A Lymphatic Perspective on Obesity and Inflammatory Arthritis: New Disease-Modifying Potential in Rheumatology
Abstract
1. Introduction
2. The Role of Obesity in Developing Rheumatoid Arthritis
3. The Role of Obesity in Developing Psoriatic Arthritis
4. The Role of Obesity in Gout
5. Treatment Response in Inflammatory Arthritis
5.1. Rheumatoid Arthritis: Divergent Mechanisms in Seropositive Versus Seronegative Disease and Treatment Specific Interactions
5.2. Psoriatic Arthritis: Mechanistic Complexity and Variable Treatment Responses
5.3. Gout: Metabolic Interference with Urate-Lowering Therapy
6. Chronic Inflammatory State Caused by Obesity
6.1. Adipose Tissue as an Active Immunometabolic Organ
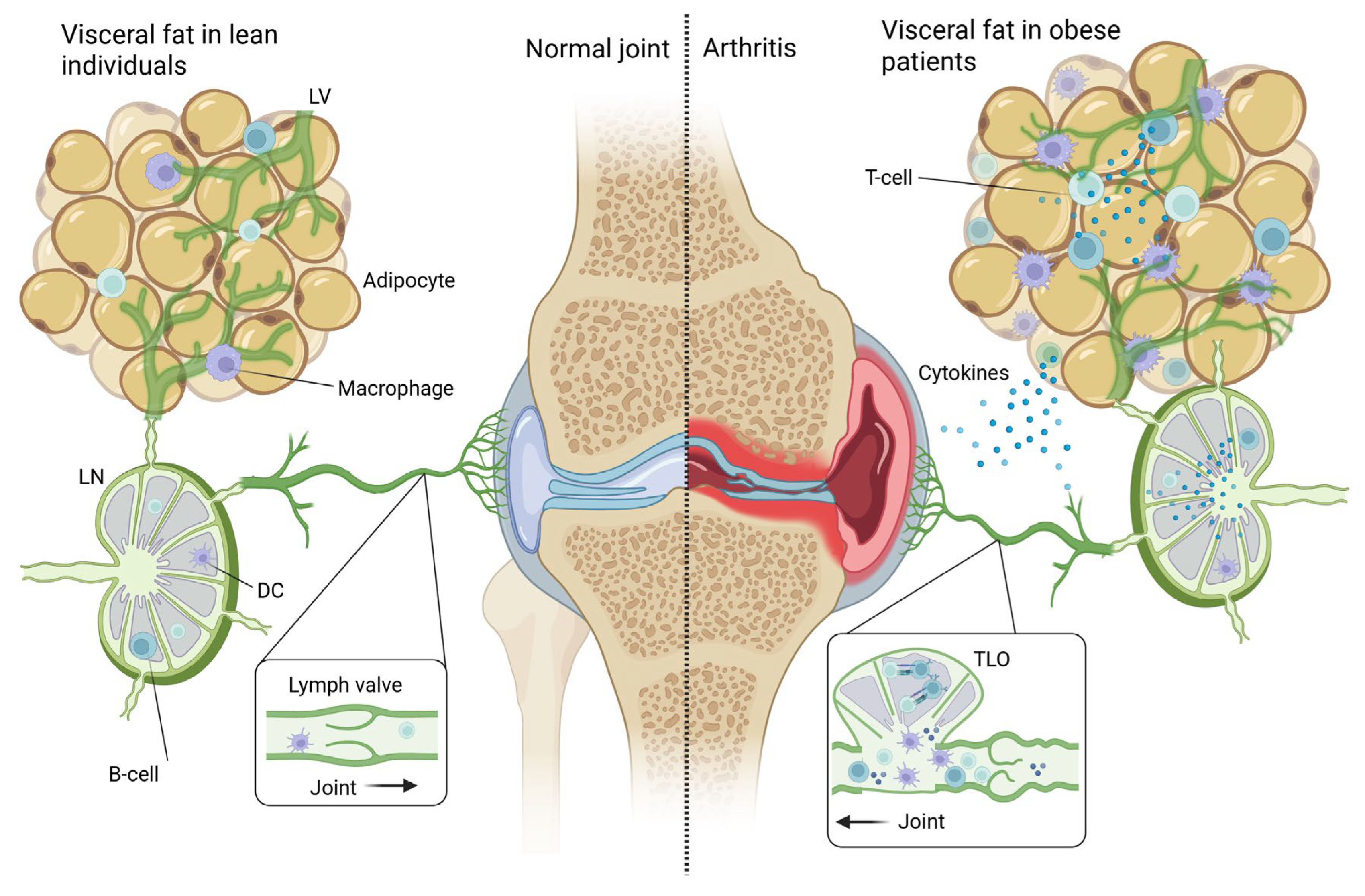
6.2. Adipose Tissue Immune Cell Populations in Obesity
6.3. Two Pathways to Arthritis: Autoimmune Versus Local Metabolic–Inflammatory Mechanisms
7. Normal Architecture of the Lymphatic System
8. Obesity Impairs Lymphatic Function
8.1. Experimental Models of Lymphatic Dysfunction in Obesity
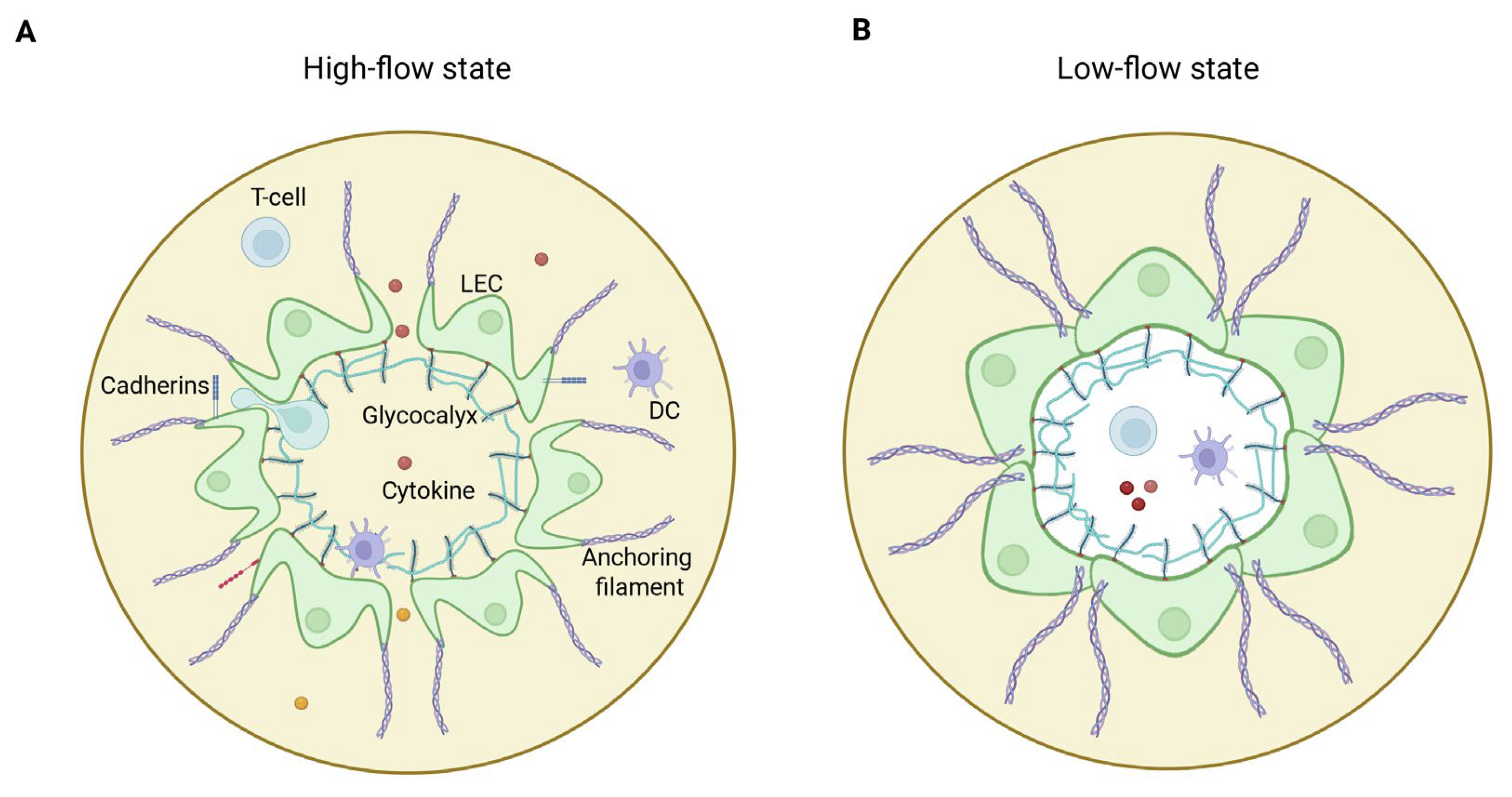
8.2. Disruption of Endothelial Cell Signaling at the Glycocalyx–Cell–Matrix Interface
8.3. Obesity-Associated Lymphatic Injury
8.4. Obesity and Lymphatic Function in Human Studies
8.5. Specific Dysfunction in Mesenteric Lymphatic Vessels
9. Obesity-Induced Local Lymphatic Injury as a Contributing Factor to Chronic Joint Inflammation
10. Incretin Analogs as Dual-Pathway Disease-Modifying Therapeutic Options
11. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACKRs | Atypical chemokine receptors |
| ACPA | Anti-citrullinated protein antibodies |
| ALND | Axillary lymph node dissection |
| BMI | Body mass index |
| BRI | Body roundness index |
| CCL21 | C-C motif chemokine ligand 21 |
| CDAI | Clinical Disease Activity Index |
| CRP | C-reactive protein |
| CSA | Clinically suspect arthralgia |
| DAPSA | Disease Activity in Psoriatic Arthritis |
| DAS28 | Disease Activity Score in 28 joints |
| DCs | Dendritic cells |
| ESR | Erythrocyte sedimentation rate |
| FOXC2 | Forkhead box protein C2 |
| GATA2 | GATA binding protein 2 |
| GLP-1 | Glucagon-like peptide-1 |
| HAQ | Health Assessment Questionnaire |
| IFN | Interferon |
| IL-1β | Interleukin-1 beta |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-13 | Interleukin-13 |
| IL-17 | Interleukin-17 |
| IL-17A | Interleukin-17A |
| iNOS | Inducible nitric oxide synthase |
| JAK | Janus kinase |
| LECs | Lymphatic endothelial cells |
| LSMCs | Lymphatic smooth muscle cells |
| MSU | Monosodium urate |
| NF-κB | Nuclear factor kappa B |
| NHANES | National Health and Nutrition Examination Survey |
| OR | Odds ratio |
| PKA/CREB | Protein kinase A/cAMP response element-binding protein |
| PROX1 | Prospero homeobox protein 1 |
| PsA | Psoriatic arthritis |
| RA | Rheumatoid arthritis |
| RF | Rheumatoid factor |
| RR | Relative risk |
| SDAI | Simplified Disease Activity Index |
| TGF-β | Transforming growth factor beta |
| Th17 | T helper 17 cells |
| TLO | Tertiary lymphoid organ |
| TLOs | Tertiary lymphoid organs |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TNF-α | Tumor necrosis factor alpha |
| ULT | Urate-lowering therapy |
| VFO | Visceral fat obesity |
| WWI | Weight-adjusted waist index |
References
- GBD 2021 Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Chen, Q.F.; Yang, W.; Zuluaga, M.; Targher, G.; Byrne, C.D.; Valenti, L.; Luo, F.; Katsouras, C.S.; Thaher, O.; et al. Burden of disease attributable to high body mass index: An analysis of data from the Global Burden of Disease Study 2021. EClinicalMedicine 2024, 76, 102848. [Google Scholar] [CrossRef] [PubMed]
- The GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Gout Collaborators. Global, regional, and national burden of gout, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2024, 6, e507–e517. [Google Scholar] [CrossRef]
- GBD 2021 Rheumatoid Arthritis Collaborators. Global, regional, and national burden of rheumatoid arthritis, 1990-2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e594–e610. [Google Scholar] [CrossRef]
- Haddad, A.; Elkayam, P.C.; Stein, N.; Feldhamer, I.; Cohen, A.D.; Saliba, W.; Zisman, D. Epidemiological trends in psoriatic arthritis: A comprehensive population-based study. Arthritis Res. Ther. 2024, 26, 108. [Google Scholar] [CrossRef]
- Crowson, C.S.; Matteson, E.L.; Davis, J.M., III; Gabriel, S.E. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res. 2013, 65, 71–77. [Google Scholar] [CrossRef]
- Dubovyk, V.; Vasileiadis, G.K.; Fatima, T.; Zhang, Y.; Kapetanovic, M.C.; Kastbom, A.; Rizk, M.; Soderbergh, A.; Zhao, S.S.; van Vollenhoven, R.F.; et al. Obesity is a risk factor for poor response to treatment in early rheumatoid arthritis: A NORD-STAR study. RMD Open 2024, 10, e004227. [Google Scholar] [CrossRef]
- Liu, Y.; Hazlewood, G.S.; Kaplan, G.G.; Eksteen, B.; Barnabe, C. Impact of Obesity on Remission and Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2017, 69, 157–165. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Hopkins, A.M.; Sorich, M.J.; Proudman, S.; Foster, D.J.R.; Wiese, M.D. Association between obesity and remission in rheumatoid arthritis patients treated with disease-modifying anti-rheumatic drugs. Sci. Rep. 2020, 10, 18634. [Google Scholar] [CrossRef]
- Sandberg, M.E.; Bengtsson, C.; Kallberg, H.; Wesley, A.; Klareskog, L.; Alfredsson, L.; Saevarsdottir, S. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 2029–2033. [Google Scholar] [CrossRef]
- Corrado, A.; Guadagni, I.; Picarelli, G.; Variola, A. Obesity and Chronic Inflammation: Implications for Rheumatoid Arthritis, Spondyloarthritis, and Ulcerative Colitis. Immun. Inflamm. Dis. 2025, 13, e70080. [Google Scholar] [CrossRef]
- Bouta, E.M.; Bell, R.D.; Rahimi, H.; Xing, L.; Wood, R.W.; Bingham, C.O., III; Ritchlin, C.T.; Schwarz, E.M. Targeting lymphatic function as a novel therapeutic intervention for rheumatoid arthritis. Nat. Rev. Rheumatol. 2018, 14, 94–106. [Google Scholar] [CrossRef]
- Zeng, Y. Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J. Cell. Mol. Med. 2017, 21, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; Mohammadi Nouri, P.M.; Hosseini, S.H.; Roper, B.; Withers, S.G.; Kizhakkedathu, J.N. Reprogramming the glycocalyx: Advances in glycoengineering for immunomodulation and regenerative medicine. Biomaterials 2025, 326, 123717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wilding, J.P.H.; Hu, J. Defective lymphatic vasculature in obesity. Obes. Rev. 2025, 26, e13922. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Sabine, A.; Agalarov, Y.; Maby-El Hajjami, H.; Jaquet, M.; Hagerling, R.; Pollmann, C.; Bebber, D.; Pfenniger, A.; Miura, N.; Dormond, O.; et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell 2012, 22, 430–445. [Google Scholar] [CrossRef]
- Yoo, H.; Lee, Y.J.; Park, C.; Son, D.; Choi, D.Y.; Park, J.H.; Choi, H.J.; La, H.W.; Choi, Y.J.; Moon, E.H.; et al. Epigenetic priming by Dot1l in lymphatic endothelial progenitors ensures normal lymphatic development and function. Cell Death Dis. 2020, 11, 14. [Google Scholar] [CrossRef]
- Magnuson, A.M.; Fouts, J.K.; Regan, D.P.; Booth, A.D.; Dow, S.W.; Foster, M.T. Adipose tissue extrinsic factor: Obesity-induced inflammation and the role of the visceral lymph node. Physiol. Behav. 2018, 190, 71–81. [Google Scholar] [CrossRef]
- Magnuson, A.M.; Regan, D.P.; Fouts, J.K.; Booth, A.D.; Dow, S.W.; Foster, M.T. Diet-induced obesity causes visceral, but not subcutaneous, lymph node hyperplasia via increases in specific immune cell populations. Cell Prolif. 2017, 50, e12365. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Wieczorek, M.; Balanescu, A.; Bischoff-Ferrari, H.A.; Boonen, A.; Cavalli, G.; de Souza, S.; de Thurah, A.; Dorner, T.E.; Moe, R.H.; et al. 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2023, 82, 48–56. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Fasshauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.M.; Jaffe, A.E.; Steele, K.E.; Schweitzer, M.A.; Magnuson, T.H.; Wolfe, A.; Wong, G.W. Cytokine, Chemokine, and Cytokine Receptor Changes Are Associated With Metabolic Improvements After Bariatric Surgery. J. Clin. Endocrinol. Metab. 2019, 104, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Zhang, Z.; Huang, Q.; Liu, L.; Que, W.; Lu, D.; Jing, S.; Gan, Y.; Liu, S.; Xiao, F. Association of regional adiposity distribution with risk of autoimmune diseases. Clin. Rheumatol. 2025, 44, 2541–2552. [Google Scholar] [CrossRef] [PubMed]
- Perera, J.; Delrosso, C.A.; Nerviani, A.; Pitzalis, C. Clinical Phenotypes, Serological Biomarkers, and Synovial Features Defining Seropositive and Seronegative Rheumatoid Arthritis: A Literature Review. Cells 2024, 13, 743. [Google Scholar] [CrossRef]
- Dong, Y.; Greenwood, D.C.; Hardie, L.J.; Cade, J.E. Measures of Adiposity and Risk of Rheumatoid Arthritis in Middle-Aged UK Women: A Prospective Cohort Study. Nutrients 2025, 17, 1557. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Zhao, W.; Zhu, Y.; Zhu, L.; Shi, R.; Wang, Z.; Pan, H.; Wang, D. The Causal Effect of Obesity on the Risk of 15 Autoimmune Diseases: A Mendelian Randomization Study. Obes. Facts 2023, 16, 598–605. [Google Scholar] [CrossRef]
- Marchand, N.E.; Sparks, J.A.; Malspeis, S.; Yoshida, K.; Prisco, L.; Zhang, X.; Costenbader, K.; Hu, F.; Karlson, E.W.; Lu, B. Long-term weight changes and risk of rheumatoid arthritis among women in a prospective cohort: A marginal structural model approach. Rheumatology 2022, 61, 1430–1439. [Google Scholar] [CrossRef]
- Ohno, T.; Aune, D.; Heath, A.K. Adiposity and the risk of rheumatoid arthritis: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2020, 10, 16006. [Google Scholar] [CrossRef]
- Hedstrom, A.K.; Klareskog, L.; Alfredsson, L. Interplay between obesity and smoking with regard to RA risk. RMD Open 2019, 5, e000856. [Google Scholar] [CrossRef]
- Dumoulin, Q.A.; Boeren, A.M.P.; Krijbolder, D.I.; Willemze, A.; de Jong, P.H.P.; van Mulligen, E.; van Steenbergen, H.W.; van der Helm-van Mil, A.H.M. When does obesity exert its effect in conferring risk of developing RA: A large study in cohorts of symptomatic persons at risk. RMD Open 2024, 10, e003785. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, J.S.; Kim, S.; Kim, Y.J.; Hong, S.; Kim, Y.G.; Lee, C.K.; Yoo, B. The association of obesity and the risk of rheumatoid arthritis according to abdominal obesity status: A nationwide population-based study in Korea. Rheumatol. Int. 2024, 44, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ogdie, A.; Merola, J.F.; Ritchlin, C. Preventing psoriatic arthritis: Focusing on patients with psoriasis at increased risk of transition. Nat. Rev. Rheumatol. 2019, 15, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Soltani-Arabshahi, R.; Wong, B.; Feng, B.J.; Goldgar, D.E.; Duffin, K.C.; Krueger, G.G. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch. Dermatol. 2010, 146, 721–726. [Google Scholar] [CrossRef]
- Eder, L.; Thavaneswaran, A.; Chandran, V.; Cook, R.J.; Gladman, D.D. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 813–817. [Google Scholar] [CrossRef]
- Li, W.; Han, J.; Qureshi, A.A. Obesity and risk of incident psoriatic arthritis in US women. Ann. Rheum. Dis. 2012, 71, 1267–1272. [Google Scholar] [CrossRef]
- Love, T.J.; Zhu, Y.; Zhang, Y.; Wall-Burns, L.; Ogdie, A.; Gelfand, J.M.; Choi, H.K. Obesity and the risk of psoriatic arthritis: A population-based study. Ann. Rheum. Dis. 2012, 71, 1273–1277. [Google Scholar] [CrossRef]
- Green, A.; Shaddick, G.; Charlton, R.; Snowball, J.; Nightingale, A.; Smith, C.; Tillett, W.; McHugh, N.; on behalf of the PROMPT study group. Modifiable risk factors and the development of psoriatic arthritis in people with psoriasis. Br. J. Dermatol. 2020, 182, 714–720. [Google Scholar] [CrossRef]
- Timsans, J.; Palomaki, A.; Kauppi, M. Gout and Hyperuricemia: A Narrative Review of Their Comorbidities and Clinical Implications. J. Clin. Med. 2024, 13, 7616. [Google Scholar] [CrossRef]
- Mohseni, M.; van der Valk, E.S.; Van der Hurk, M.J.B.; Savas, M.; Boon, M.R.; van Rossum, E.F.C. Corticosteroid Use and Long-Term Changes in Weight and Waist Circumference: The Lifelines Cohort Study. J. Clin. Endocrinol. Metab. 2025, dgaf166. [Google Scholar] [CrossRef]
- Zou, Y.; Li, F.; Huang, Q.; Zhao, Z.; Li, S.; Liu, H. Associations of weight change patterns with hyperuricemia risk in U.S. adults. Sci. Rep. 2025, 15, 21124. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.Y.; Lee, J.H.; Jung, S.M.; Suh, Y.S.; Koh, J.H.; Kwok, S.K.; Ju, J.H.; Park, K.S.; Park, S.H. Visceral fat obesity is highly associated with primary gout in a metabolically obese but normal weighted population: A case control study. Arthritis Res. Ther. 2015, 17, 79. [Google Scholar] [CrossRef]
- Mao, T.; He, Q.; Yang, J.; Jia, L.; Xu, G. Relationship between gout, hyperuricemia, and obesity-does central obesity play a significant role?-a study based on the NHANES database. Diabetol. Metab. Syndr. 2024, 16, 24. [Google Scholar] [CrossRef]
- Eun, Y.; Kim, I.Y.; Han, K.; Lee, K.N.; Lee, D.Y.; Shin, D.W.; Kang, S.; Lee, S.; Cha, H.S.; Koh, E.M.; et al. Association between female reproductive factors and gout: A nationwide population-based cohort study of 1 million postmenopausal women. Arthritis Res. Ther. 2021, 23, 304. [Google Scholar] [CrossRef]
- DeMarco, M.A.; Maynard, J.W.; Huizinga, M.M.; Baer, A.N.; Kottgen, A.; Gelber, A.C.; Coresh, J. Obesity and younger age at gout onset in a community-based cohort. Arthritis Care Res. 2011, 63, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 2015, 11, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.; Han, K.; Lee, S.W.; Kim, K.; Kang, S.; Lee, S.; Cha, H.S.; Koh, E.M.; Kim, H.; Lee, J. Increased risk of incident gout in young men with metabolic syndrome: A nationwide population-based cohort study of 3.5 million men. Front. Med. 2022, 9, 1010391. [Google Scholar] [CrossRef] [PubMed]
- Hollander, N.K.D.; Boeren, A.M.P.; van der Helm-van Mil, A.H.M.; van Steenbergen, H.W. Patients with obesity have more inflamed joints and higher CRP levels during the disease course in ACPA-positive RA but not in ACPA-negative RA. Arthritis Res. Ther. 2024, 26, 42. [Google Scholar] [CrossRef]
- Agrawal, S.; Gollapudi, S.; Su, H.; Gupta, S. Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011, 31, 472–478. [Google Scholar] [CrossRef]
- Slisere, B.; Arisova, M.; Aizbalte, O.; Salmina, M.M.; Zolovs, M.; Levensteins, M.; Mukans, M.; Troickis, I.; Meija, L.; Lejnieks, A.; et al. Distinct B cell profiles characterise healthy weight and obesity pre- and post-bariatric surgery. Int. J. Obes. 2023, 47, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Valentino, T.R.; Chen, N.; Makhijani, P.; Khan, S.; Winer, S.; Revelo, X.S.; Winer, D.A. The role of autoantibodies in bridging obesity, aging, and immunosenescence. Immun. Ageing. 2024, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Karpouzas, G.A.; Gonzalez-Gay, M.A.; Corrales, A.; Myasoedova, E.; Rantapaa-Dahlqvist, S.; Sfikakis, P.P.; Dessein, P.; Hitchon, C.; Pascual-Ramos, V.; Contreras-Yanez, I.; et al. Influence of body mass index on cardiovascular risk in rheumatoid arthritis varies across anti-citrullinated protein antibody status and biologic use. RMD Open 2025, 11, e005464. [Google Scholar] [CrossRef] [PubMed]
- Novella-Navarro, M.; Genre, F.; Hernandez-Breijo, B.; Remuzgo-Martinez, S.; Martinez-Feito, A.; Peiteado, D.; Monjo, I.; Gonzalez-Gay, M.A.; Plasencia-Rodriguez, C.; Balsa, A. Obesity and response to biological therapy in rheumatoid arthritis: The role of body mass index and adipose tissue cytokines. Clin. Exp. Rheumatol. 2022, 40, 1726–1732. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Dikranian, A.H.; Gonzalez-Gay, M.A.; Wellborne, F.; Alvaro-Gracia, J.M.; Takiya, L.; Stockert, L.; Paulissen, J.; Shi, H.; Tatulych, S.; Curtis, J.R. Efficacy of tofacitinib in patients with rheumatoid arthritis stratified by baseline body mass index: An analysis of pooled data from phase 3 studies. RMD Open 2022, 8, e002103. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Eder, L.; Orbai, A.M.; Coates, L.C.; de Wit, M.; Smolen, J.S.; Kiltz, U.; Palominos, P.; Canete, J.D.; Scrivo, R.; et al. Association between obesity and likelihood of remission or low disease activity status in psoriatic arthritis applying index-based and patient-based definitions of remission: A cross-sectional study. RMD Open 2023, 9, e003157. [Google Scholar] [CrossRef]
- Ogdie, A.; Palmer, J.L.; Greenberg, J.; Curtis, J.R.; Harrold, L.R.; Solomon, D.H.; Kavanaugh, A.; Kremer, J.M.; Mease, P.J. Predictors of Achieving Remission among Patients with Psoriatic Arthritis Initiating a Tumor Necrosis Factor Inhibitor. J. Rheumatol. 2019, 46, 475–482. [Google Scholar] [CrossRef]
- Pantano, I.; Iacono, D.; Favalli, E.G.; Scalise, G.; Costa, L.; Caso, F.; Guggino, G.; Scarpa, R.; Ciccia, F. Secukinumab efficacy in patients with PsA is not dependent on patients’ body mass index. Ann. Rheum. Dis. 2022, 81, e42. [Google Scholar] [CrossRef]
- Giles, J.T.; Ogdie, A.; Gomez Reino, J.J.; Helliwell, P.; Germino, R.; Stockert, L.; Young, P.; Joseph, W.; Mundayat, R.; Graham, D.; et al. Impact of baseline body mass index on the efficacy and safety of tofacitinib in patients with psoriatic arthritis. RMD Open 2021, 7, e001486. [Google Scholar] [CrossRef]
- Dodington, D.W.; Desai, H.R.; Woo, M. JAK/STAT—Emerging Players in Metabolism. Trends Endocrinol. Metab. 2018, 29, 55–65. [Google Scholar] [CrossRef]
- Cheng, Z.; Xu, X.; Qi, H.; Li, X.; Li, Y.; Jiang, C.; Miao, X.; Ji, X.; Wang, Y.; Dong, B.; et al. Obesity reduces the urate-lowering efficacy among patients with primary gout: A prospective cohort study. Rheumatology 2025, 64, 3500–3508. [Google Scholar] [CrossRef]
- DiSpirito, J.R.; Mathis, D. Immunological contributions to adipose tissue homeostasis. Semin. Immunol. 2015, 27, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Caer, C.; Rouault, C.; Le Roy, T.; Poitou, C.; Aron-Wisnewsky, J.; Torcivia, A.; Bichet, J.C.; Clement, K.; Guerre-Millo, M.; Andre, S. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep. 2017, 7, 3000. [Google Scholar] [CrossRef]
- Faust, H.J.; Chang, M.H.; Jonsson, A.H.; Theisen, E.; LaMarche, N.M.; Trim, W.V.; Lynch, L.; Nigrovic, P.A.; Brenner, M.B. Adipose tissue harbors pathogenic T cells in obesity that exacerbate inflammatory arthritis. J. Exp. Med. 2025, 222, eadl4909. [Google Scholar] [CrossRef]
- Elkins, C.; Ye, C.; Sivasami, P.; Mulpur, R.; Diaz-Saldana, P.P.; Peng, A.; Xu, M.; Chiang, Y.P.; Moll, S.; Rivera-Rodriguez, D.E.; et al. Obesity reshapes regulatory T cells in the visceral adipose tissue by disrupting cellular cholesterol homeostasis. Sci. Immunol. 2025, 10, eadl4909. [Google Scholar] [CrossRef]
- Shantaram, D.; Hoyd, R.; Blaszczak, A.M.; Antwi, L.; Jalilvand, A.; Wright, V.P.; Liu, J.; Smith, A.J.; Bradley, D.; Lafuse, W.; et al. Obesity-associated microbiomes instigate visceral adipose tissue inflammation by recruitment of distinct neutrophils. Nat. Commun. 2024, 15, 5434. [Google Scholar] [CrossRef]
- Conroy, M.J.; Fitzgerald, V.; Doyle, S.L.; Channon, S.; Useckaite, Z.; Gilmartin, N.; O’Farrelly, C.; Ravi, N.; Reynolds, J.V.; Lysaght, J. The microenvironment of visceral adipose tissue and liver alter natural killer cell viability and function. J. Leukoc. Biol. 2016, 100, 1435–1442. [Google Scholar] [CrossRef]
- Sasaki, T.; Moro, K.; Kubota, T.; Kubota, N.; Kato, T.; Ohno, H.; Nakae, S.; Saito, H.; Koyasu, S. Innate Lymphoid Cells in the Induction of Obesity. Cell Rep. 2019, 28, 202–217.e207. [Google Scholar] [CrossRef] [PubMed]
- Daley, A.D.; Benezech, C. Fat-associated lymphoid clusters: Supporting visceral adipose tissue B cell function in immunity and metabolism. Immunol. Rev. 2024, 324, 78–94. [Google Scholar] [CrossRef] [PubMed]
- van der Zalm, I.J.B.; van der Valk, E.S.; Wester, V.L.; Nagtzaam, N.M.A.; van Rossum, E.F.C.; Leenen, P.J.M.; Dik, W.A. Obesity-associated T-cell and macrophage activation improve partly after a lifestyle intervention. Int. J. Obes. 2020, 44, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef]
- Gianesini, S.; Rimondi, E.; Raffetto, J.D.; Melloni, E.; Pellati, A.; Menegatti, E.; Avruscio, G.P.; Bassetto, F.; Costa, A.L.; Rockson, S. Human collecting lymphatic glycocalyx identification by electron microscopy and immunohistochemistry. Sci. Rep. 2023, 13, 3022. [Google Scholar] [CrossRef]
- Moore, K.H.; Murphy, H.A.; George, E.M. The glycocalyx: A central regulator of vascular function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R508–R518. [Google Scholar] [CrossRef]
- den Braanker, H.; van Stigt, A.C.; Kok, M.R.; Lubberts, E.; Bisoendial, R.J. Single-Cell RNA Sequencing Reveals Heterogeneity and Functional Diversity of Lymphatic Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 11976. [Google Scholar] [CrossRef]
- Teijeira, A.; Hunter, M.C.; Russo, E.; Proulx, S.T.; Frei, T.; Debes, G.F.; Coles, M.; Melero, I.; Detmar, M.; Rouzaut, A.; et al. T Cell Migration from Inflamed Skin to Draining Lymph Nodes Requires Intralymphatic Crawling Supported by ICAM-1/LFA-1 Interactions. Cell Rep. 2017, 18, 857–865. [Google Scholar] [CrossRef]
- Kazenwadel, J.; Betterman, K.L.; Chong, C.E.; Stokes, P.H.; Lee, Y.K.; Secker, G.A.; Agalarov, Y.; Demir, C.S.; Lawrence, D.M.; Sutton, D.L.; et al. GATA2 is required for lymphatic vessel valve development and maintenance. J. Clin. Investig. 2015, 125, 2979–2994. [Google Scholar] [CrossRef]
- Blum, K.S.; Karaman, S.; Proulx, S.T.; Ochsenbein, A.M.; Luciani, P.L.; Leroux, J.-C.; Wolfrum, C.; Detmar, M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS ONE 2014, 9, e94713. [Google Scholar] [CrossRef] [PubMed]
- Rehal, S.; Kataru, R.P.; Hespe, G.E.; Ly, C.L.; Mehrara, B.J. Regulation of lymphatic function and injury by nitrosative stress in obese mice. Mol. Metab. 2020, 30, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Weitman, E.S.; Aschen, S.Z.; Farias-Eisner, G.; Albano, N.J.; Cuzzone, D.A.; Ghanta, S.; Zampell, J.C.; Thorek, D.; Mehrara, B.J. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS ONE 2013, 8, e70703. [Google Scholar] [CrossRef]
- Savetsky, I.L.; Torrisi, J.S.; Cuzzone, D.A.; Ghanta, S.; Albano, N.J.; Gardenier, J.C.; Joseph, W.J.; Mehrara, B.J. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H165–H172. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.D.; Hespe, G.E.; Kataru, R.P.; García Nores, G.D.; Savetsky, I.L.; Torrisi, J.S.; Mehrara, B.J. Obesity-induced lymphatic dysfunction is reversible with weight loss: Involvement of iNOS and perilymphatic inflammation. J. Physiol. 2016, 594, 7073–7087. [Google Scholar] [CrossRef]
- García Nores, G.D.; Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Hespe, G.E.; Torrisi, J.S.; Gardenier, J.C.; Savetsky, I.L.; Aschen, S.Z.; Mehrara, B.J. Obesity but not high-fat diet impairs lymphatic function. Int. J. Obes. 2016, 40, 1582–1590. [Google Scholar] [CrossRef]
- Savetsky, I.L.; Ghanta, S.; Gardenier, J.C.; Torrisi, J.S.; Garcia Nores, G.D.; Hespe, G.E.; Nitti, M.D.; Kataru, R.P.; Mehrara, B.J. Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 2015, 10, e0126908. [Google Scholar] [CrossRef]
- Kataru, R.P.; Kim, H.; Jang, C.; Choi, D.K.; Koh, B.I.; Kim, M.; Gollamudi, S.; Kim, Y.K.; Lee, S.H.; Koh, G.Y. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 2011, 34, 96–107. [Google Scholar] [CrossRef]
- Fukasawa, K.; Hanada, K.; Ichikawa, K.; Hirashima, M.; Takagi, T.; Itoh, S.; Watabe, T.; Itoh, F. Endothelial-specific depletion of TGF-beta signaling affects lymphatic function. Inflamm. Regen. 2021, 41, 35. [Google Scholar] [CrossRef]
- Fancher, I.S.; Le Master, E.; Ahn, S.J.; Adamos, C.; Lee, J.C.; Berdyshev, E.; Dull, R.O.; Phillips, S.A.; Levitan, I. Impairment of Flow-Sensitive Inwardly Rectifying K(+) Channels via Disruption of Glycocalyx Mediates Obesity-Induced Endothelial Dysfunction. Arter. Thromb. Vasc. Biol. 2020, 40, e240–e255. [Google Scholar] [CrossRef]
- Sato, A.; Kamekura, R.; Kawata, K.; Kawada, M.; Jitsukawa, S.; Yamashita, K.; Sato, N.; Himi, T.; Ichimiya, S. Novel Mechanisms of Compromised Lymphatic Endothelial Cell Homeostasis in Obesity: The Role of Leptin in Lymphatic Endothelial Cell Tube Formation and Proliferation. PLoS ONE 2016, 11, e0158408. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Rutkowski, J.M.; Helft, J.; Reddy, S.T.; Swartz, M.A.; Randolph, G.J.; Angeli, V. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am. J. Pathol. 2009, 175, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Arngrim, N.; Simonsen, L.; Holst, J.J.; Bülow, J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: A possible link between obesity and local tissue inflammation? Int. J. Obes. 2013, 37, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.K.; Grant, F.D.; Slavin, S.A. Obesity-induced lymphedema: Clinical and lymphoscintigraphic features. Plast. Reconstr. Surg. 2015, 135, 1715–1719. [Google Scholar] [CrossRef]
- Greene, A.K.; Sudduth, C.L. Lower extremity lymphatic function predicted by body mass index: A lymphoscintigraphic study of obesity and lipedema. Int. J. Obes. 2021, 45, 369–373. [Google Scholar] [CrossRef]
- Cao, E.; Watt, M.J.; Nowell, C.J.; Quach, T.; Simpson, J.S.; De Melo Ferreira, V.; Agarwal, S.; Chu, H.; Srivastava, A.; Anderson, D.; et al. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nat. Metab. 2021, 3, 1175–1188. [Google Scholar] [CrossRef]
- Mikrani, R.; Styles, I.K.; Hoang, T.A.; Abdallah, M.; Senyschyn, D.; Porter, C.J.H.; Cao, E.; Trevaskis, N.L. Obesity-associated mesenteric lymph leakage impairs the trafficking of lipids, lipophilic drugs and antigens from the intestine to mesenteric lymph nodes. Eur. J. Pharm. Biopharm. 2022, 180, 319–331. [Google Scholar] [CrossRef]
- Harvey, N.L.; Srinivasan, R.S.; Dillard, M.E.; Johnson, N.C.; Witte, M.H.; Boyd, K.; Sleeman, M.W.; Oliver, G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005, 37, 1072–1081. [Google Scholar] [CrossRef]
- Lin, X.; Bell, R.D.; Catheline, S.E.; Takano, T.; McDavid, A.; Jonason, J.H.; Schwarz, E.M.; Xing, L. Targeting Synovial Lymphatic Function as a Novel Therapeutic Intervention for Age-Related Osteoarthritis in Mice. Arthritis Rheumatol. 2023, 75, 923–936. [Google Scholar] [CrossRef]
- Guo, R.; Zhou, Q.; Proulx, S.T.; Wood, R.; Ji, R.C.; Ritchlin, C.T.; Pytowski, B.; Zhu, Z.; Wang, Y.J.; Schwarz, E.M.; et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009, 60, 2666–2676. [Google Scholar] [CrossRef]
- Bouta, E.M.; Kuzin, I.; de Mesy Bentley, K.; Wood, R.W.; Rahimi, H.; Ji, R.C.; Ritchlin, C.T.; Bottaro, A.; Xing, L.; Schwarz, E.M. Brief Report: Treatment of Tumor Necrosis Factor-Transgenic Mice With Anti-Tumor Necrosis Factor Restores Lymphatic Contractions, Repairs Lymphatic Vessels, and May Increase Monocyte/Macrophage Egress. Arthritis Rheumatol. 2017, 69, 1187–1193. [Google Scholar] [CrossRef]
- Bell, R.D.; Rahimi, H.; Kenney, H.M.; Lieberman, A.A.; Wood, R.W.; Schwarz, E.M.; Ritchlin, C.T. Altered Lymphatic Vessel Anatomy and Markedly Diminished Lymph Clearance in Affected Hands of Patients With Active Rheumatoid Arthritis. Arthritis Rheumatol. 2020, 72, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.B.; Rasmussen, J.C.; Fife, C.E.; Shaitelman, S.F.; Sevick-Muraca, E.M. The Development and Treatment of Lymphatic Dysfunction in Cancer Patients and Survivors. Cancers 2020, 12, 2280. [Google Scholar] [CrossRef] [PubMed]
- Thurlings, R.M.; Wijbrandts, C.A.; Mebius, R.E.; Cantaert, T.; Dinant, H.J.; van der Pouw-Kraan, T.C.; Verweij, C.L.; Baeten, D.; Tak, P.P. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum. 2008, 58, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Czepielewski, R.S.; Erlich, E.C.; Onufer, E.J.; Young, S.; Saunders, B.T.; Han, Y.H.; Wohltmann, M.; Wang, P.L.; Kim, K.W.; Kumar, S.; et al. Ileitis-associated tertiary lymphoid organs arise at lymphatic valves and impede mesenteric lymph flow in response to tumor necrosis factor. Immunity 2021, 54, 2795–2811.e2799. [Google Scholar] [CrossRef]
- Geng, X.; Chen, L.; Ahmed, Z.; Formigari, G.P.; Ho, Y.C.; Del Gaudio, I.; Datilo, M.N.; Azartash-Namin, Z.J.; Heron, C.; Shan, X.; et al. S1PR1 regulates lymphatic valve development and tertiary lymphoid organ formation in the ileum. J. Exp. Med. 2025, 222, e20241799. [Google Scholar] [CrossRef]
- Keane, K.; Stephens, M.; Roizes, S.; Xue, J.; Liao, S.; von der Weid, P.Y. The spatiotemporal development of mesenteric lymphatic changes in the TNF(DeltaARE/+) mouse model of terminal ileitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2025, 328, G624–G643. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Bilgin, E.; Venerito, V.; Bogdanos, D.P. Glucagon-Like Peptide-1 (GLP-1) receptor agonists in rheumatology: A review of current evidence and future directions. Autoimmun. Rev. 2025, 24, 103864. [Google Scholar] [CrossRef]
- Chen, J.; Xie, J.J.; Shi, K.S.; Gu, Y.T.; Wu, C.C.; Xuan, J.; Ren, Y.; Chen, L.; Wu, Y.S.; Zhang, X.L.; et al. Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis. 2018, 9, 212. [Google Scholar] [CrossRef]
- Que, Q.; Guo, X.; Zhan, L.; Chen, S.; Zhang, Z.; Ni, X.; Ye, B.; Wan, S. The GLP-1 agonist, liraglutide, ameliorates inflammation through the activation of the PKA/CREB pathway in a rat model of knee osteoarthritis. J. Inflamm. 2019, 16, 13. [Google Scholar] [CrossRef]
- Brown, S.; Tadros, A.B.; Montagna, G.; Bell, T.; Crowley, F.; Gallagher, E.J.; Dayan, J.H. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) may reduce the risk of developing cancer-related lymphedema following axillary lymph node dissection (ALND). Front. Pharmacol. 2024, 15, 1457363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
den Braanker, H.; van der Valk, E.S.; Bisoendial, R.J. A Lymphatic Perspective on Obesity and Inflammatory Arthritis: New Disease-Modifying Potential in Rheumatology. J. Clin. Med. 2025, 14, 7641. https://doi.org/10.3390/jcm14217641
den Braanker H, van der Valk ES, Bisoendial RJ. A Lymphatic Perspective on Obesity and Inflammatory Arthritis: New Disease-Modifying Potential in Rheumatology. Journal of Clinical Medicine. 2025; 14(21):7641. https://doi.org/10.3390/jcm14217641
Chicago/Turabian Styleden Braanker, Hannah, Eline S. van der Valk, and Radjesh J. Bisoendial. 2025. "A Lymphatic Perspective on Obesity and Inflammatory Arthritis: New Disease-Modifying Potential in Rheumatology" Journal of Clinical Medicine 14, no. 21: 7641. https://doi.org/10.3390/jcm14217641
APA Styleden Braanker, H., van der Valk, E. S., & Bisoendial, R. J. (2025). A Lymphatic Perspective on Obesity and Inflammatory Arthritis: New Disease-Modifying Potential in Rheumatology. Journal of Clinical Medicine, 14(21), 7641. https://doi.org/10.3390/jcm14217641






