The Polyhedral Matrix Configuration (PMC) Technique: A Retrospective Cohort Study of Geometric Standardization of Acellular Dermal Matrix Wrapping and Operative Efficiency in Prepectoral Breast Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Ethical Considerations
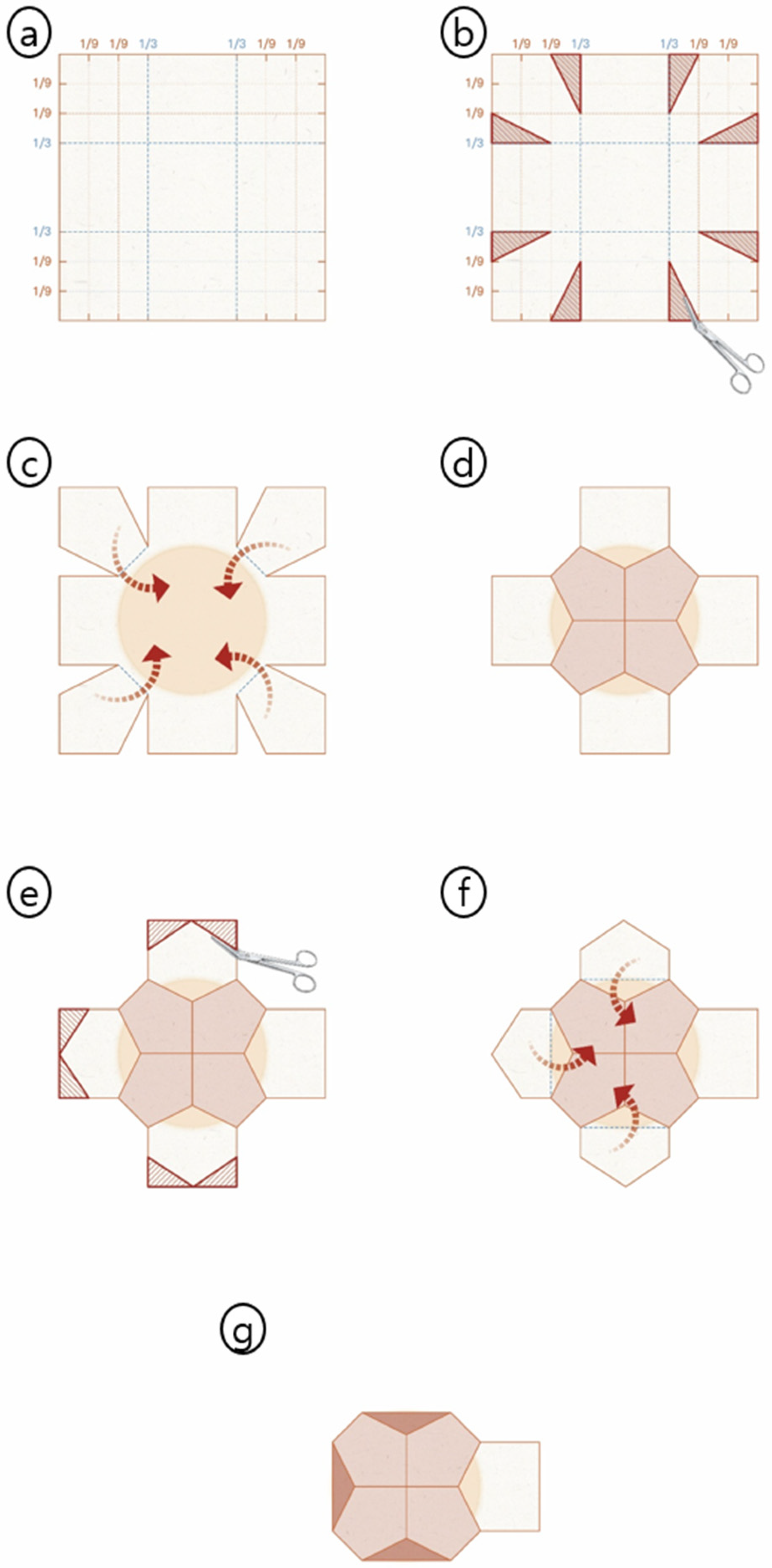
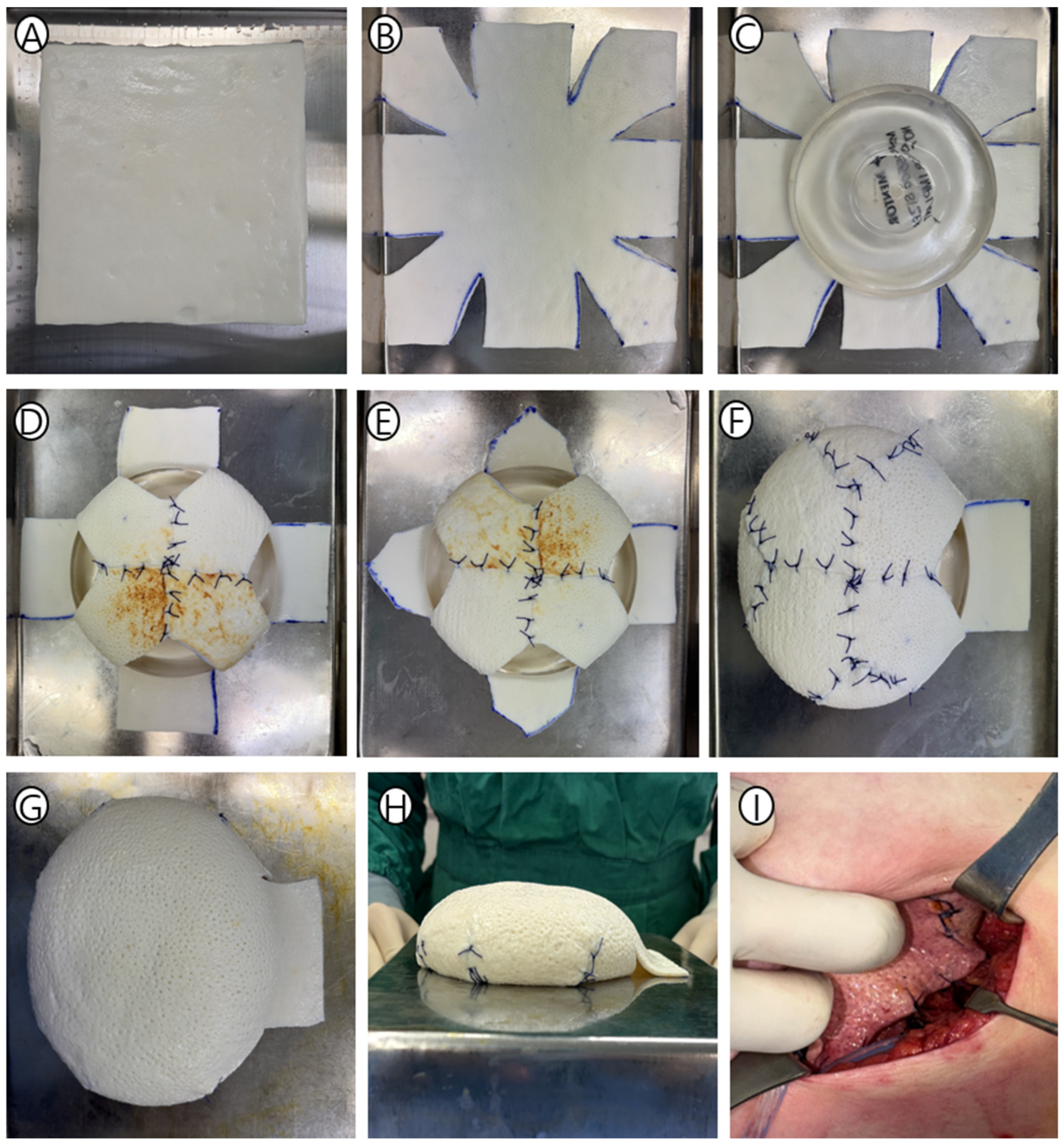
2.3. The PMC Design: “Surgical Origami” Principles
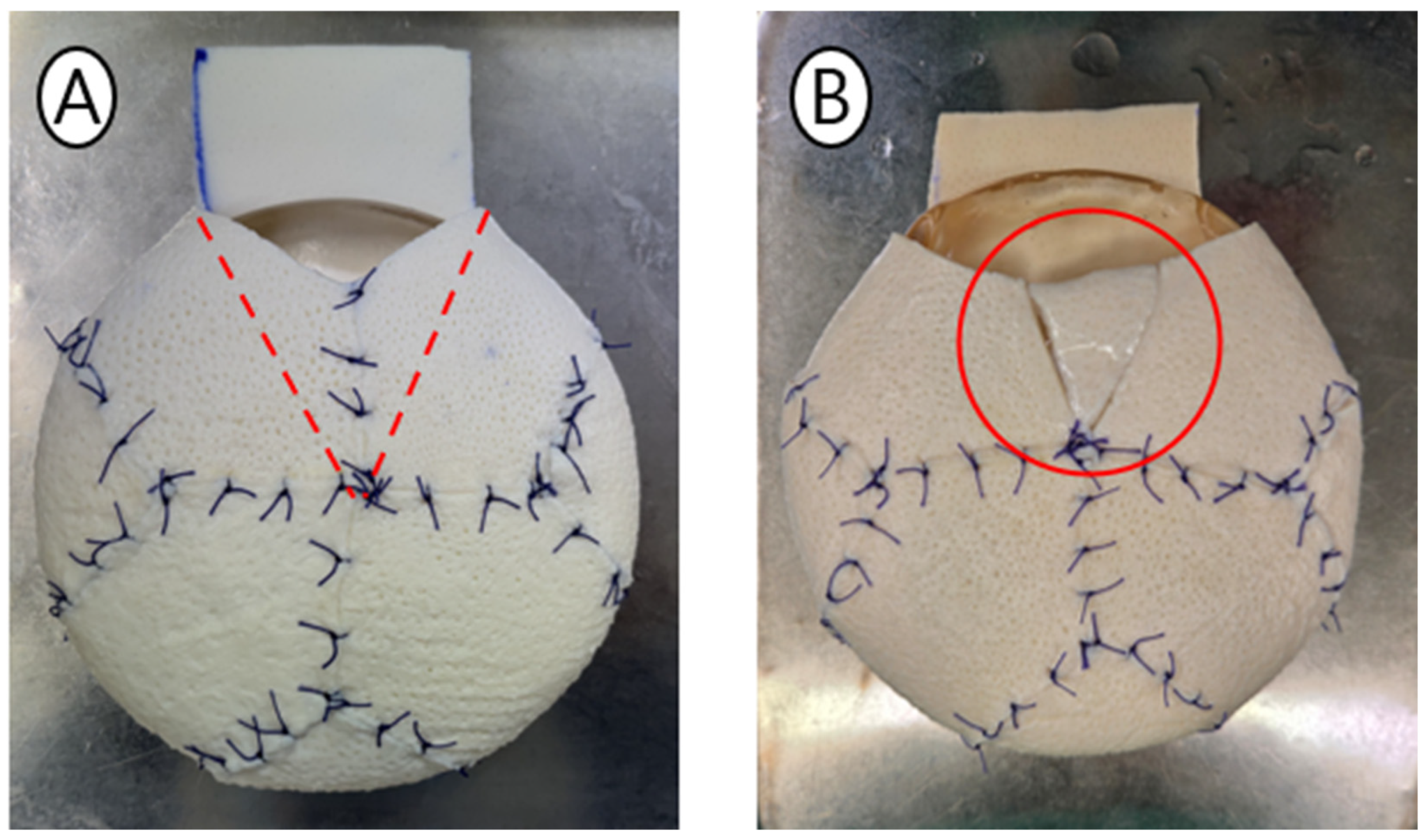
2.4. Detailed Surgical Technique
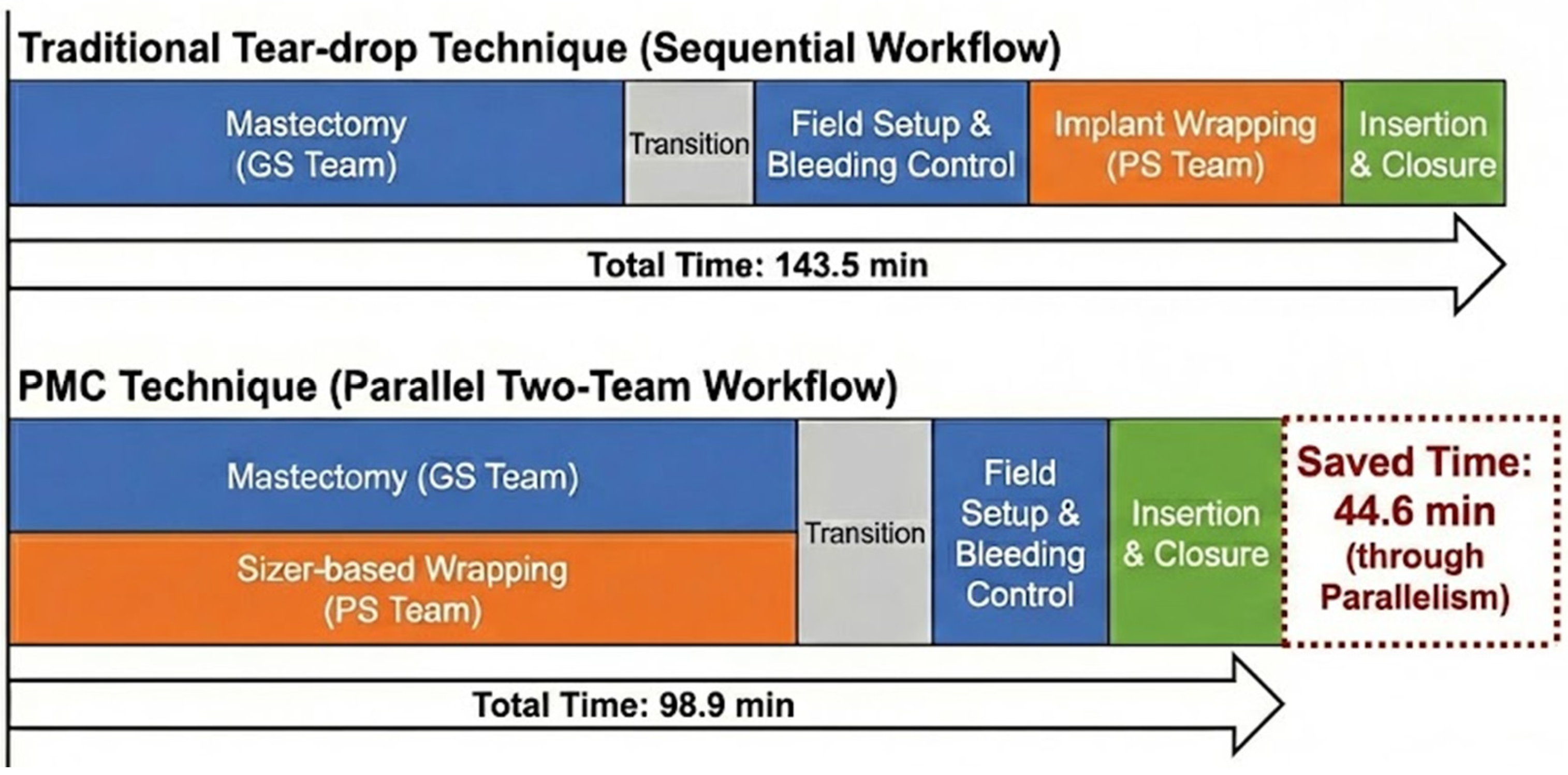
2.5. Operative Workflow: Design-Enabled Parallelization
2.6. Statistical Analysis
- Start point: Completion of mastectomy and hemostasis, marked by the general surgery team stepping back from the operative field.
- End point: Completion of skin closure.
- Data source: Prospectively recorded from electronic anesthesia records, with times documented by circulating nurses at each phase transition.
- Included phases: All reconstructive procedures, including ADM positioning, implant insertion, fixation suturing, drain placement, and wound closure.
3. Results
3.1. Patient Demographics
3.2. Operative Time and Workflow Efficiency
3.3. Safety and Aesthetic Outcomes
4. Discussion
4.1. Geometric Standardization and Operative Efficiency
4.2. Zero-Overlap Design: Structural and Aesthetic Outcomes
4.3. Robotic Mastectomy and PMC Workflow
4.4. Patient-Reported Outcomes
4.5. Alternative Wrapping Techniques
4.6. Surgical Education and Future Applications
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sigalove, S.; Maxwell, G.P.; Sigalove, N.M.; Storm-Dickerson, T.L.; Pope, N.; Rice, J.; Gabriel, A. Prepectoral implant-based breast reconstruction: Rationale, indications, and preliminary results. Plast. Reconstr. Surg. 2017, 139, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Walia, G.S.B.; Aston, J.B.; Bello, R.; Mackert, G.A.; Pedreira, R.A.B.; Cho, B.H.; Carl, H.M.B.; Rada, E.M.; Rosson, G.D.; Sacks, J.M.M. Prepectoral versus subpectoral tissue expander placement: A clinical and quality of life outcomes study. Plast. Reconstr. Surg.–Glob. Open 2018, 6, e1731. [Google Scholar] [CrossRef] [PubMed]
- Reitsamer, R.; Peintinger, F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: A new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Yoo, A.; King, V.; Jao, B.; Wang, H.; Rammos, C.; Elwood, E. Prepectoral immediate direct-to-implant breast reconstruction with anterior AlloDerm coverage. Plast. Reconstr. Surg. 2017, 140, 31S–38S. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Ayeni, O.A.; Hughes, K.B.; Lee, B.T.; Slavin, S.A.; Lin, S.J. Acellular dermal matrices in breast surgery: A comprehensive review. Ann. Plast. Surg. 2013, 70, 732–738. [Google Scholar] [CrossRef]
- Hong, H.K.; Kim, Y.H.; Lee, J.S.; Lee, J.Y.; Park, H.Y.; Yang, J.D. Prepectoral breast reconstruction with complete anterior implant coverage using a single, large, square-shaped acellular dermal matrix. BMC Surg. 2022, 22, 234. [Google Scholar] [CrossRef]
- Pittman, T.A.; Abbate, O.A.; Economides, J.M. The P1 method: Prepectoral breast reconstruction to minimize the palpable implant edge and upper pole rippling. Ann. Plast. Surg. 2018, 80, 487–492. [Google Scholar] [CrossRef]
- Escandón, J.M.; Sweitzer, K.; Christiano, J.G.; Gooch, J.C.; Olzinski, A.T.; Prieto, P.A.; Skinner, K.A.; Langstein, H.N.; Manrique, O.J. Subpectoral versus prepectoral two-stage breast reconstruction: A propensity score-matched analysis of 30-day morbidity and long-term outcomes. J. Plast. Reconstr. Aesthet. Surg. 2023, 76, 76–87. [Google Scholar] [CrossRef]
- Pusic, A.L.; Matros, E.; Fine, N.; Buchel, E.; Gordillo, G.M.; Hamill, J.B.; Kim, H.M.; Qi, J.; Albornoz, C.; Klassen, A.F.; et al. Patient-reported outcomes 1 year after immediate breast reconstruction: Results of the mastectomy reconstruction outcomes consortium study. J. Clin. Oncol. 2017, 35, 2499–2506. [Google Scholar] [CrossRef]
- Cohen, W.A.; Mundy, L.R.; Ballard, T.N.; Klassen, A.; Cano, S.J.; Browne, J.; Pusic, A.L. The BREAST-Q in surgical research: A review of the literature 2009–2015. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 149–162. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, B.S.; Park, J.H.; Yi, H.S.; Kim, H.Y.; Choi, J.H.; Jung, S.U.; Kim, Y.S. “Tear-Drop Appearance” Wrap: A Novel Implant Coverage Method for Creating Natural Contour in Prepectoral Prosthetic-Based Breast Reconstruction. J. Clin. Med. 2022, 11, 4592. [Google Scholar] [CrossRef]
- Hill, E.J.R.; Buck, D.W., 2nd. The “Butterfly” Wrap: A Simplified Technique for Consistent Prosthesis Coverage in Prepectoral Breast Reconstruction. Plast. Reconstr. Surg.–Glob. Open 2018, 6, e2007. [Google Scholar] [CrossRef]
- Paydar, K.Z.; Wirth, G.A.; Mowlds, D.S. Prepectoral breast reconstruction with fenestrated acellular dermal matrix: A novel design. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e1712. [Google Scholar] [CrossRef]
- Sbitany, H. Important considerations for performing prepectoral breast reconstruction. Plast. Reconstr. Surg. 2017, 140, 7S–13S. [Google Scholar] [CrossRef]
- Vidya, R.; Cawthorn, S.J. Innovations for the future of breast surgery. Br. J. Surg. 2021, 108, 908–914. [Google Scholar] [CrossRef]
- Tampaki, E.C.; Tampakis, A. Breast reconstruction: Necessity for further standardization of the current surgical techniques attempting to facilitate scientific evaluation and select tailored procedures. Breast Care 2021, 16, 574–583. [Google Scholar] [CrossRef]
- Todd, A.R.; Rahmani, A.; Elmi, M.; Matthews, J.; Pao, C.; Macadam, S.A. Improved operative efficiency and surgical times in autologous breast reconstruction: A 15-year single-center retrospective review. Plast. Reconstr. Surg.–Glob. Open 2023, 11, 5231. [Google Scholar] [CrossRef]
- Allam, O.; Ripley, K.; Alperovich, M.; Sbitany, H. Future of autologous breast reconstruction: A review of novel technological innovations. Plast. Aesthet. Res. 2024, 11, 19. [Google Scholar] [CrossRef]
- Canizares, O.; Mayo, J.; Soto, E.; Allen, R.J.; Sadeghi, A. Optimizing efficiency in deep inferior epigastric perforator flap breast reconstruction. Ann. Plast. Surg. 2015, 75, 186–192. [Google Scholar] [CrossRef]
- Chopra, K.; Buckingham, B.; Matthews, J.; Sabino, J.; Tadisina, K.K.; Silverman, R.P.; Goldberg, N.H.; Slezak, S.; Singh, D.P. Acellular dermal matrix reduces capsule formation in two-stage breast reconstruction. Int. Wound J. 2017, 14, 414–419. [Google Scholar] [CrossRef]
- Longo, B.; Giacalone, M.; D’Orsi, G.; Farcomeni, A.; Gagliano, E.; Vannucchi, L.; Vanni, G.; Buonomo, C.O.; Cervelli, V. Immediate Hybrid Breast Reconstruction: Dual-Plane Approach Using Prepectoral Implants and Retropectoral Fat Grafting. Plast. Reconstr. Surg. 2025, 156, 209–222. [Google Scholar] [CrossRef]
- Chandarana, M.; Harries, S.; National Braxon Audit Study Group. Multicentre study of prepectoral breast reconstruction using acellular dermal matrix. BJS Open 2020, 4, 71–77. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.S.; Lee, J.H.; Lee, J.W.; Lee, J.; Park, H.Y.; Yang, J.D. Prepectoral breast reconstruction with complete implant coverage using double-crossed acellular dermal matrixs. Gland. Surg. 2019, 8, 748–757. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, S.; Zhang, Y. Complications After Prepectoral Versus Subpectoral Breast Reconstruction in Patients Receiving Postmastectomy Radiation Therapy: A Systematic Review and Meta-Analysis. Aesthet. Plast. Surg. 2024, 48, 4421–4429. [Google Scholar] [CrossRef]
- Bernini, M.; Calabrese, C.; Cecconi, L.; Santi, C.; Gjondedaj, U.; Roselli, J.; Nori, J.; Fausto, A.; Orzalesi, L.; Casella, D. Subcutaneous direct-to-implant breast reconstruction: Surgical, functional, and aesthetic results after long-term follow-up. Plast. Reconstr. Surg.–Glob. Open 2015, 3, e574. [Google Scholar] [CrossRef]
- Salgarello, M.; Pagliara, D.; Barone Adesi, L.; Visconti, G.; Wild, J.B.; Matey, P. Direct to Implant Breast Reconstruction with Prepectoral Micropolyurethane Foam-Coated Implant: Analysis of Patient Satisfaction. Clin. Breast Cancer 2021, 21, e454–e461. [Google Scholar] [CrossRef]
- Khan, M.T.; Won, B.W.; Baumgardner, K.; Lue, M.; Montorfano, L.; Hosein, R.C.; Wang, H.T.; Martinez, R.A. Robotic-Assisted Harvest of Deep Inferior Epigastric Flap for Breast Reconstruction. Ann. Plast. Surg. 2022, 89, 703–708. [Google Scholar] [CrossRef]
- Roy, M.K.; Smith, D.; Ramakrishnan, V. Robotic-assisted breast reconstruction: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2023, 76, 30–40. [Google Scholar]
- Laporta, R.; Longo, B.; Sorotos, M.; Farcomeni, A.; Amorosi, V.; di Pompeo, F.S. Time-dependent factors in DIEP flap breast reconstruction. Microsurgery 2017, 37, 793–799. [Google Scholar] [CrossRef]
- Berna, G.; Cawthorn, S.J.; Papaccio, G.; Balestrieri, N. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: A new muscle-sparing breast reconstruction. ANZ J. Surg. 2017, 87, 493–498. [Google Scholar] [CrossRef]
- Mégevand, V.; Scampa, M.; McEvoy, H.; Kalbermatten, D.F.; Oranges, C.M. Comparison of Outcomes Following Prepectoral and Subpectoral Implants for Breast Reconstruction: Systematic Review and Meta-Analysis. Cancers 2022, 14, 4223. [Google Scholar] [CrossRef]
- Khan, A.; Tasoulis, M.K.; Teoh, V.; Tanska, A.; Edmonds, R.; Gui, G. Pre-pectoral one-stage breast reconstruction with anterior biological acellular dermal matrix coverage. Gland. Surg. 2021, 10, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.; Chambers, A.; Govindajulu, S.; Sahu, A.; Warr, R.; Cawthorn, S. Early complications and implant loss in implant-based breast reconstruction with and without acellular dermal matrix (Tecnoss Protexa®): A comparative study. Eur. J. Surg. Oncol. 2015, 41, 113–119. [Google Scholar] [CrossRef]
- Macadam, S.A.; Bovill, E.S.; Buchel, E.W.; Lennox, P.A. Evidence-based medicine: Autologous breast reconstruction. Plast. Reconstr. Surg. 2017, 139, 204e–229e. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y. Acellular dermal matrices in primary breast reconstruction: Principles, concepts, and indications. Plast. Reconstr. Surg. 2012, 130, 44S–53S. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, G.; Giacalone, M.; Calicchia, A.; Gagliano, E.; Vannucchi, L.; Vanni, G.; Buonomo, O.C.; Cervelli, V.; Longo, B. BIA-ALCL and BIA-SCC: Updates on Clinical Features and Genetic Mutations for Latest Recommendations. Medicina 2024, 60, 793. [Google Scholar] [CrossRef]
- Clemens, M.W.; Medeiros, L.J.; Butler, C.E.; Hunt, K.K.; Fanale, M.A.; Horwitz, S.; Weisenburger, D.D.; Liu, J.; Morgan, E.A.; Kanagal-Shamanna, R.; et al. Complete Surgical Excision Is Essential for the Management of Patients with Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J. Clin. Oncol. 2016, 34, 160–168. [Google Scholar] [CrossRef]

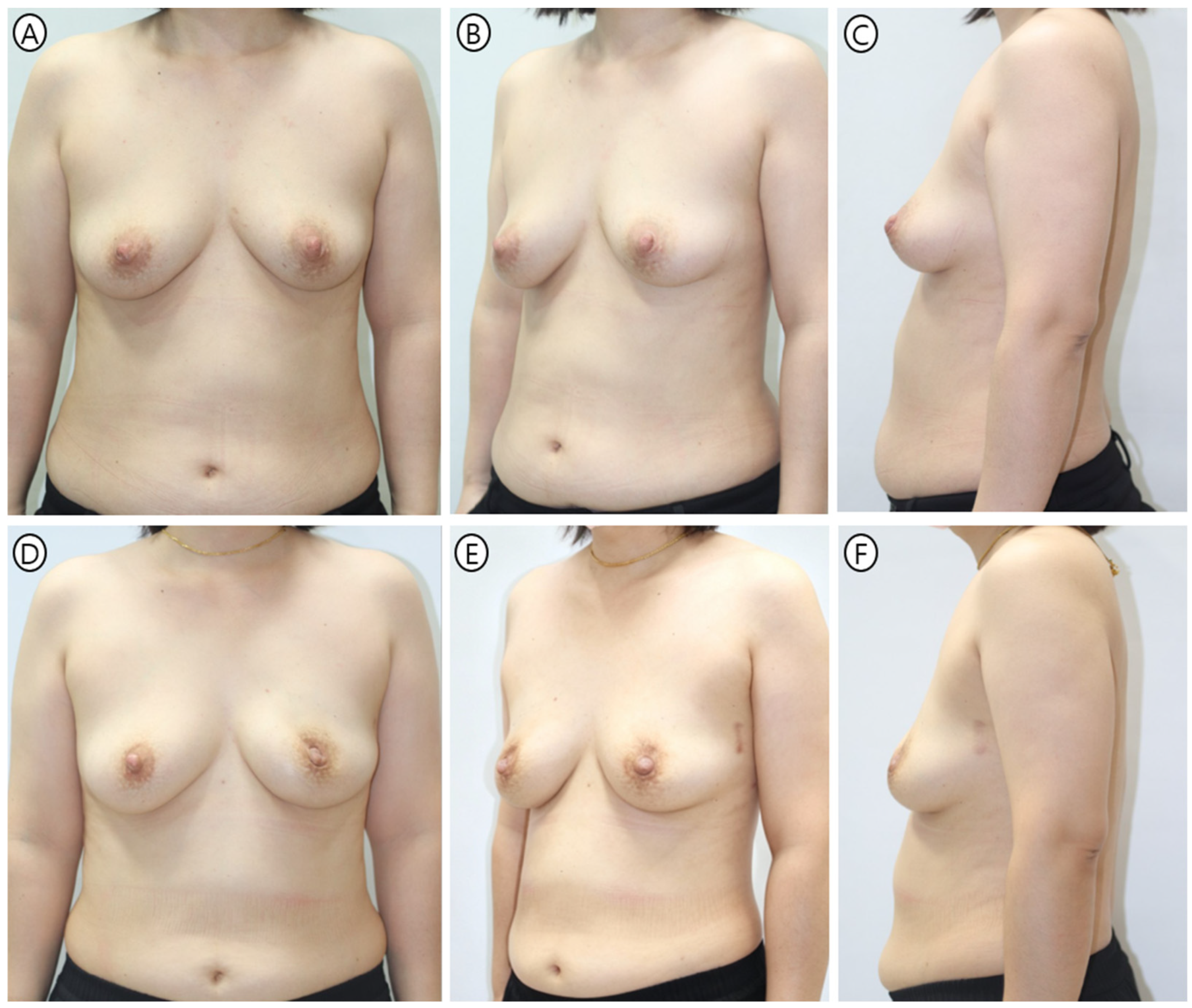
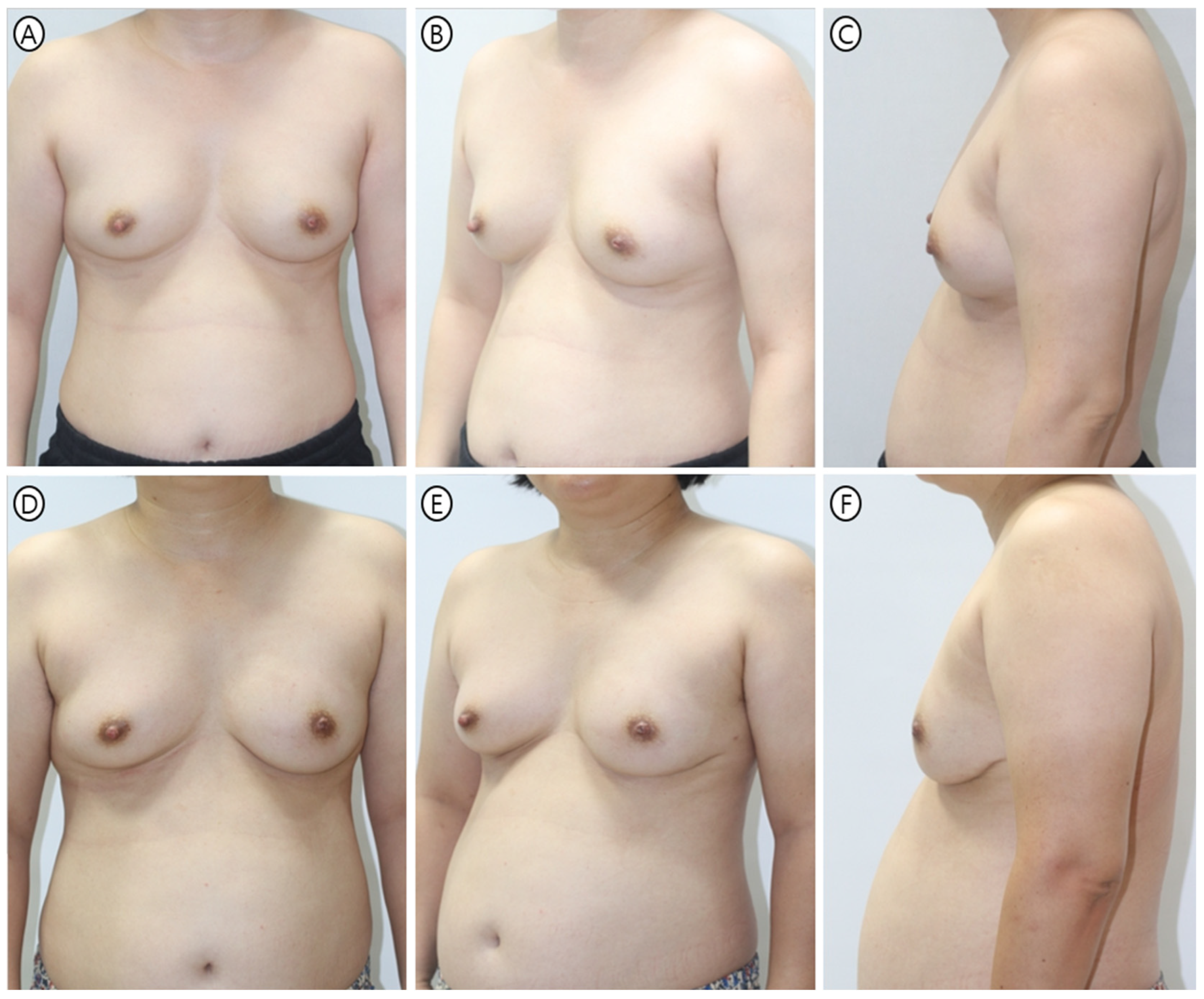
| Variable | Tear-Drop Group (n = 155) | PMC Group (n = 72) | p-Value |
|---|---|---|---|
| Age (years) | 49.5 ± 8.2 | 50.1 ± 7.9 | 0.584 |
| BMI (kg/m2) | 23.8 ± 3.4 | 23.5 ± 3.1 | 0.512 |
| Comorbidities | |||
| Diabetes Mellitus | 11 (7.1%) | 5 (6.9%) | 0.999 ^ |
| Hypertension | 19 (12.3%) | 8 (11.1%) | 0.805 |
| Current Smoker | 5 (3.2%) | 2 (2.8%) | 0.999 ^ |
| Mastectomy Type | <0.001 * | ||
| Conventional (CNSM) | 97 (62.6%) | 23 (31.9%) | |
| Robot-Assisted (RANSM) | 58 (37.4%) | 49 (68.1%) | |
| Surgical Factors | |||
| Specimen Weight (g) | 315.2 ± 102.5 | 308.4 ± 98.1 | 0.645 |
| Implant Volume (cc) | 285.6 ± 85.4 | 295.2 ± 90.1 | 0.438 |
| ADM Size (cm2) | 324.8 ± 17.5 | 323.6 ± 16.2 | 0.624 |
| Variable | Tear-Drop Group (n = 155) | PMC Group (n = 72) | 95% CI | p-Value |
|---|---|---|---|---|
| Operative Time (min) | ||||
| Total Operative Time (Overall) | 268.4 ± 58.2 | 252.6 ± 42.8 | 3.2–28.4 | 0.028 * |
| Plastic Surgery Time (Overall) | 143.5 ± 42.9 | 98.9 ± 28.8 | 35.2–54.0 | <0.001 ** |
| PS Time in CNSM | 134.6 ± 45.3 | 101.8 ± 22.7 | 20.1–45.5 | <0.001 ** |
| PS Time in RANSM | 158.4 ± 33.9 | 97.6 ± 31.4 | 47.3–74.3 | <0.001 ** |
| Mean PS Time Saving (Adjusted) | Ref. | −44.6 min | 35.2–54.0 | <0.001 ** |
| Recovery Metrics | ||||
| Hospital LOS (days) | 14.3 ± 3.8 | 14.1 ± 3.5 | −0.9–1.3 | 0.684 |
| LOS in CNSM Subgroup | 13.9 ± 3.2 | 13.8 ± 3.1 | −1.2–1.4 | 0.812 |
| LOS in RANSM Subgroup | 14.8 ± 4.1 | 14.5 ± 3.9 | −1.4–2.0 | 0.597 |
| Drain Removal (days) | 13.5 ± 3.6 | 13.2 ± 3.3 | −0.8–1.4 | 0.541 |
| Variable | Tear-Drop Group (n = 155) | PMC Group (n = 72) | p-Value |
|---|---|---|---|
| Overall Complications | 26 (16.8%) | 10 (13.9%) | 0.582 |
| Major Complications (CD ≥ III) | 4 (2.6%) | 2 (2.8%) | 0.999 ^ |
| Implant Loss | 2 (1.3%) | 1 (1.4%) | 0.999 ^ |
| Skin Necrosis (Full Thickness) | 2 (1.3%) | 1 (1.4%) | 0.999 ^ |
| Minor Complications (CD I–II) | 22 (14.2%) | 8 (11.1%) | 0.518 |
| Seroma (Requiring Aspiration) | 10 (6.5%) | 4 (5.6%) | 0.784 ^ |
| Skin Necrosis (Partial/Linear) | 8 (5.2%) | 3 (4.2%) | 0.999 ^ |
| Infection (Oral Antibiotics) | 3 (1.9%) | 1 (1.4%) | 0.999 ^ |
| Red Breast Syndrome | 1 (0.6%) | 0 (0.0%) | 0.999 ^ |
| Aesthetic Outcomes | |||
| Rippling (Palpable) | 21 (13.5%) | 3 (4.2%) | 0.032 * |
| Scale (0–100) | Tear-Drop Group (n = 155) | PMC Group (n = 72) | Diff. | 95% CI | p-Value |
|---|---|---|---|---|---|
| Satisfaction with Breasts | 72.5 ± 14.1 | 79.8 ± 12.4 | +7.3 | 3.1–11.5 | 0.001 * |
| Psychosocial Well-being | 75.8 ± 15.2 | 78.2 ± 14.8 | +2.4 | −1.9–6.7 | 0.265 |
| Sexual Well-being | 64.2 ± 19.5 | 67.5 ± 18.2 | +3.3 | −2.1–8.7 | 0.218 |
| Physical Well-being (Chest) | 86.9 ± 10.5 | 90.4 ± 8.1 | +3.5 | 0.8–6.2 | 0.012 * |
| AIS Item | Tear-Drop Group (n = 155) | PMC Group (n = 72) | Mean Diff. | 95% CI | p-Value |
|---|---|---|---|---|---|
| Breast Volume Adequacy | 4.12 ± 0.62 | 4.18 ± 0.61 | +0.06 | −0.12–0.24 | 0.274 |
| Breast Shape Naturalness | 3.86 ± 0.82 | 4.21 ± 0.58 | +0.35 | 0.14–0.56 | 0.001 * |
| Bilateral Symmetry | 3.78 ± 0.88 | 3.95 ± 0.74 | +0.17 | −0.06–0.40 | 0.148 |
| Contour Smoothness (Rippling) | 3.71 ± 0.79 | 4.26 ± 0.52 | +0.55 | 0.35–0.75 | <0.001 * |
| Nipple–Areolar Complex | 4.02 ± 0.64 | 4.10 ± 0.58 | +0.08 | −0.10–0.26 | 0.382 |
| Total AIS Score | 19.49 ± 3.25 | 20.70 ± 2.44 | +1.21 | 0.48–1.94 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yi, H.-s.; Park, J.-j.; Park, J.-h.; Kim, Y.-s. The Polyhedral Matrix Configuration (PMC) Technique: A Retrospective Cohort Study of Geometric Standardization of Acellular Dermal Matrix Wrapping and Operative Efficiency in Prepectoral Breast Reconstruction. J. Clin. Med. 2026, 15, 1226. https://doi.org/10.3390/jcm15031226
Yi H-s, Park J-j, Park J-h, Kim Y-s. The Polyhedral Matrix Configuration (PMC) Technique: A Retrospective Cohort Study of Geometric Standardization of Acellular Dermal Matrix Wrapping and Operative Efficiency in Prepectoral Breast Reconstruction. Journal of Clinical Medicine. 2026; 15(3):1226. https://doi.org/10.3390/jcm15031226
Chicago/Turabian StyleYi, Hyung-suk, Jeong-jin Park, Jin-hyung Park, and Yoon-soo Kim. 2026. "The Polyhedral Matrix Configuration (PMC) Technique: A Retrospective Cohort Study of Geometric Standardization of Acellular Dermal Matrix Wrapping and Operative Efficiency in Prepectoral Breast Reconstruction" Journal of Clinical Medicine 15, no. 3: 1226. https://doi.org/10.3390/jcm15031226
APA StyleYi, H.-s., Park, J.-j., Park, J.-h., & Kim, Y.-s. (2026). The Polyhedral Matrix Configuration (PMC) Technique: A Retrospective Cohort Study of Geometric Standardization of Acellular Dermal Matrix Wrapping and Operative Efficiency in Prepectoral Breast Reconstruction. Journal of Clinical Medicine, 15(3), 1226. https://doi.org/10.3390/jcm15031226






