Caval Valve Implantation Procedure in 7 Cases of Torrential Tricuspid Regurgitation and Step-by-Step Description of the Procedure. Anatol. J. Cardiol. 2025, 29, 261. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Loureiro, R.; Sánchez-Recalde, A.; Amat-Santos, I.J.; Cruz-González, I.; Baz, J.A.; Pascual, I.; Mascherbauer, J.; Abdul-Jawad Altisent, O.; Nombela-Franco, L.; Pan, M. 6-month outcomes of the TricValve system in patients with tricuspid regurgitation: The TRICUS EURO study. Cardiovasc. Interv. 2022, 15, 1366–1377. [Google Scholar]
- Chen, M.; Moschovitis, A.; Taramasso, M. Pacemaker lead dislocation during TricValve procedure with an extremely small superior vena cava. Int. J. Cardiovasc. Imaging 2024, 40, 1149–1151. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Aboulhosn, J.A.; Dvir, D.; Whisenant, B.; Zhang, Y.; Eicken, A.; Ribichini, F.; Tzifa, A.; Hainstock, M.R.; Martin, M.H. Mid-term valve-related outcomes after transcatheter tricuspid valve-in-valve or valve-in-ring replacement. J. Am. Coll. Cardiol. 2019, 73, 148–157. [Google Scholar] [CrossRef]
- Deharo, J.-C.; Dreyfus, J.; Bongiorni, M.-G.; Burri, H.; Defaye, P.; Glikson, M.; Lever, N.; Mangieri, A.; Mondésert, B.; Nielsen, J.C. Management of patients with transvalvular right ventricular leads undergoing transcatheter tricuspid valve interventions: A scientific statement of the European Heart Rhythm Association and the European Association of Percutaneous Cardiovascular Interventions of the ESC endorsed by the Heart Rhythm Society, the Asian Pacific Heart Rhythm Society and the Canadian Heart Rhythm Society. Europace 2025, 27, euaf061. [Google Scholar]
- Anderson, J.H.; McElhinney, D.B.; Aboulhosn, J.; Zhang, Y.; Ribichini, F.; Eicken, A.; Whisenant, B.; Jones, T.; Kornowski, R.; Dvir, D. Management and outcomes of transvenous pacing leads in patients undergoing transcatheter tricuspid valve replacement. Cardiovasc. Interv. 2020, 13, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.-M.; Clancy, J.; Deharo, J.-C.; Ellenbogen, K.A. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.A.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A. The European lead extraction controlled (ELECTRa) study: A European heart rhythm association (EHRA) registry of transvenous lead extraction outcomes. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Kutarski, A. Transvenous lead extraction SAFeTY score for risk stratification and proper patient selection for removal procedures using mechanical tools. J. Clin. Med. 2020, 9, 361. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, e51–e156. [Google Scholar] [PubMed]
- Anwaruddin, S.; Desai, N.D.; Vemulapalli, S.; Marquis-Gravel, G.; Li, Z.; Kosinski, A.; Reardon, M.J. Evaluating out-of-hospital 30-day mortality after transfemoral transcatheter aortic valve replacement: An STS/ACC TVT analysis. Cardiovasc. Interv. 2021, 14, 261–274. [Google Scholar]
- Kutarski, A.; Jacheć, W.; Tułecki, Ł.; Czajkowski, M.; Nowosielecka, D.; Stefańczyk, P.; Tomków, K.; Polewczyk, A. Disparities in transvenous lead extraction in young adults. Sci. Rep. 2022, 12, 9601. [Google Scholar] [CrossRef]
- Polewczyk, A.; Jacheć, W.; Nowosielecka, D.; Tomaszewski, A.; Brzozowski, W.; Szczęśniak-Stańczyk, D.; Duda, K.; Kutarski, A. Lead dependent tricuspid valve dysfunction-risk factors, improvement after transvenous lead extraction and long-term prognosis. J. Clin. Med. 2021, 11, 89. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. EP Eur. 2018, 20, 1217. [Google Scholar] [CrossRef]
- Grimm, W.; Grimm, K.; Greene, B.; Parahuleva, M. Predictors of pacing-dependency in patients with cardiovascular implantable electronic devices. Cardiol. J. 2021, 28, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Kini, V.; Soufi, M.K.; Deo, R.; Epstein, A.E.; Bala, R.; Riley, M.; Groeneveld, P.W.; Shalaby, A.; Dixit, S. Appropriateness of primary prevention implantable cardioverter-defibrillators at the time of generator replacement: Are indications still met? J. Am. Coll. Cardiol. 2014, 63, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Blauwet, L.A.; Cha, Y.-M.; Connolly, H.M.; Brady, P.A.; Dearani, J.A.; Espinosa, R.E. Bioprosthetic tricuspid valve regurgitation associated with pacemaker or defibrillator lead implantation. J. Am. Coll. Cardiol. 2012, 59, 813–818. [Google Scholar] [CrossRef]
- Hanafy, D.A.; Soesanto, A.M.; Setianto, B.; Immanuel, S.; Raharjo, S.B.; Herqutanto; Amir, M.; Yuniadi, Y. Identification of pacemaker lead position using fluoroscopy to avoid significant tricuspid regurgitation. J. Clin. Med. 2023, 12, 4782. [Google Scholar] [CrossRef]
- Noheria, A.; Van Zyl, M.; Scott, L.R.; Srivathsan, K.; Madhavan, M.; Asirvatham, S.J.; McLeod, C.J. Single-site ventricular pacing via the coronary sinus in patients with tricuspid valve disease. EP Eur. 2018, 20, 636–642. [Google Scholar] [CrossRef]
- Hanna, D.B.; Verghese, D.; Patel, S.; Wang, D.D.; Sharma, D. Coronary Sinus Pacing After Tricuspid Valve Intervention: Single-Center Case Series. Case Rep. 2025, 30, 104560. [Google Scholar]
- Fink, T.; Eitz, T.; Sciacca, V.; Rudolph, V.; Sohns, C.; Sommer, P.; Imnadze, G. Transfemoral leadless pacemaker implantation after interventional or surgical tricuspid valve repair. Europace 2024, 26, euae111. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Garweg, C.; Clementy, N.; Al-Samadi, F.; Iacopino, S.; Martinez-Sande, J.L.; Roberts, P.R.; Tondo, C.; Johansen, J.B.; Vinolas-Prat, X. Leadless pacemakers at 5-year follow-up: The Micra transcatheter pacing system post-approval registry. Eur. Heart J. 2024, 45, 1241–1251. [Google Scholar] [CrossRef]
- Sampognaro, J.R.; Lewis, R.K.; Black-Maier, E.; Pokorney, S.D.; Hegland, D.D.; Piccini, J.P. Cases of Azygous Coil Extraction. Heart Rhythm O2 2022, 3, 65–69. [Google Scholar] [CrossRef]
- Friedman, P.; Murgatroyd, F.; Boersma, L.V.; Manlucu, J.; Knight, B.P.; Clémenty, N.; Leclercq, C.; Amin, A.; Merkely, B.; Birgersdotter-Green, U.M. Performance and safety of the extravascular implantable cardioverter defibrillator through long-term follow-up: Final results from the pivotal study. Circulation 2025, 151, 322–332. [Google Scholar] [CrossRef]
- Gold, M.R.; Lambiase, P.D.; El-Chami, M.F.; Knops, R.E.; Aasbo, J.D.; Bongiorni, M.G.; Russo, A.M.; Deharo, J.-C.; Burke, M.C.; Dinerman, J. Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 2021, 143, 7–17. [Google Scholar] [CrossRef]
- Jaganathan, N.; Goel, V.; Sorrentino, R.; Gallo, D.; Devarapalli, M. Extravascular Implantable Cardioverter-Defibrillators: A Systematic Review of Emerging Evidence. Cureus 2025, 17, e85359. [Google Scholar] [CrossRef]
- Knops, R.E.; Lloyd, M.S.; Roberts, P.R.; Wright, D.J.; Boersma, L.V.; Doshi, R.; Friedman, P.A.; Neuzil, P.; Blomström-Lundqvist, C.; Bongiorni, M.G. A modular communicative leadless pacing–defibrillator system. N. Engl. J. Med. 2024, 391, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lim, K.; Park, S.-J.; Park, J.-S.; Kim, J.Y.; Chung, S.; Jung, D.-S.; Park, K.-M.; On, Y.K.; Kim, J.S. Thoracoscopic implantation of epicardial left ventricular lead for cardiac resynchronization therapy. J. Cardiovasc. Dev. Dis. 2022, 9, 160. [Google Scholar] [CrossRef]
- Doll, N.; Piorkowski, C.; Czesla, M.; Kallenbach, M.; Rastan, A.; Arya, A.; Mohr, F. Epicardial versus transvenous left ventricular lead placement in patients receiving cardiac resynchronization therapy: Results from a randomized prospective study. Thorac. Cardiovasc. Surg. 2008, 56, 256–261. [Google Scholar] [CrossRef]
- Huntley, G.D.; Deshmukh, A.J.; Warnes, C.A.; Kapa, S.; Egbe, A.C. Longitudinal outcomes of epicardial and endocardial pacemaker leads in the adult Fontan patient. Pediatr. Cardiol. 2018, 39, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.J.; Jost, C.H.A.; Warnes, C.A.; Hodge, D.; Hyberger, L.; Connolly, H.M.; Asirvatham, S.J.; Dearani, J.A.; Hayes, D.L.; Ammash, N.M. Epicardial versus endocardial permanent pacing in adults with congenital heart disease. J. Interv. Card. Electrophysiol. 2010, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Crossley, G.H.; Boyle, A.; Vitense, H.; Chang, Y.; Mead, R.H.; Investigators, C. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: The value of wireless remote monitoring with automatic clinician alerts. J. Am. Coll. Cardiol. 2011, 57, 1181–1189. [Google Scholar] [CrossRef]
- Varma, N.; Epstein, A.E.; Irimpen, A.; Schweikert, R.; Love, C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: The Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010, 122, 325–332. [Google Scholar] [CrossRef]
- Slotwiner, D.; Varma, N.; Akar, J.G.; Annas, G.; Beardsall, M.; Fogel, R.I.; Galizio, N.O.; Glotzer, T.V.; Leahy, R.A.; Love, C.J. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015, 12, e69–e100. [Google Scholar] [CrossRef]
- Mekary, W.; Ibrahim, R.; Lloyd, M.S.; Bhatia, N.K.; Westerman, S.B.; Shah, A.D.; Byku, I.; Gleason, P.; Greenbaum, A.; Babaliaros, V. Pacing considerations in patients undergoing transcatheter tricuspid valve replacement: Insights from a tertiary care center. Heart Rhythm 2025, 23, 143–148. [Google Scholar] [CrossRef]
- Peigh, G.; Al-Kazaz, M.; Davidson, L.J.; Gerçek, M.; Potratz, M.; Malaisrie, S.C.; Finke, R.; Meng, Z.; Baldridge, A.S.; Gao, J. Outcomes of entrapped right ventricular pacing or defibrillator leads following transcatheter tricuspid valve replacement. Cardiovasc. Interv. 2025, 18, 1762–1772. [Google Scholar]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.-C. European heart rhythm association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—Endorsed by the heart rhythm society (HRS), the Asia Pacific heart rhythm society (APHRS), the Latin American heart rhythm society (LAHRS), international society for cardiovascular infectious diseases (ISCVID) and the European society of clinical microbiology and infectious diseases (ESCMID) in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur. J. Cardio-Thorac. Surg. 2020, 57, e1–e31. [Google Scholar]
- Sorajja, P.; Whisenant, B.; Hamid, N.; Naik, H.; Makkar, R.; Tadros, P.; Price, M.J.; Singh, G.; Fam, N.; Kar, S. Transcatheter repair for patients with tricuspid regurgitation. N. Engl. J. Med. 2023, 388, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Esquer Garrigos, Z.; Rizwan Sohail, M.; Havers-Borgersen, E.; Krahn, A.D.; Chu, V.H.; Radke, C.S.; Avari-Silva, J.; El-Chami, M.F.; Miro, J.M. Update on cardiovascular implantable electronic device infections and their prevention, diagnosis, and management: A scientific statement from the American Heart Association. Circulation 2024, 149, e201–e216. [Google Scholar] [CrossRef] [PubMed]
- Bhuta, S.; Colak, S.; Arif, A.I.; Afzal, M.R. Snaring via a femoral approach to facilitate transvenous lead extraction of an infected right ventricular lead jailed by a bioprosthetic tricuspid valve. J. Innov. Card. Rhythm Manag. 2024, 15, 6066. [Google Scholar] [CrossRef]
- Tan, E.M.; DeSimone, D.C.; Sohail, M.R.; Baddour, L.M.; Wilson, W.R.; Steckelberg, J.M.; Virk, A. Outcomes in patients with cardiovascular implantable electronic device infection managed with chronic antibiotic suppression. Clin. Infect. Dis. 2017, 64, 1516–1521. [Google Scholar] [CrossRef]
- Topaz, M.; Chorin, E.; Schwartz, A.L.; Hochstadt, A.; Shotan, A.; Ashkenazi, I.; Kazatsker, M.; Carmel, N.-N.; Topaz, G.; Oron, Y. Regional antibiotic delivery for implanted cardiovascular electronic device infections. J. Am. Coll. Cardiol. 2023, 81, 119–133. [Google Scholar] [CrossRef]
- Auricchio, A.; Regoli, F.; Conte, G.; Caputo, M.L. Key lessons from the ELECTRa registry in the modern era of transvenous lead extraction. Arrhythmia Electrophysiol. Rev. 2017, 6, 111. [Google Scholar] [CrossRef]
- Dan, G.-A. 2021 ESC guidelines on cardiac pacing and cardiac resynchronisation therapy. Eur. Cardiol. Rev. 2021, 16, e55. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.M.; Desai, M.Y.; Do, M.M.; Butler, J.; Chung, M.K.; Epstein, A.E.; Guglin, M.E.; Levy, W.C.; Piccini, J.P.; Bhave, N.M. ACC/AHA/ASE/HFSA/HRS/SCAI/SCCT/SCMR 2025 Appropriate Use Criteria for Implantable Cardioverter-Defibrillators, Cardiac Resynchronization Therapy, and Pacing. J. Am. Coll. Cardiol. 2025, 85, 1213–1285. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.V.; El-Chami, M.; Steinwender, C.; Lambiase, P.; Murgatroyd, F.; Mela, T.; Theuns, D.A.; Khelae, S.K.; Kalil, C.; Zabala, F. Practical considerations, indications, and future perspectives for leadless and extravascular cardiac implantable electronic devices: A position paper by EHRA/HRS/LAHRS/APHRS. Europace 2022, 24, 1691–1708. [Google Scholar] [CrossRef] [PubMed]
- Wijesuriya, N.; Elliott, M.K.; Mehta, V.; Sidhu, B.S.; Behar, J.M.; Niederer, S.; Rinaldi, C.A. Leadless left ventricular endocardial pacing for cardiac resynchronization therapy: A systematic review and meta-analysis. Heart Rhythm 2022, 19, 1176–1183. [Google Scholar] [CrossRef]
Figure 1.
Phenotypes of tricuspid regurgitation (TR) in patients with cardiac implantable electronic device (CIED) leads and distinguishing CIED-induced, CIED-incidental, and mixed TR. Abbreviations: 3D, three-dimensional; AF, atrial fibrillation; CIED, cardiac implantable electronic device; LV, left ventricle; PH, pulmonary hypertension; RA, right atrium; RV, right ventricle; TR, tricuspid regurgitation; TV, tricuspid valve.
Figure 1.
Phenotypes of tricuspid regurgitation (TR) in patients with cardiac implantable electronic device (CIED) leads and distinguishing CIED-induced, CIED-incidental, and mixed TR. Abbreviations: 3D, three-dimensional; AF, atrial fibrillation; CIED, cardiac implantable electronic device; LV, left ventricle; PH, pulmonary hypertension; RA, right atrium; RV, right ventricle; TR, tricuspid regurgitation; TV, tricuspid valve.
Figure 2.
Proposed composition of a Heart Team managing patients with transvalvular right ventricular leads undergoing transcatheter tricuspid valve interventions. Abbreviations: TLE: transvenous lead extraction; CIED: cardiac implantable electronic device; TR: tricuspid regurgitation; TTVI: transcatheter tricuspid valve intervention; TTV: transcatheter tricuspid valve; SUD: substance use disorder.
Figure 2.
Proposed composition of a Heart Team managing patients with transvalvular right ventricular leads undergoing transcatheter tricuspid valve interventions. Abbreviations: TLE: transvenous lead extraction; CIED: cardiac implantable electronic device; TR: tricuspid regurgitation; TTVI: transcatheter tricuspid valve intervention; TTV: transcatheter tricuspid valve; SUD: substance use disorder.
Figure 3.
Proposed algorithm for the management of TTVI candidates with symptomatic severe TR and a CIED lead crossing the TV, adapted from the 2025 ECAVI/EHRA scientific statement on Management of patients with transvalvular right ventricular leads undergoing transcatheter tricuspid valve interventions. Abbreviations: 3D: three-dimensional; CIED: cardiac implantable electronic device; CT: computed tomography; RV: right ventricular; T-TEER: transcatheter tricuspid edge-to-edge repair; TEE: transesophageal echocardiography; TLE: transvenous lead extraction; TR: tricuspid regurgitation; TTE: transthoracic echocardiography; TTVI: transcatheter tricuspid valve intervention; TTVR: transcatheter tricuspid valve replacement; TV: tricuspid valve.
Figure 3.
Proposed algorithm for the management of TTVI candidates with symptomatic severe TR and a CIED lead crossing the TV, adapted from the 2025 ECAVI/EHRA scientific statement on Management of patients with transvalvular right ventricular leads undergoing transcatheter tricuspid valve interventions. Abbreviations: 3D: three-dimensional; CIED: cardiac implantable electronic device; CT: computed tomography; RV: right ventricular; T-TEER: transcatheter tricuspid edge-to-edge repair; TEE: transesophageal echocardiography; TLE: transvenous lead extraction; TR: tricuspid regurgitation; TTE: transthoracic echocardiography; TTVI: transcatheter tricuspid valve intervention; TTVR: transcatheter tricuspid valve replacement; TV: tricuspid valve.
Figure 4.
Non-transvalvular pacing and defibrillation options after transcatheter transvalvular interventions. Abbreviations: TTVI: transcatheter tricuspid valve intervention; ICD: implantable cardioverter-defibrillator; S-ICD: subcutaneous implantable cardioverter-defibrillator; EV-ICD: extravascular implantable cardioverter-defibrillator.
Figure 4.
Non-transvalvular pacing and defibrillation options after transcatheter transvalvular interventions. Abbreviations: TTVI: transcatheter tricuspid valve intervention; ICD: implantable cardioverter-defibrillator; S-ICD: subcutaneous implantable cardioverter-defibrillator; EV-ICD: extravascular implantable cardioverter-defibrillator.
Table 1.
Multidisciplinary Heart Team composition and roles.
Table 1.
Multidisciplinary Heart Team composition and roles.
| Team Member | Primary Role | Secondary/Adjunct Role |
|---|
| Structural/Interventional Cardiologist | Procedural candidacy assessment integrating anatomic suitability, frailty, and expected clinical benefit; TTVI platform selection (repair vs. replacement; annuloplasty vs. leaflet-based); procedural execution | Co-ownership of lead-management strategy with EP to minimize lead-related procedural hazards; intraprocedural troubleshooting; preservation of downstream rhythm-management options |
| Electrophysiologist (TLE expertise) | Lead management strategy (extract/reposition/retain/jail); device interrogation, including rhythm dependence, arrhythmia burden, and ICD therapy history; extraction risk appraisal | Pre-specification of contingency pacing/defibrillation pathways (leadless pacing, CS-based CRT, extravascular ICD); intraoperative CIED programming; post-procedural device surveillance and troubleshooting |
| Advanced Imaging Specialist | Rigorous TR mechanism adjudication (CIED-induced vs. CIED-incidental); integration of 3D echo and CT for lead–leaflet interaction assessment and anatomical planning | Intraprocedural guidance (device steering, leaflet grasping, complication recognition); coordination of post-intervention imaging surveillance for prosthetic function and hardware interaction |
| Heart Failure Specialist | Longitudinal clinical context including symptom trajectory, functional status, and HF hospitalization history; medical therapy optimization (diuretics, neurohormonal agents) | Alignment of procedural goals with realistic expectations in comorbid TR populations; post-procedural HF regimen adjustment; long-term surveillance and prognostication |
| Cardiac Surgeon | Comprehensive surgical risk assessment (redo sternotomy, multivalve disease, endocarditis); surgical backup for procedural complications | Contingency planning when transcatheter options are limited; primary operator role when open intervention is most appropriate; hybrid procedure collaboration |
| Infectious Disease Specialist | Evaluation of suspected/confirmed CIED infection; antimicrobial strategy development (agent selection, duration) | Coordination with EP regarding extraction timing and reimplantation; long-term suppressive therapy planning; post-procedural infection surveillance |
| Addiction Medicine Specialist | Substance use disorder assessment and staging; medication-assisted treatment initiation/optimization; relapse risk stratification | Coordination of long-term recovery support and harm reduction; linkage to outpatient addiction services; input on procedural timing relative to recovery stability |
| Palliative Care Specialist | Goals-of-care discussions; facilitation of shared decision-making when risk–benefit is uncertain; advance care planning, including ICD deactivation discussions | Symptom-focused management in frail/highly comorbid patients; psychosocial support for patients and families; coordination with hospice services when appropriate |
Table 2.
TTVI Strategies and interactions with CIED leads. T-TEER emerges as the most “lead-friendly” TTVI strategy, presenting minimal risk to lead integrity and preserving future options. TTVR, while offering potent TR elimination, is associated with a high risk for the development of complete heart block and creates an irreversible “jailed” state with a demonstrated risk of insulation damage. TTVIV presents the highest risk of mechanical lead failure due to the metal-on-metal interface.
Table 2.
TTVI Strategies and interactions with CIED leads. T-TEER emerges as the most “lead-friendly” TTVI strategy, presenting minimal risk to lead integrity and preserving future options. TTVR, while offering potent TR elimination, is associated with a high risk for the development of complete heart block and creates an irreversible “jailed” state with a demonstrated risk of insulation damage. TTVIV presents the highest risk of mechanical lead failure due to the metal-on-metal interface.
| Strategy | Primary Mechanism | Lead Jailing Risk | Lead Interaction Type | New PPM Rate | Lead Extraction Feasibility Post-Proc | Key Lead-Related Concern |
|---|
| T-TEER | Leaflet Approximation | Low | “Innocent Bystander” or Displaced | <1–3% | Preserved | Grasping/Entanglement (Rare) |
| TTVR | Orthotopic Replacement | High (Obligate) | Metal-on-Tissue Compression | ~27.8% | Precluded | Insulation Breach (13%); High PPM rate; No future access |
| Annuloplasty | Annular Reduction | Moderate | Impingement/Anchor collision | Low | Difficult but Possible | Anchor penetration of lead; Septal crowding |
| CAVI | Heterotopic Valve | High (SVC) | Stent Jailing (SVC segment) | Low | Precluded (High Risk) | Loss of venous access; SVC entrapment |
| TTVIV | Valve-in-Valve | Obligate | Metal-on-Metal Compression | Low | Precluded | High rate of lead fracture (10.7%); Shearing forces |
Table 3.
Risk factors for cardiac implantable electronic device (CIED) infection can be categorized into three main domains: patient-related, device-related, and procedure-related.
Table 3.
Risk factors for cardiac implantable electronic device (CIED) infection can be categorized into three main domains: patient-related, device-related, and procedure-related.
| Risk Factors for CIED Infections |
|---|
| Patient-Related | Device-Related | Procedure-Related |
|---|
| Multiple transvenous leads Repeated prior procedures Device type (CRT > ICD > pacemaker) Abdominal pocket
| |
Table 4.
Comparison of Guideline Recommendations for CIED Management in Patients with Tricuspid Valve Disease.
Table 4.
Comparison of Guideline Recommendations for CIED Management in Patients with Tricuspid Valve Disease.
| Clinical Domain | 2025 EHRA/EAPCI Statement | 2025 AUC/2022 Leadless Position Paper | 2021 ESC Pacing Guidelines | 2020 ACC/AHA VHD Guidelines | 2017 HRS Lead Extraction Consensus |
|---|
| TR and CIED interaction | Central focus; distinguishes CIED-induced vs. CIED-incidental and mixed TR; defines “red flags” for lead jailing | Not the primary focus | Recognized as a complication; echocardiography-centered work-up | Acknowledged but limited mechanistic detail | Indirectly addressed (extraction may improve TR) |
| Heart Team/Multidisciplinary approach | Mandated for all TTVI candidates | Endorsed for complex candidacy decisions | Recommended for complex scenarios | Required for valvular intervention | Emphasized for high-risk extraction; institutional preparedness required |
| Lead management strategy | TLE viable even in elderly; risk stratification framework provided | Extraction risk favors a leadless/extravascular approach | Epicardial leads at TV surgery (Class IIa, Level C); CS pacing for mechanical TV prostheses | Not addressed | Core document: procedural endpoints, safety standards, program infrastructure |
| Alternative pacing/ICD systems | Leadless pacing, CS pacing, epicardial leads, S-ICD/EV-ICD endorsed | Leadless: AUC score 6 post-TV surgery; candidacy framework for leadless/S-ICD/EV-ICD | CS and epicardial strategies acknowledged | Not addressed | Not the primary focus |
| Monitoring and follow-up | Frequent follow-up essential for jailed leads, especially PM-dependent/ICD patients | Standard device follow-up | Remote monitoring recommended (Class I, Level A) | Not addressed | Post-extraction surveillance addressed |
Table 5.
Future directions in the management of patients with CIEDs undergoing TTVI.
Table 5.
Future directions in the management of patients with CIEDs undergoing TTVI.
| Research Priorities |
| Domain | Current Limitation | Future Direction | Potential Impact |
| Evidence generation | No RCTs comparing extraction vs. lead preservation; limited long-term jailed-lead data | Multicenter randomized trials or prospective registries with prespecified treatment algorithms and standardized endpoints | Guideline-level recommendations for extract-vs-jail decisions |
| Terminology standardization | Inconsistent definitions of CIED-induced vs. CIED-incidental TR across studies | Consensus terminology distinguishing direct mechanical interference from functional mechanisms | Improved cross-study comparability and treatment effect estimation |
| Predictive modeling | No validated tools to identify patients benefiting from extraction or stratify procedural risk | AI/ML algorithms integrating clinical, imaging, and device variables for individualized decision support | Personalized extract-vs-jail recommendations with quantified uncertainty |
| Collaborative infrastructure | Fragmented data collection; variable imaging protocols; short follow-up | International registries with harmonized protocols, dedicated jailed-lead cohorts, and ≥2-year follow-up | Robust evidence base for guideline development and quality benchmarking |
| Technological Innovations |
| Domain | Current Limitation | Future Direction | Potential Impact |
| Implantation strategies | Lead placement without systematic consideration of TV interaction | Real-time 3D echo/ICE-guided implantation to avoid leaflet-interfering trajectories | Primary prevention of CIED-induced TR |
| Leadless and extravascular platforms | Limited physiologic pacing options without transvalvular hardware | Modular leadless PM + S-ICD/EV-ICD systems; leadless CSP; fully leadless CRT | Complete valve-sparing device therapy across pacing and defibrillation indications |
| TTVI device design | Current devices are not engineered for lead coexistence | Lead-accommodating features: commissural alignment strategies, dedicated lead channels | Safe jailing with preserved lead function and valve durability |
| Smart lead technology | Lead–valve interaction detected only after clinical TR develops | Sensor-equipped leads detecting early mechanical stress, impedance changes, or micro-motion | Preemptive intervention before symptomatic TR |
| Computational planning | Empiric decision-making without patient-specific simulation | Digital twin modeling to simulate extraction risk, jailing outcomes, and device–anatomy interaction | Virtual procedural planning and complication prediction |
| Regenerative and gene therapy | No biologic approaches to CIED-induced valve injury | Tissue-engineered leaflet repair; gene therapy targeting fibrosis; biocompatible lead coatings | Restoration of native valve function; prevention of lead-related fibrosis |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |