Towards New Strategies: Case Report and Review of the Literature—Effective Use of JAK Inhibitor Baricitinib in a 4-Year-Old Boy with Anti-MDA5 Antibody-Positive Juvenile Dermatomyositis
Abstract
1. Introduction
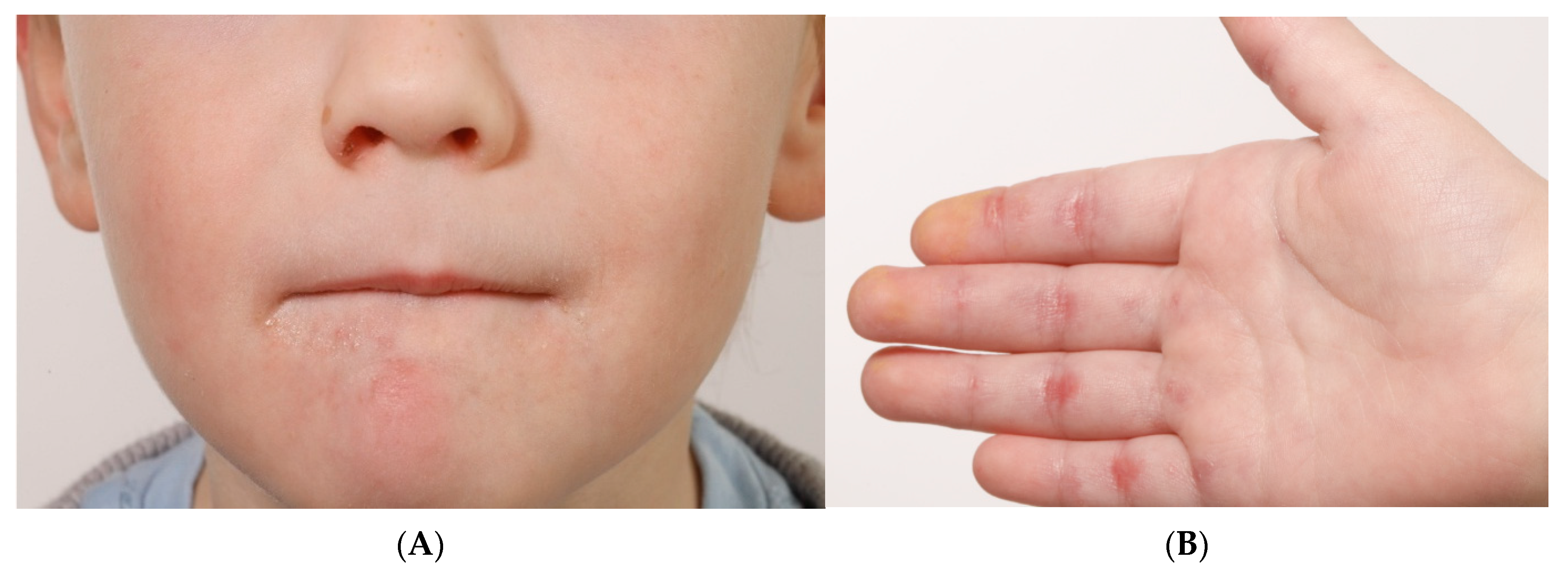
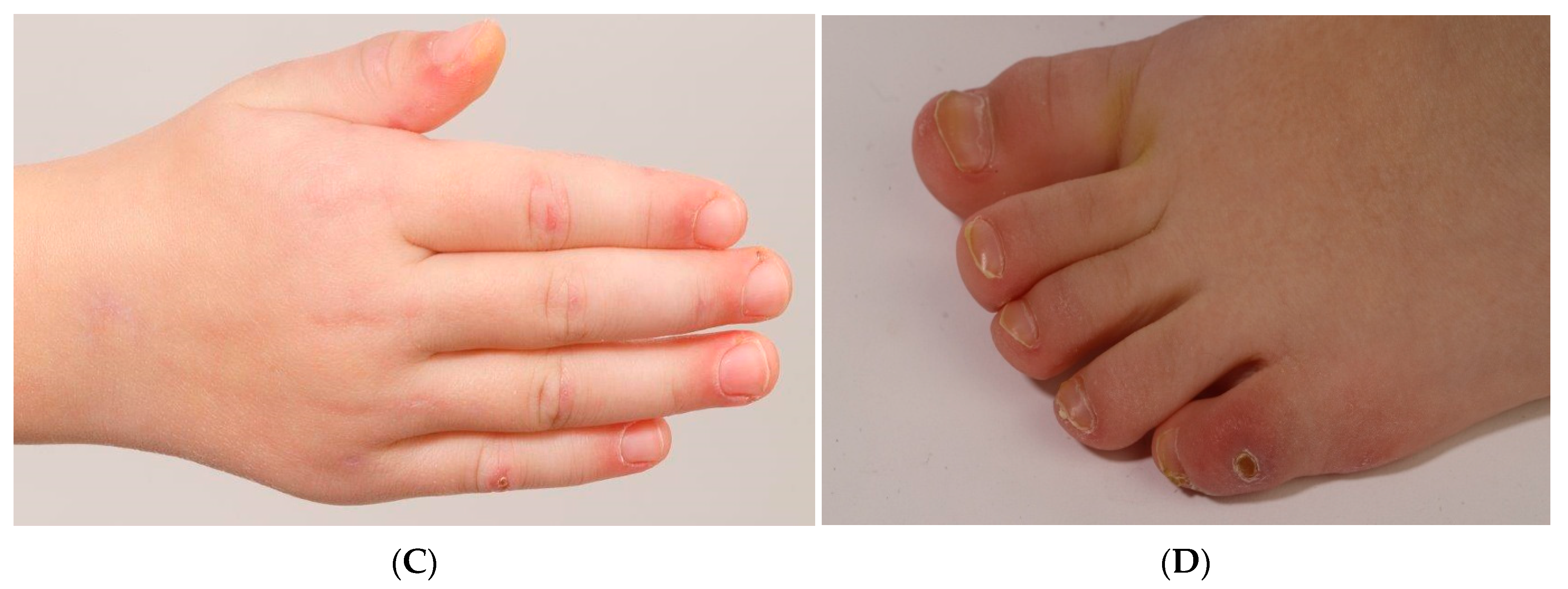
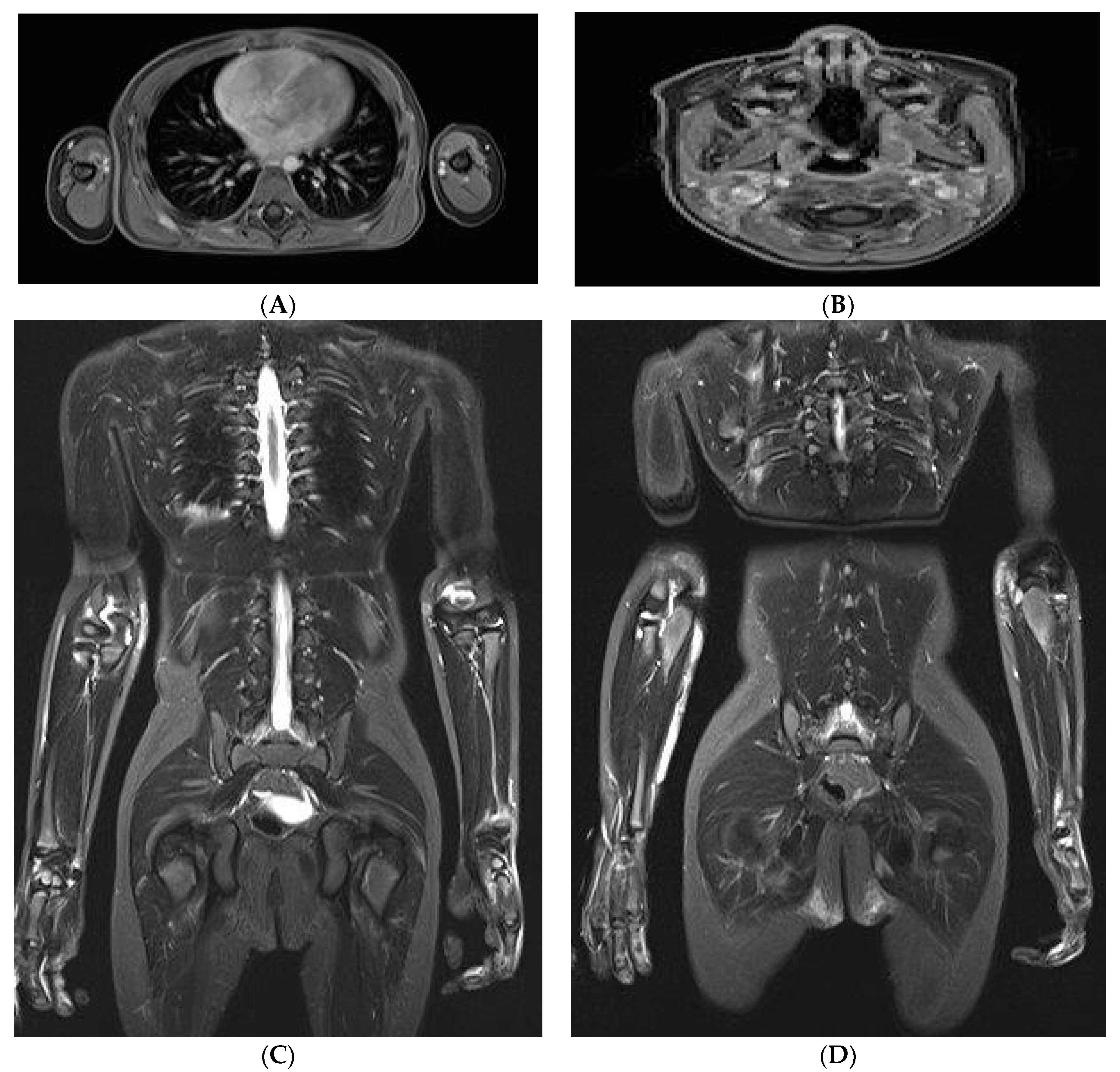
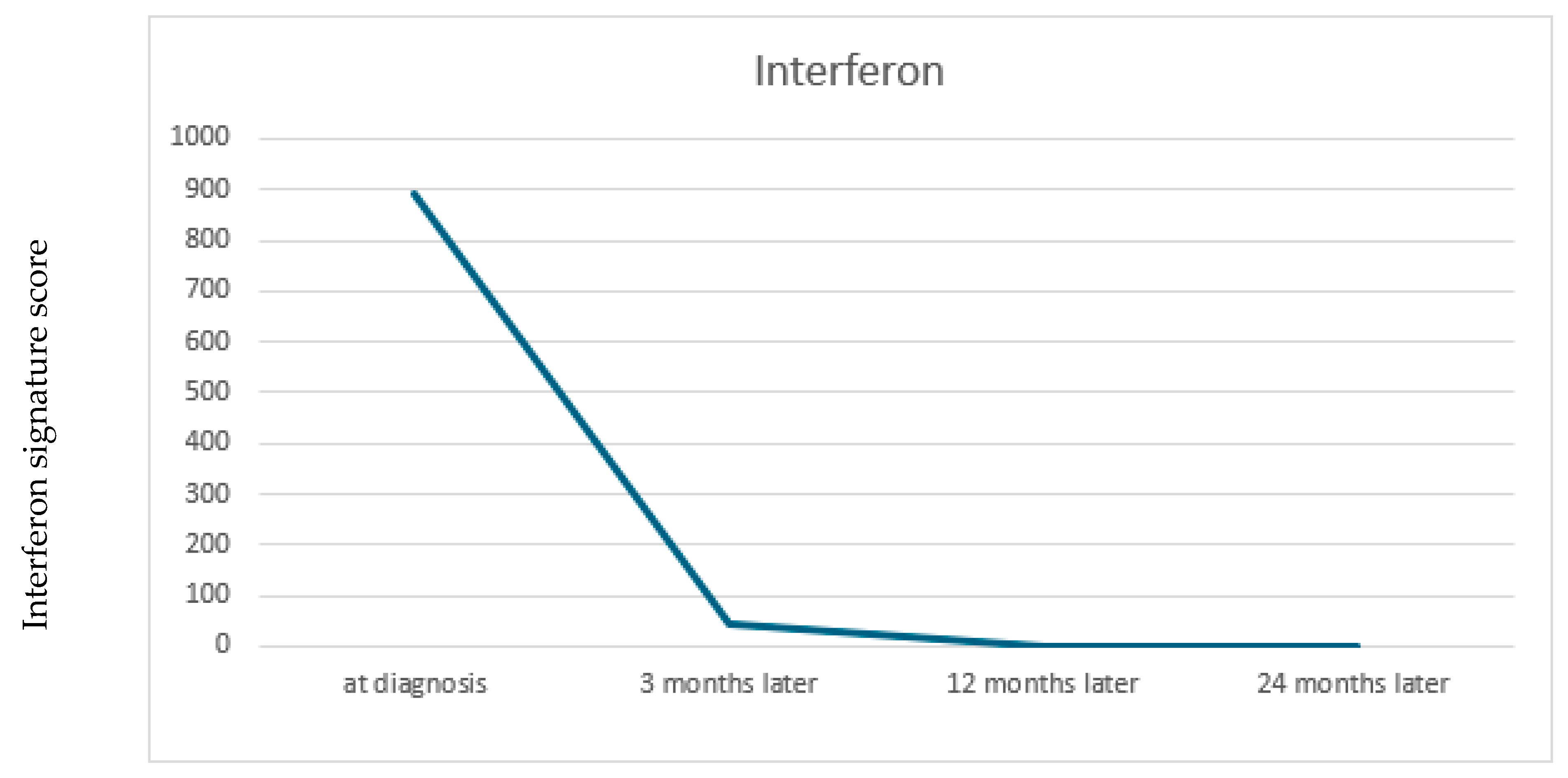
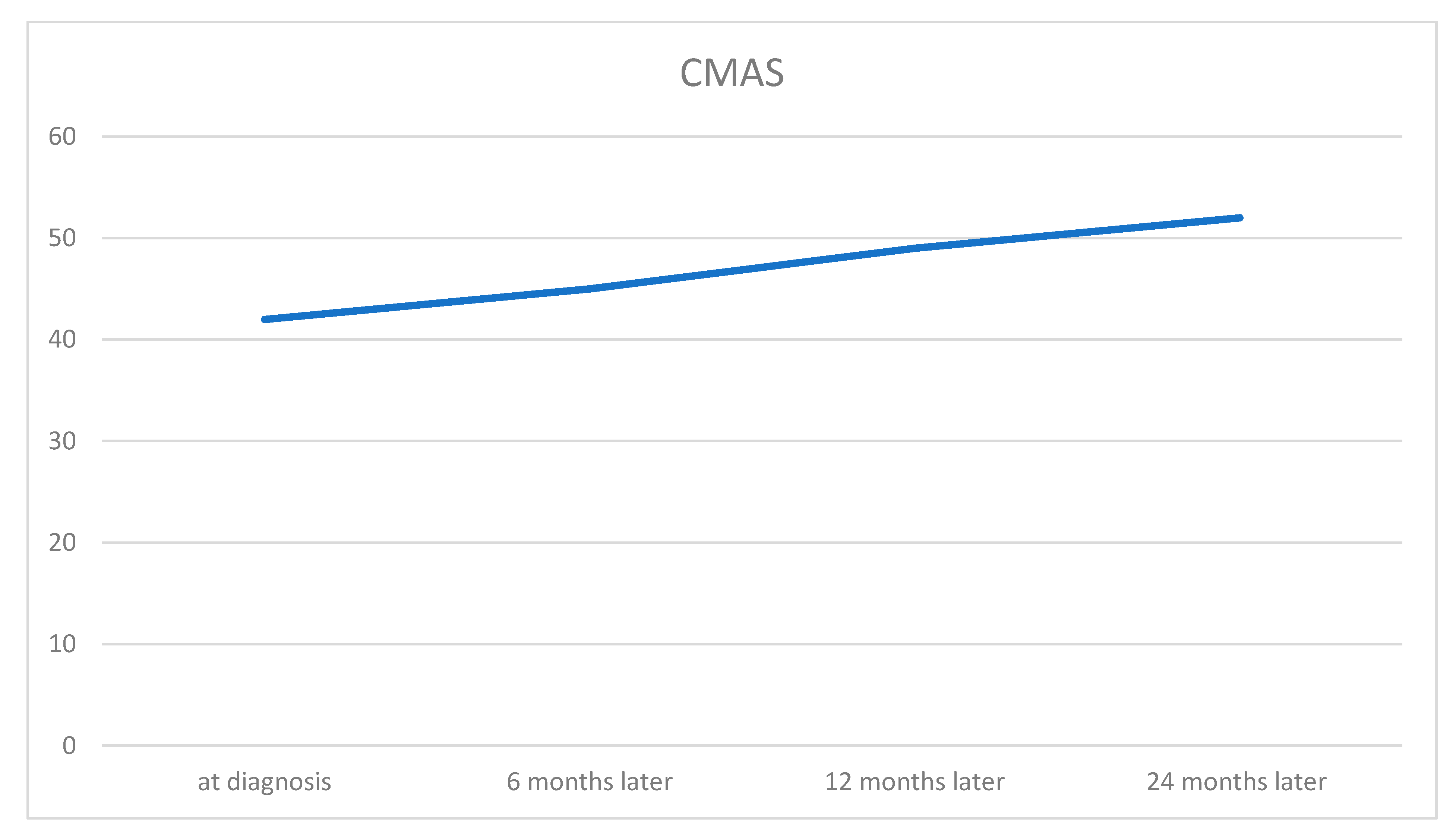
2. Case Report
3. Discussion
4. Conclusions
| Study | Author(s) | Year | Patient Characteristics | Treatment | Efficacy | Safety |
|---|---|---|---|---|---|---|
| [11] | Aeschlimann et al. | 2018 | 13-year-old female, refractory JDM, anti-NXP2+ | Ruxolitinib | Sustained remission, improved muscle strength, reduced inflammation | No major adverse events |
| [27] | Papadopoulou et al. | 2019 | 11-year-old, refractory JDM | Baricitinib | Strength, skin disease improved; steroid tapering | No adverse events, relapse after stopping |
| [35] | Sabbagh et al. | 2019 | Anti-MDA5+ JDM, refractory | Tofacitinib | Muscle, skin, lung function improved | No adverse events |
| [36] | Melki et al. | 2020 | JIIM patients with/without anti-MDA5, 4 cases | Various therapies, 4 patients Ruxolitinib | Required for remission in severe skin cases | Not specified |
| [37] | Sozeri & Demir | 2020 | 2 pediatric patients (JDM, refractory calcinosis) | Tofacitinib | Complete resolution of calcinosis in one patient, moderate improvement in the other | Not mentioned |
| [23] | Chan Ng et al. | 2021 | Pediatric JDM, rapidly progressive ILD | Tofacitinib | Remission achieved, ILD biomarkers improved | No adverse events |
| [22] | Ding et al. | 2021 | 25 JDM patients (mean age 7.2 ± 4.0) | Tofacitinib (n = 7), Ruxolitinib (n = 18) | Rash resolved (96%), muscle strength improved (40%) | No major adverse events |
| [20] | Heinen et al. | 2021 | 14-year-old male, NXP2+ JDM | Ruxolitinib | Improved strength, reduced inflammation | No major adverse events |
| [38] | Kim et al. | 2021 | 4 refractory JDM cases (ages 5.8–20.7) | Baricitinib | Strength, corticosteroid tapering improved | Not specified |
| [39] | Quintana-Ortega et al. | 2022 | 11-year-old, anti-MDA5+ JDM, ILD | Tofacitinib | No response | Fatal SARS-CoV-2 complication |
| [25] | Agud-Dios et al. | 2022 | 5-year-old male, JDM, calcinosis, contractures | Baricitinib | Improved muscle strength, calcinosis, mobility | No major adverse events |
| [29] | Kostik et al. | 2022 | 2 JDM patients (6-month follow-up) | Tofacitinib | One complete, one partial response | Lymphadenitis |
| [28] | Le Voyer et al. | 2022 | 10 JDM cases (9 refractory, 1 new-onset) | Ruxolitinib (n = 7), Baricitinib (n = 3) | 5 achieved inactive disease, steroids reduced | Herpes zoster, skin abscesses |
| [40] | Stewart et al. | 2022 | 1 pediatric patient (JDM, MAS as initial manifestation) | IVIG, steroids, mycophenolate, anakinra, tofacitinib | Resolution of MAS, improvement in multi-organ involvement | Not mentioned |
| [13] | Strauss et al. | 2023 | 4-year-old patient with MDA5 antibody | Ruxolitinib | Fast and sustained remission | No major adverse events |
| [21] | Huang et al. | 2023 | 9 (previously unreported) JDM patients | Ruxolitinib (n = 6), Baricitinib (n = 3) | Rash, muscle strength, and lab markers improved; 39.6% stopped steroids | Leukopenia, cough |
| [24] | Kaplan et al. | 2023 | Anti-MDA5+ JDM, ILD, cardiac involvement | Tofacitinib | Disease control with steroid tapering | Not specified |
[26] | Mastrolia et al. | 2023 | Refractory JDM, calcinosis | Baricitinib | Disease and calcinosis improved | Not specified |
| [41] | Wang et al. | 2023 | 20 children with refractory/severe JDM | Baricitinib (n = 20) + steroids + immunosuppressants | 95% improvement in skin rash, better muscle strength, reduced disease activity, 49% steroid reduction at 24 weeks | No serious side effects reported |
| [42] | Xue Y | 2023 | 9 anti-MDA5-positive children with JDM and ILD | Tofacitinib | 55.5% showed ILD improvement; 44.5% had worsened ILD; high IgG and T-cell levels correlated with poor response | Not mentioned |
| [43] | Zhang J | 2023 | A total of eighty-eight patients with JDM | Tofacitinib | Skin and muscle symptoms improved markedly. Nearly half achieved complete response, six remained on tofacitinib monotherapy. Lung disease improved in 60%, and calcinosis in 75% (complete resolution in 25%). | Only one patient had herpes zoster infection 9 months after initiation. After drug withdrawal, herpes recovered and tofacitinib was given again. |
| [44] | Yu et al. | 2023 | 3 children with refractory JDM | Tofacitinib | Improved muscle strength, skin lesions, quality of life, steroid tapering | No severe adverse events |
| [45] | Xiangyuan C. et al. | 2024 | 12-year-old girl with JDM who developed multiple GI perforations | Tofacitinib | Leading to gradual improvement in muscle strength and reduction in inflammation | No severe adverse events |
| [46] | Kinkor M et al. | 2024 | 14-month-old female with anti-MDA5 | Tofacitinib | Remission of skin and muscle disease | Not mentioned |
| [47] | Huang B et al. | 2024 | 11-year-old girl with juvenile dermatomyositis (JDM), anti-MDA5 antibodies and multiple skin ulcers | Tofacitinib | At the 2-month follow-up visit, early healing of the ulcers Within 8 months, the skin ulcers healed, and muscle enzyme markers and ESR returned to normal. | Not mentioned |
| [48] | Bader-Meunier B et al. | 2025 | Thirty-nine patients with JDM | Various therapies | A significant decrease in the median Type 1 IFN score and serum IFN-α from the diagnosis of JDM to the 6-month follow-up | JAKi-related adverse events consisted of infections in nine patients (including five herpes zoster infections) and weight gain in three patients. |
| [49] | Arguelles Balas D et al. | 2025 | 9-year-old Spanish boy | Tofacitinib | Remission with tofacitinib monotherapy following the failure of previous therapies | No AEs related to tofacitinib have been observed. |
Author Contributions
Funding
Institutional Review Board approval
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marie, I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr. Rheumatol. Rep. 2012, 14, 275–285. [Google Scholar] [CrossRef]
- Cassidy, J.T.; Petty, R.E.; Laxer, R.M.; Lindsley, C.B. Textbook of Pediatric Rheumatology E-Book; Saunders/Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Baechler, E.C.; Bauer, J.W.; Slattery, C.A.; Ortmann, W.A.; Espe, K.J.; Novitzke, J.; Ytterberg, S.R.; Gregersen, P.K.; Behrens, T.W.; Reed, A.M. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol. Med. 2007, 13, 59–68. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Derfoul, A.; Pak, K.; Plotz, P.; Miller, F.W.; Milisenda, J.C.; Grau-Junyent, J.M.; Selva-O’Callaghan, A.; Paik, J.; et al. Identification of distinctive interferon gene signatures in different types of myositis. Neurology 2019, 93, e1193–e1204. [Google Scholar] [CrossRef]
- Wallwork, R.S.; Paik, J.J.; Kim, H. Current evidence for janus kinase inhibitors in adult and juvenile dermatomyositis and key comparisons. Expert Opin. Pharmacother. 2024, 25, 1625–1645. [Google Scholar] [CrossRef]
- Casal-Dominguez, M.; Pinal-Fernandez, I.; Mammen, A.L. Inhibiting interferon pathways in dermatomyositis: Rationale and preliminary evidence. Curr. Treat. Opt. Rheumatol. 2021, 7, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Luo, Y.; O’Shea, J.J.; Nakayamada, S. Janus kinase-targeting therapies in rheumatology: A mechanisms-based approach. Nat. Rev. Rheumatol. 2022, 18, 133–145. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Yuan, W.; Zhang, S.; He, X.; Ji, J. Risk factors for mortality in anti-MDA5 antibody-positive dermatomyositis with interstitial lung disease: A systematic review and meta-analysis. Front. Immunol. 2025, 16, 1628748. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Cheng, L.; Huang, Y.; Yan, S.; Li, H.; Zhan, H.; Li, Y. Roles of biomarkers in anti-MDA5-positive dermatomyositis, associated interstitial lung disease, and rapidly progressive interstitial lung disease. J. Clin. Lab. Anal. 2022, 36, e24726. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.M.; Giannini, E.H.; Bowyer, S.L.; Kim, S.; Lang, B.; Lindsley, C.B.; Pachman, L.M.; Pilkington, C.; Reed, A.M.; Rennebohm, R.M.; et al. Protocols for the initial treatment of moderately severe juvenile dermatomyositis: Results of a Children’s Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res. 2010, 62, 219–225. [Google Scholar] [CrossRef]
- Aeschlimann, F.A.; Fremond, M.L.; Duffy, D.; Rice, G.I.; Charuel, J.L.; Bondet, V.; Saire, E.; Neven, B.; Bodemer, C.; Balu, L.; et al. A child with severe juvenile dermatomyositis treated with ruxolitinib. Brain 2018, 141, e80. [Google Scholar] [CrossRef][Green Version]
- Hinze, C.H.; Oommen, P.T.; Dressler, F.; Urban, A.; Weller-Heinemann, F.; Speth, F.; Lainka, E.; Brunner, J.; Fesq, H.; Foell, D.; et al. Development of practice and consensus-based strategies including a treat-to-target approach for the management of moderate and severe juvenile dermatomyositis in Germany and Austria. Pediatr. Rheumatol. Online J. 2018, 16, 40. [Google Scholar] [CrossRef]
- Strauss, T.; Gunther, C.; Schnabel, A.; Wolf, C.; Hahn, G.; Lee-Kirsch, M.A.; Bruck, N. Rapid and sustained response to JAK inhibition in a child with severe MDA5 + juvenile dermatomyositis. Pediatr. Rheumatol. Online J. 2023, 21, 104. [Google Scholar] [CrossRef]
- Hinze, C. Juvenile dermatomyositis-what’s new? Z. Rheumatol. 2019, 78, 627–635. [Google Scholar] [CrossRef]
- Sherman, M.A.; Nicolai, R.; Datyner, E.K.; Rosina, S.; Hamilton, A.; Ardalan, K.; Bader-Meunier, B.; Brown, A.G.; Jansen, M.H.A.; Kim, S.; et al. Approach to Janus kinase inhibition for juvenile dermatomyositis among CARRA and PReS providers. Rheumatology 2025, 64, 4732–4737. [Google Scholar] [CrossRef]
- Ladislau, L.; Suarez-Calvet, X.; Toquet, S.; Landon-Cardinal, O.; Amelin, D.; Depp, M.; Rodero, M.P.; Hathazi, D.; Duffy, D.; Bondet, V.; et al. JAK inhibitor improves type I interferon induced damage: Proof of concept in dermatomyositis. Brain 2018, 141, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Huard, C.; Gulla, S.V.; Bennett, D.V.; Coyle, A.J.; Vleugels, R.A.; Greenberg, S.A. Correlation of cutaneous disease activity with type 1 interferon gene signature and interferon beta in dermatomyositis. Br. J. Dermatol. 2017, 176, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, S.R.; Reugebrink, M.; Evers, S.W.; Moreau, T.R.J.; Bondet, V.; Armbrust, W.; van den Berg, J.M.; Hissink Muller, P.C.E.; Kamphuis, S.; Schatorje, E.; et al. Targeting interferon responses in juvenile dermatomyositis: Siglec-1 as an in vitro biomarker for JAK inhibitor efficacy. Rheumatology 2025, 64, 5132–5141. [Google Scholar] [CrossRef]
- EMA. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant (accessed on 8 January 2020).
- Heinen, A.; Schnabel, A.; Bruck, N.; Smitka, M.; Wolf, C.; Lucas, N.; Dollinger, S.; Hahn, G.; Gunther, C.; Berner, R.; et al. Interferon signature guiding therapeutic decision making: Ruxolitinib as first-line therapy for severe juvenile dermatomyositis? Rheumatology 2021, 60, e136–e138. [Google Scholar] [CrossRef]
- Huang, B.; Wang, X.; Niu, Y.; Ding, Y.; Wang, X.; Tan, Q.; Li, Y.; Liu, Y.; Chi, Y.; Wang, Y.; et al. Long-term follow-up of Janus-kinase inhibitor and novel active disease biomarker in juvenile dermatomyositis. Rheumatology 2023, 62, 1227–1237. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, B.; Wang, Y.; Hou, J.; Chi, Y.; Zhou, Z.; Li, J. Janus kinase inhibitor significantly improved rash and muscle strength in juvenile dermatomyositis. Ann. Rheum. Dis. 2021, 80, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Chan Ng, P.L.P.; Mopur, A.; Goh, D.Y.T.; Ramamurthy, M.B.; Lim, M.T.C.; Lim, L.K.; Ooi, P.L.; Ang, E.Y. Janus kinase inhibition in induction treatment of anti-MDA5 juvenile dermatomyositis-associated rapidly progressive interstitial lung disease. Int. J. Rheum. Dis. 2022, 25, 228–231. [Google Scholar] [CrossRef]
- Kaplan, M.M.; Celikel, E.; Gungorer, V.; Ekici Tekin, Z.; Gursu, H.A.; Polat, S.E.; Cinel, G.; Celikel Acar, B. Cardiac involvement in a case of juvenile dermatomyositis with positive anti-melanoma differentiation associated protein 5 antibody. Int. J. Rheum. Dis. 2023, 26, 1582–1585. [Google Scholar] [CrossRef]
- Agud-Dios, M.; Arroyo-Andres, J.; Rubio-Muniz, C.; Zarco-Olivo, C.; Calleja-Algarra, A.; de Inocencio, J.; Perez, S.I.P. Juvenile dermatomyositis-associated calcinosis successfully treated with combined immunosuppressive, bisphosphonate, oral baricitinib and physical therapy. Dermatol. Ther. 2022, 35, e15960. [Google Scholar] [CrossRef]
- Mastrolia, M.V.; Orsini, S.I.; Marrani, E.; Maccora, I.; Pagnini, I.; Simonini, G. Efficacy of Janus kinase inhibitor baricitinib in the treatment of refractory juvenile dermatomyositis complicated by calcinosis. Clin. Exp. Rheumatol. 2023, 41, 402–403. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Hong, Y.; Omoyinmi, E.; Brogan, P.A.; Eleftheriou, D. Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain 2019, 142, e8. [Google Scholar] [CrossRef] [PubMed]
- Le Voyer, T.; Gitiaux, C.; Authier, F.J.; Bodemer, C.; Melki, I.; Quartier, P.; Aeschlimann, F.; Isapof, A.; Herbeuval, J.P.; Bondet, V.; et al. JAK inhibitors are effective in a subset of patients with juvenile dermatomyositis: A monocentric retrospective study. Rheumatology 2021, 60, 5801–5808. [Google Scholar] [CrossRef]
- Kostik, M.M.; Raupov, R.K.; Suspitsin, E.N.; Isupova, E.A.; Gaidar, E.V.; Gabrusskaya, T.V.; Kaneva, M.A.; Snegireva, L.S.; Likhacheva, T.S.; Miulkidzhan, R.S.; et al. The Safety and Efficacy of Tofacitinib in 24 Cases of Pediatric Rheumatic Diseases: Single Centre Experience. Front. Pediatr. 2022, 10, 820586. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Ortega, C.; Remesal, A.; Ruiz de Valbuena, M.; de la Serna, O.; Laplaza-Gonzalez, M.; Alvarez-Rojas, E.; Udaondo, C.; Alcobendas, R.; Murias, S. Fatal outcome of anti-MDA5 juvenile dermatomyositis in a paediatric COVID-19 patient: A case report. Mod. Rheumatol. Case Rep. 2021, 5, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862, Erratum in Nat. Rev. Drug Discov. 2018, 17, 78. https://doi.org/10.1038/nrd.2017.267. [Google Scholar] [CrossRef] [PubMed]
- Mikaeili, B.; Alqahtani, Z.A.; Hincapie, A.L.; Guo, J.J. Safety of Janus kinase inhibitors in rheumatoid arthritis: A disproportionality analysis using FAERS database from 2012 to 2022. Clin. Rheumatol. 2025, 44, 1467–1474. [Google Scholar] [CrossRef]
- Winthrop, K. Erratum: The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Bellutti Enders, F.; Bader-Meunier, B.; Baildam, E.; Constantin, T.; Dolezalova, P.; Feldman, B.M.; Lahdenne, P.; Magnusson, B.; Nistala, K.; Ozen, S.; et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann. Rheum. Dis. 2017, 76, 329–340. [Google Scholar] [CrossRef]
- Sabbagh, S.; Almeida de Jesus, A.; Hwang, S.; Kuehn, H.S.; Kim, H.; Jung, L.; Carrasco, R.; Rosenzweig, S.; Goldbach-Mansky, R.; Rider, L.G. Treatment of anti-MDA5 autoantibody-positive juvenile dermatomyositis using tofacitinib. Brain 2019, 142, e59. [Google Scholar] [CrossRef] [PubMed]
- Melki, I.; Devilliers, H.; Gitiaux, C.; Bondet, V.; Duffy, D.; Charuel, J.L.; Miyara, M.; Bokov, P.; Kheniche, A.; Kwon, T.; et al. Anti-MDA5 juvenile idiopathic inflammatory myopathy: A specific subgroup defined by differentially enhanced interferon-alpha signalling. Rheumatology 2020, 59, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Sozeri, B.; Demir, F. A striking treatment option for recalcitrant calcinosis in juvenile dermatomyositis: Tofacitinib citrate. Rheumatology 2020, 59, e140–e141. [Google Scholar] [CrossRef]
- Kim, H.; Dill, S.; O’Brien, M.; Vian, L.; Li, X.; Manukyan, M.; Jain, M.; Adeojo, L.W.; George, J.; Perez, M.; et al. Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann. Rheum. Dis. 2021, 80, 406–408. [Google Scholar] [CrossRef]
- Quintana-Ortega, C.; Prieto-Moreno Pfeifer, A.; Palomino Lozano, L.; Lancharro, A.; Saavedra Lozano, J.; Villa-Garcia, A.J.; Seoane-Reula, E. Colchicine as rescue treatment in two pediatric patients with chronic recurrent multifocal osteomyelitis (CRMO). Mod. Rheumatol. Case Rep. 2023, 7, 215–218. [Google Scholar] [CrossRef]
- Stewart, J.A.; Price, T.; Moser, S.; Mullikin, D.; Bryan, A. Progressive, refractory macrophage activation syndrome as the initial presentation of anti-MDA5 antibody positive juvenile dermatomyositis: A case report and literature review. Pediatr. Rheumatol. Online J. 2022, 20, 16. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhang, Y.; Tang, T.; Luo, C.; Liu, M.Y.; Xu, L.; Wang, L.; Tang, X.M. Anti-nuclear matrix protein 2+ juvenile dermatomyositis with severe skin ulcer and infection: A case report and literature review. World J. Clin. Cases 2022, 10, 3579–3586. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, J.; Deng, J.; Kuang, W.; Wang, J.; Tan, X.; Li, C.; Li, S.; Li, C. Efficiency of tofacitinib in refractory interstitial lung disease among anti-MDA5 positive juvenile dermatomyositis patients. Ann. Rheum. Dis. 2023, 82, 1499–1501. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Shi, X.; Li, S.; Liu, C.; Li, X.; Lu, M.; Deng, J.; Tan, X.; Guan, W.; et al. Janus kinase inhibitor, tofacitinib, in refractory juvenile dermatomyositis: A retrospective multi-central study in China. Arthritis Res. Ther. 2023, 25, 204. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, L.; Quan, M.; Zhang, T.; Song, H. Successful management with Janus kinase inhibitor tofacitinib in refractory juvenile dermatomyositis: A pilot study and literature review. Rheumatology 2021, 60, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Xiangyuan, C.; Xiaoling, Z.; Guangchao, S.; Huasong, Z.; Dexin, L. Juvenile dermatomyositis complications: Navigating gastrointestinal perforations and treatment challenges, a case report. Front. Pediatr. 2024, 12, 1419355. [Google Scholar] [CrossRef]
- Kinkor, M.; Hameed, S.; Kats, A.; Slowik, V.; Fox, E.; Ibarra, M. 14-month-old female with anti-MDA5 juvenile dermatomyositis complicated by liver disease: A case report. Pediatr. Rheumatol. Online J. 2024, 22, 86. [Google Scholar] [CrossRef]
- Huang, B.; Huang, W.; Hao, S. Cutaneous ulceration in juvenile dermatomyositis with anti-melanoma differentiation-associated gene 5. Pediatr. Dermatol. 2024, 41, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Bader-Meunier, B.; Moreau, T.R.J.; Aeschlimann, F.; Authier, F.J.; Charuel, J.L.; Jouen, F.; Boyer, O.; Bodemer, C.; Welfringer-Morin, A.; Bondet, V.; et al. Myositis-specific autoantibody subtypes are associated with response to Janus kinase inhibitors in patients with juvenile dermatomyositis. Rheumatology 2025, 64, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Arguelles Balas, D.; Barral Mena, E.; Martin Diaz, M.A.; Calvo-Aranda, E. Tofacitinib monotherapy as maintenance treatment in juvenile dermatomyositis: A case report. RMD Open 2025, 11, e005651. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Buzoianu, O.; Satirer, Ö.; Kuemmerle-Deschner, J.B.; Reiser, C. Towards New Strategies: Case Report and Review of the Literature—Effective Use of JAK Inhibitor Baricitinib in a 4-Year-Old Boy with Anti-MDA5 Antibody-Positive Juvenile Dermatomyositis. J. Clin. Med. 2026, 15, 709. https://doi.org/10.3390/jcm15020709
Buzoianu O, Satirer Ö, Kuemmerle-Deschner JB, Reiser C. Towards New Strategies: Case Report and Review of the Literature—Effective Use of JAK Inhibitor Baricitinib in a 4-Year-Old Boy with Anti-MDA5 Antibody-Positive Juvenile Dermatomyositis. Journal of Clinical Medicine. 2026; 15(2):709. https://doi.org/10.3390/jcm15020709
Chicago/Turabian StyleBuzoianu, Oana, Özlem Satirer, Jasmin B. Kuemmerle-Deschner, and Christiane Reiser. 2026. "Towards New Strategies: Case Report and Review of the Literature—Effective Use of JAK Inhibitor Baricitinib in a 4-Year-Old Boy with Anti-MDA5 Antibody-Positive Juvenile Dermatomyositis" Journal of Clinical Medicine 15, no. 2: 709. https://doi.org/10.3390/jcm15020709
APA StyleBuzoianu, O., Satirer, Ö., Kuemmerle-Deschner, J. B., & Reiser, C. (2026). Towards New Strategies: Case Report and Review of the Literature—Effective Use of JAK Inhibitor Baricitinib in a 4-Year-Old Boy with Anti-MDA5 Antibody-Positive Juvenile Dermatomyositis. Journal of Clinical Medicine, 15(2), 709. https://doi.org/10.3390/jcm15020709





