Prognostic Impact of Combinational Elastography in Patients with Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Protocol
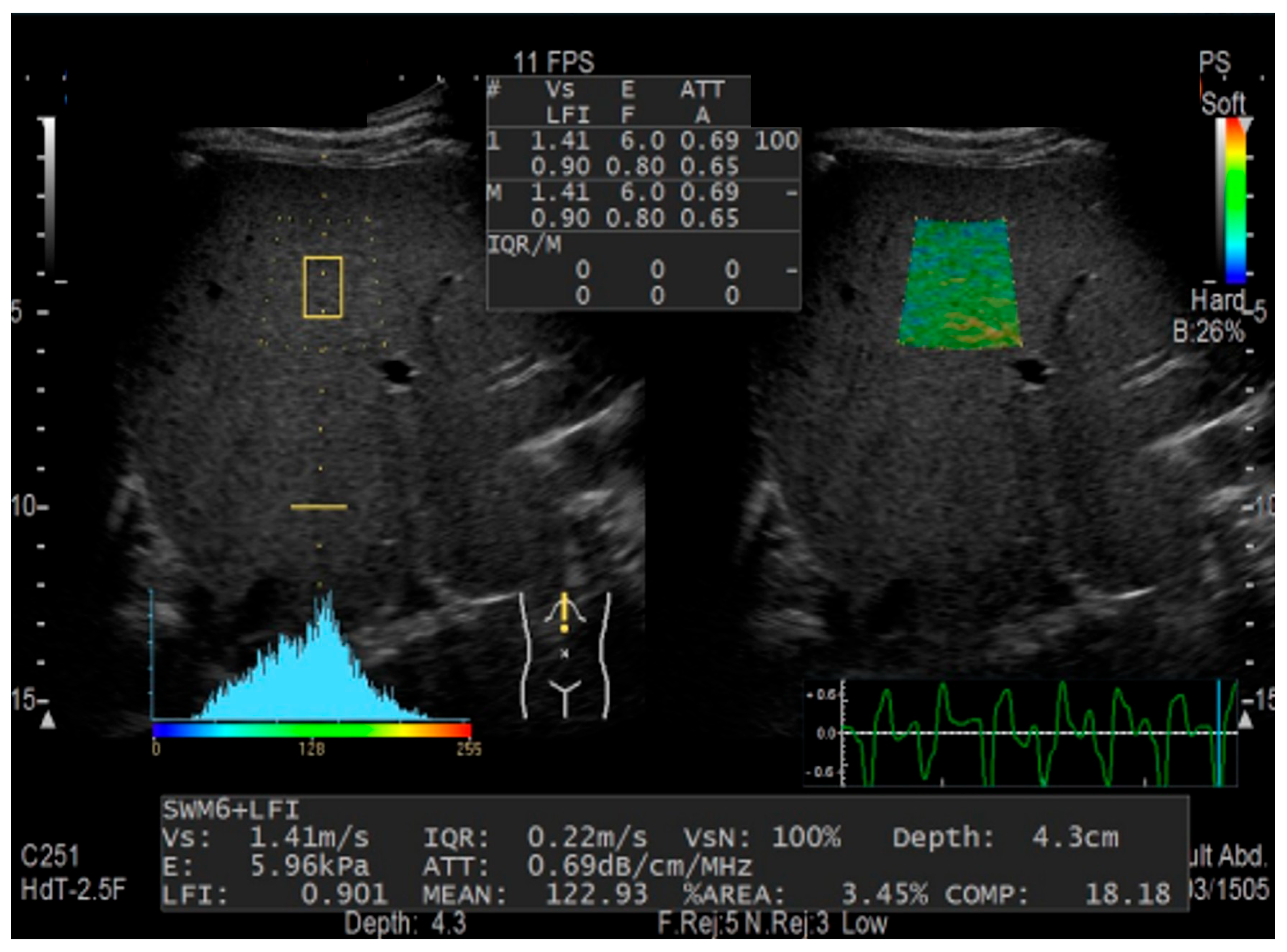
2.2. Combinational Elastography
2.3. Laboratory Tests, Echocardiography, and Composite Congestion Score
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
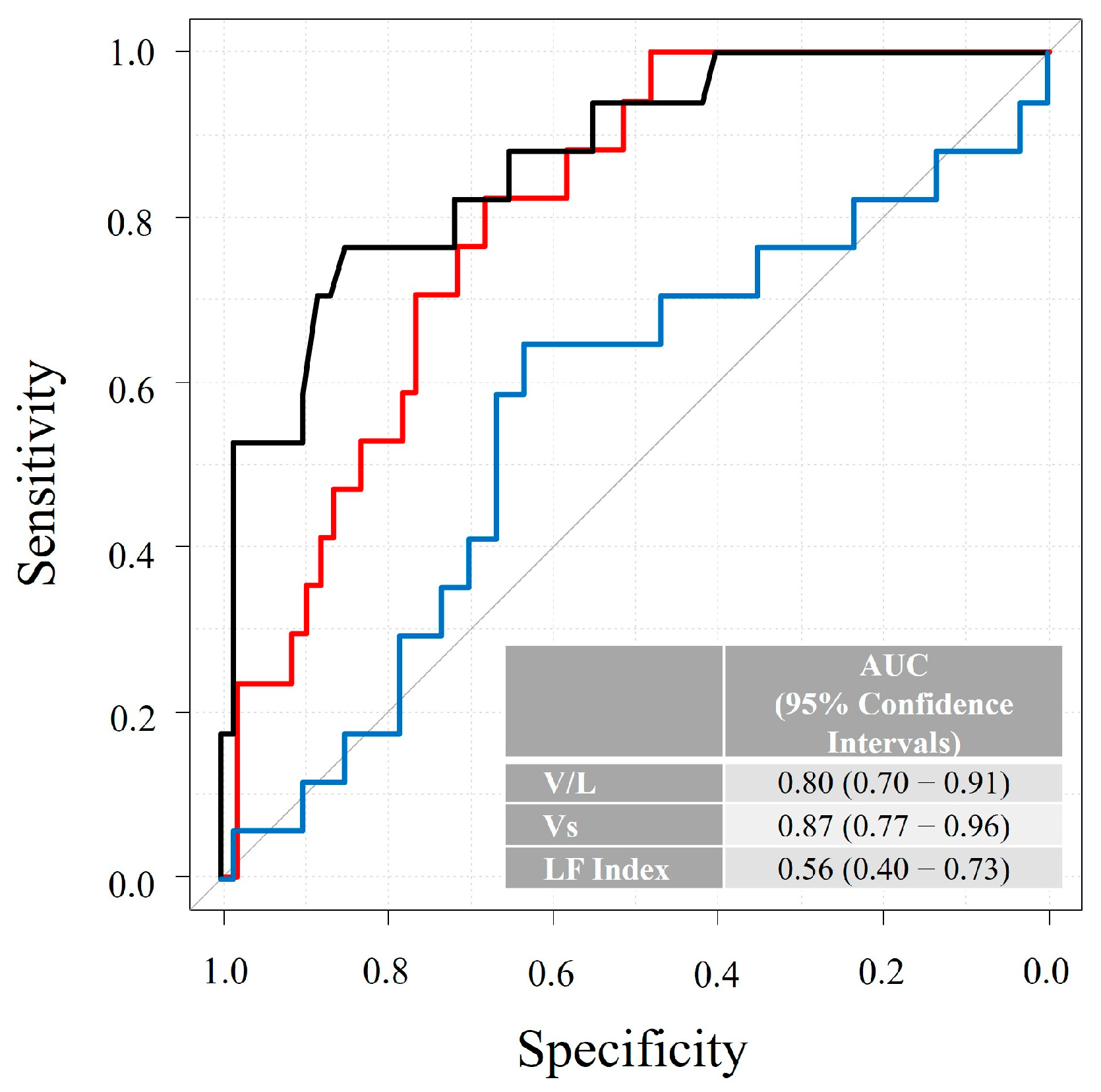
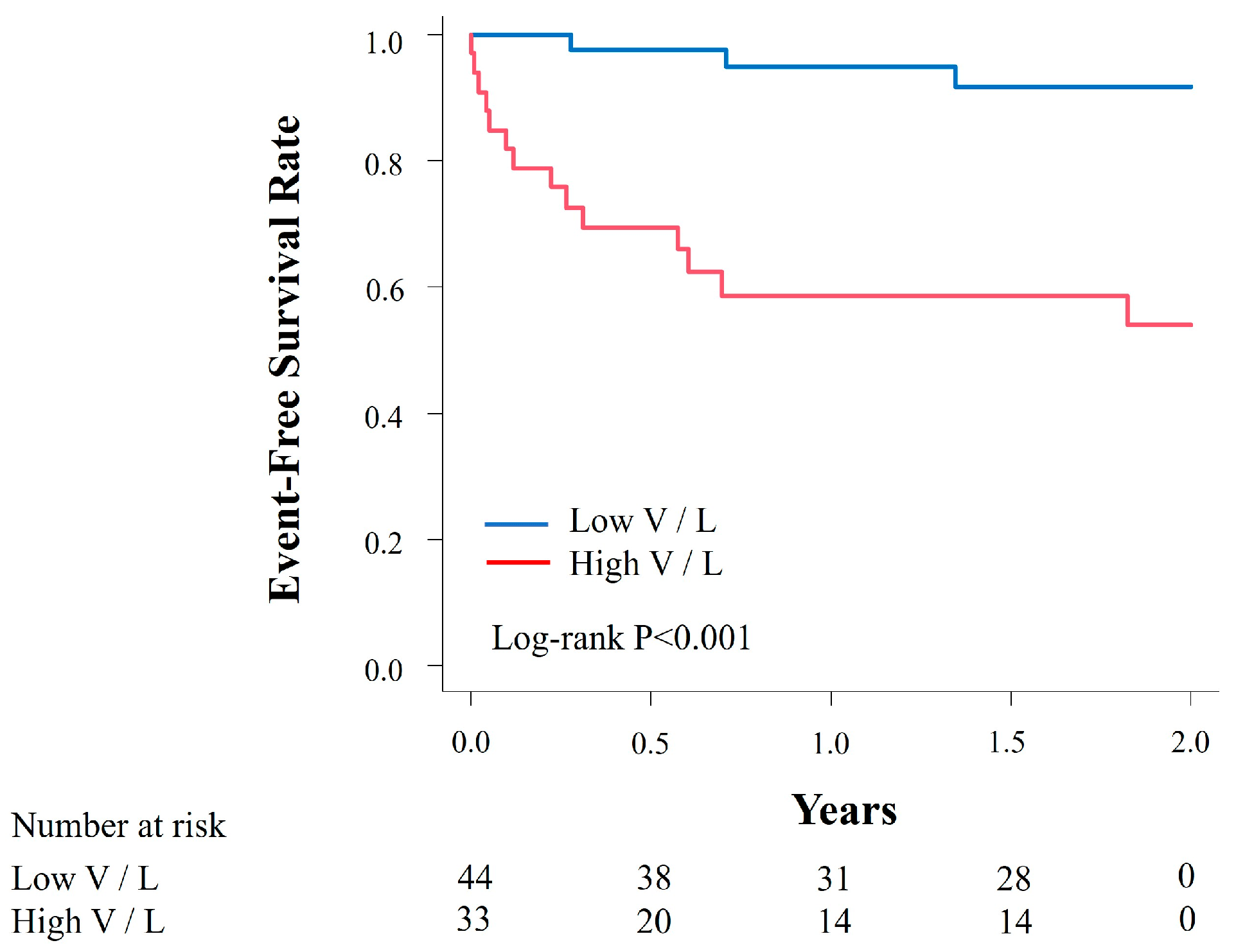
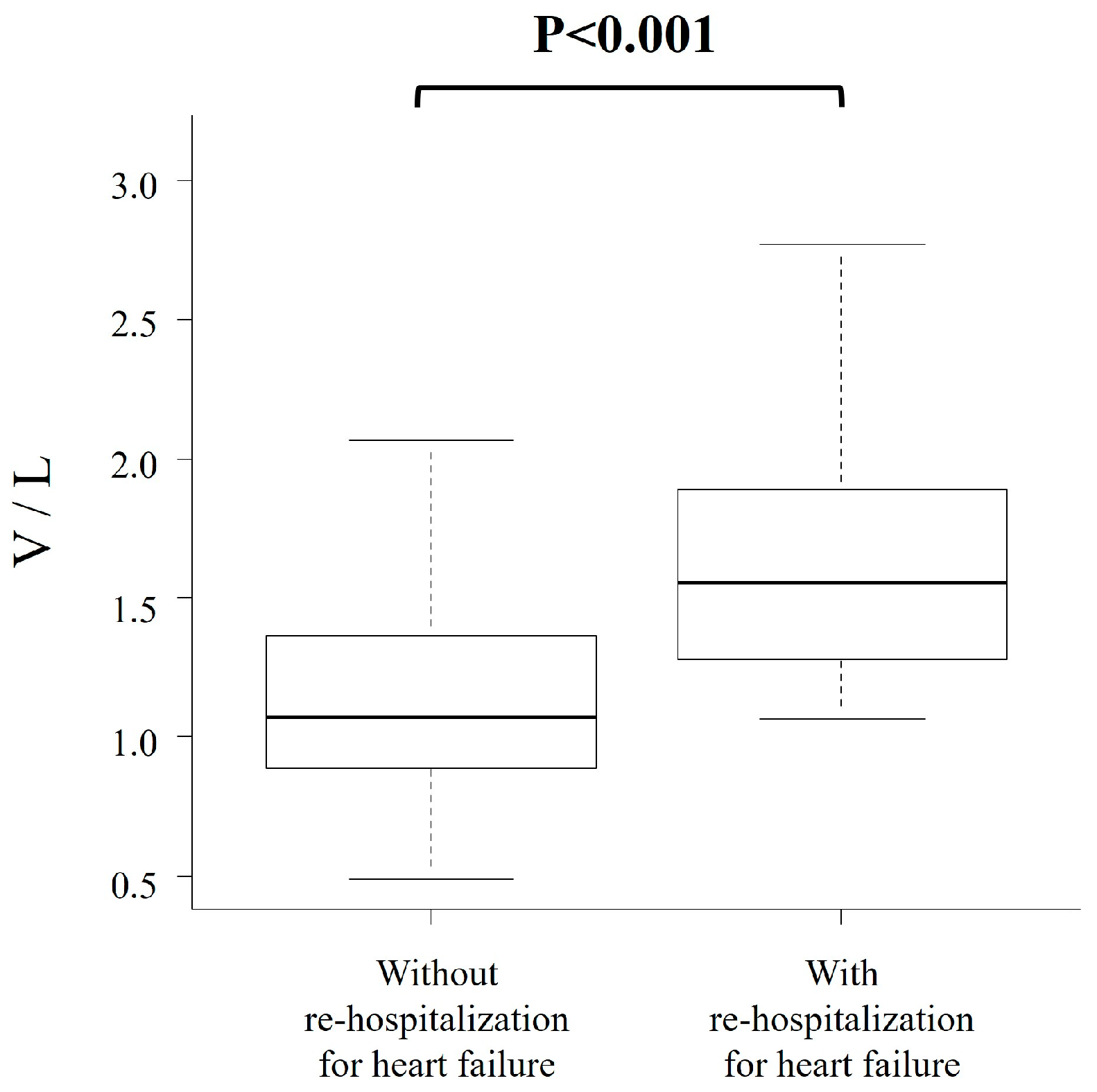
3.2. Combinational Elastography and Clinical Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| CCS | composite congestion score |
| HF | heart failure |
| IVC | inferior vena cava |
| LF index | liver fibrosis index |
| ROC | receiver operating characteristic |
| Vs | Vs value |
| V/L | Vs value/liver fibrosis index |
References
- Laribi, S.; Mebazaa, A. Cardiohepatic syndrome: Liver injury in decompensated heart failure. Curr. Heart Fail. Rep. 2014, 11, 236–240. [Google Scholar] [CrossRef]
- Poelzl, G.; Auer, J. Cardiohepatic syndrome. Curr. Heart Fail. Rep. 2015, 12, 68–78. [Google Scholar] [CrossRef]
- Miller, W.L. Congestion/decongestion in heart failure: What does it mean, how do we assess it, and what are we missing?—Is there utility in measuring volume? Heart Fail. Rev. 2024, 29, 1187–1199. [Google Scholar] [CrossRef]
- Yada, N.; Kudo, M.; Morikawa, H.; Fujimoto, K.; Kato, M.; Kawada, N. Assessment of liver fibrosis with real-time tissue elastography in chronic viral hepatitis. Oncology 2013, 84, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: Liver. Ultrasound Med. Biol. 2015, 41, 1161–1179. [Google Scholar] [CrossRef]
- Yada, N.; Sakurai, T.; Minami, T.; Arizumi, T.; Takita, M.; Hagiwara, S.; Ida, H.; Ueshima, K.; Nishida, N.; Kudo, M. Influence of liver inflammation on liver stiffness measurement in patients with autoimmune hepatitis evaluation by combinational elastography. Oncology 2017, 92, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Ito, S.; Sato, S.; Tobita, H.; Endo, A.; Yoshitomi, H.; Tanabe, K. Evaluation of hepatic congestion in patients with heart failure using shear wave and strain imaging. J. Echocardiogr. 2020, 18, 260–261. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ito, S.; Endo, A.; Yoshitomi, H.; Tanabe, K. Combinational elastography. Int. Heart J. 2022, 63, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Sakamoto, T.; Endo, A.; Yoshitomi, H.; Tanabe, K. Combinational elastography prior to pericardiectomy to assess liver condition in patients with constrictive pericarditis. J. Cardiol. Cases 2024, 29, 254–257. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the heart failure society of America, heart failure association of the European Society of Cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kato, M.; Tonomura, A.; Yada, N.; Tatsumi, C.; Oshita, M.; Wada, S.; Ueshima, K.; Ishida, T.; Furuta, T.; et al. Non-invasive evaluation method of the liver fibrosis using real-time tissue elastography—Usefulness of judgement liver fibrosis stage by liver fibrosis index (LF Index). Kanzo 2010, 51, 539–541. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kato, M.; Kudo, M.; Yada, N.; Shiina, T.; Ueshima, K.; Yamada, Y.; Ishida, T.; Azuma, M.; Yamasaki, M.; et al. Novel image analysis method using ultrasound elastography for noninvasive evaluation of hepatic fibrosis in patients with chronic hepatitis C. Oncology 2013, 84, 3–12. [Google Scholar] [CrossRef]
- Yada, N.; Sakurai, T.; Minami, T.; Arizumi, T.; Takita, M.; Hagiwara, S.; Ueshima, K.; Ida, H.; Nishida, N.; Kudo, M. A newly developed shear wave elastography modality: With a unique reliability index. Oncology 2015, 89, 53–59. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the Everest trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Taniguchi, T.; Sakata, Y.; Ohtani, T.; Mizote, I.; Takeda, Y.; Asano, Y.; Masuda, M.; Minamiguchi, H.; Kanzaki, M.; Ichibori, Y.; et al. Usefulness of transient elastography for noninvasive and reliable estimation of right-sided filling pressure in heart failure. Am. J. Cardiol. 2014, 113, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Yoshitani, T.; Asakawa, N.; Sakakibara, M.; Noguchi, K.; Tokuda, Y.; Kamiya, K.; Iwano, H.; Yamada, S.; Kudou, Y.; Nishida, M.; et al. Value of virtual touch quantification elastography for assessing liver congestion in patients with heart failure. Circ. J. 2016, 80, 1187–1195. [Google Scholar] [CrossRef]
- Saito, Y.; Kato, M.; Nagashima, K.; Monno, K.; Aizawa, Y.; Okumura, Y.; Matsumoto, N.; Moriyama, M.; Hirayama, A. Prognostic relevance of liver stiffness assessed by transient elastography in patients with acute decompensated heart failure. Circ. J. 2018, 82, 1822–1829. [Google Scholar] [CrossRef]
- Sakamoto, T.; Tanabe, K. Assessment of organ congestion in patients with heart failure by ultrasonography. J. Echocardiogr. 2022, 20, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Seo, Y.; Sai, S.; Machino-Ohtsuka, T.; Yamamoto, M.; Ishizu, T.; Kawakami, Y.; Aonuma, K. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016, 4, 674–682. [Google Scholar] [CrossRef]
- Sakamoto, T.; Asanuma, T.; Sasaki, H.; Kawahara, H.; Uchida, K.; Endo, A.; Yoshitomi, H.; Tanabe, K. Diagnostic value of lung ultrasound B-lines for evaluating left ventricular filling pressure. Cardiovasc. Ultrasound 2025, 23, 6. [Google Scholar] [CrossRef]
- Sakamoto, T.; Uchida, K.; Endo, A.; Yoshitomi, H.; Tanabe, K. Gallbladder wall thickness-based assessment of organ congestion in patients with heart failure. Circ. Rep. 2022, 4, 166–172. [Google Scholar] [CrossRef]
- Saito, Y.; Matsumoto, N.; Aizawa, Y.; Fukamachi, D.; Kitano, D.; Kazuto, T.; Tamaki, T.; Fujito, H.; Sezai, A.; Okumura, Y. Clinical significance of spleen stiffness in patients with acute decompensated heart failure. ESC Heart Fail. 2020, 7, 4005–4014. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ishii, S.; Fujita, T.; Iida, Y.; Kaida, T.; Nabeta, T.; Maekawa, E.; Yanagisawa, T.; Koitabashi, T.; Takeuchi, I.; et al. Prognostic impact of intestinal wall thickening in hospitalized patients with heart failure. Int. J. Cardiol. 2017, 230, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, V.; Del Punta, L.; De Biase, N.; Pellicori, P.; Gargani, L.; Dini, F.L.; Armenia, S.; Li Vigni, M.; Maremmani, D.; Masi, S.; et al. Integrative assessment of congestion in heart failure using ultrasound imaging. Intern. Emerg. Med. 2025, 20, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.; Barghash, M.H.; Giustino, G.; Alvarez-Garcia, J.; Konje, S.; Parikh, A.; Ullman, J.; Keith, B.; Donehey, J.; Mitter, S.S.; et al. Early use of remote dielectric sensing after hospitalization to reduce heart failure readmissions. ESC Heart Fail. 2021, 8, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [CrossRef]
- Iijima, H.; Tada, T.; Kumada, T.; Kobayashi, N.; Yoshida, M.; Aoki, T.; Nishimura, T.; Nakano, C.; Ishii, A.; Takashima, T.; et al. Comparison of liver stiffness assessment by transient elastography and shear wave elastography using six ultrasound devices. Hepatol. Res. 2019, 49, 676–686. [Google Scholar] [CrossRef]
| n = 77 | |
|---|---|
| Age, years | 79 (73–86) |
| Male, n (%) | 41 (52) |
| Body mass index, kg/m2 | 21 (19–24) |
| Systolic blood pressure, mmHg | 113 (99–131) |
| Heart rate, bpm | 70 (64–82) |
| New York Heart Association class I/II/III/IV | 50/22/3/2 |
| Medical history, n (%) | |
| Hypertension | 63 (82) |
| Dyslipidemia | 34 (44) |
| Diabetes mellitus | 16 (21) |
| Atrial fibrillation | 31 (40) |
| Chronic kidney disease | 42 (55) |
| HF etiology, n (%) | |
| Cardiomyopathy | 27 (35) |
| Valvular heart disease | 15 (19) |
| Ischemia | 13 (17) |
| Others | 22 (29) |
| Medications | |
| ACEI or ARB, n (%) | 45 (58) |
| β-blocker, n (%) | 41 (53) |
| Mineral corticoid-receptor antagonists, n (%) | 31 (40) |
| Loop diuretics, n (%) | 54 (70) |
| n = 77 | |
|---|---|
| Laboratory data | |
| Hemoglobin, g/dL | 12 (10–13) |
| Platelet, ×103/μL | 20 (16–24) |
| Aspartate aminotransferase, U/L | 23 (19–31) |
| Alanine aminotransferase, U/L | 20 (13–29) |
| Gamma-glutamyl transpeptidase, U/L | 33 (19–58) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 45 (31–68) |
| Brain natriuretic peptide, pg/mL | 308 (108–543) |
| Echocardiography | |
| Left ventricular end-diastolic volume, mL | 75 (59–117) |
| Left ventricular end-systolic volume, mL | 36 (23–68) |
| Left ventricular ejection fraction, % | 49 (36–64) |
| Left atrial volume index, mL/m2 | 46 (34–61) |
| E/A | 0.8 (0.6–1.6) |
| E/e’ | 13 (10–17) |
| Left ventricular outflow tract velocity-time integral, cm | 16 (12–20) |
| Tricuspid regurgitation peak gradient, mmHg | 26 (20–35) |
| Maximal inferior vena cava diameter, mm | 14 (11–19) |
| Composite congestion score | 1 (0–2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sakamoto, T.; Yamasaki, S.; Okada, T.; Endo, A.; Yoshitomi, H.; Sato, S.; Tanabe, K. Prognostic Impact of Combinational Elastography in Patients with Heart Failure. J. Clin. Med. 2026, 15, 478. https://doi.org/10.3390/jcm15020478
Sakamoto T, Yamasaki S, Okada T, Endo A, Yoshitomi H, Sato S, Tanabe K. Prognostic Impact of Combinational Elastography in Patients with Heart Failure. Journal of Clinical Medicine. 2026; 15(2):478. https://doi.org/10.3390/jcm15020478
Chicago/Turabian StyleSakamoto, Takahiro, Seita Yamasaki, Taiji Okada, Akihiro Endo, Hiroyuki Yoshitomi, Shuichi Sato, and Kazuaki Tanabe. 2026. "Prognostic Impact of Combinational Elastography in Patients with Heart Failure" Journal of Clinical Medicine 15, no. 2: 478. https://doi.org/10.3390/jcm15020478
APA StyleSakamoto, T., Yamasaki, S., Okada, T., Endo, A., Yoshitomi, H., Sato, S., & Tanabe, K. (2026). Prognostic Impact of Combinational Elastography in Patients with Heart Failure. Journal of Clinical Medicine, 15(2), 478. https://doi.org/10.3390/jcm15020478





