Minimally Invasive Cardiac Surgery: A State-of-the-Art Review
Abstract
1. Introduction
2. Methodology
3. Minimally Invasive Cardiac Surgery: Current Status
4. Origin and Evolution of MICS
4.1. Mini-Sternotomy
4.2. Right Mini-Thoracotomy
4.3. Left Mini-Thoracotomy: TA TAVI and MIDCAB
4.4. Totally Endoscopic Technique
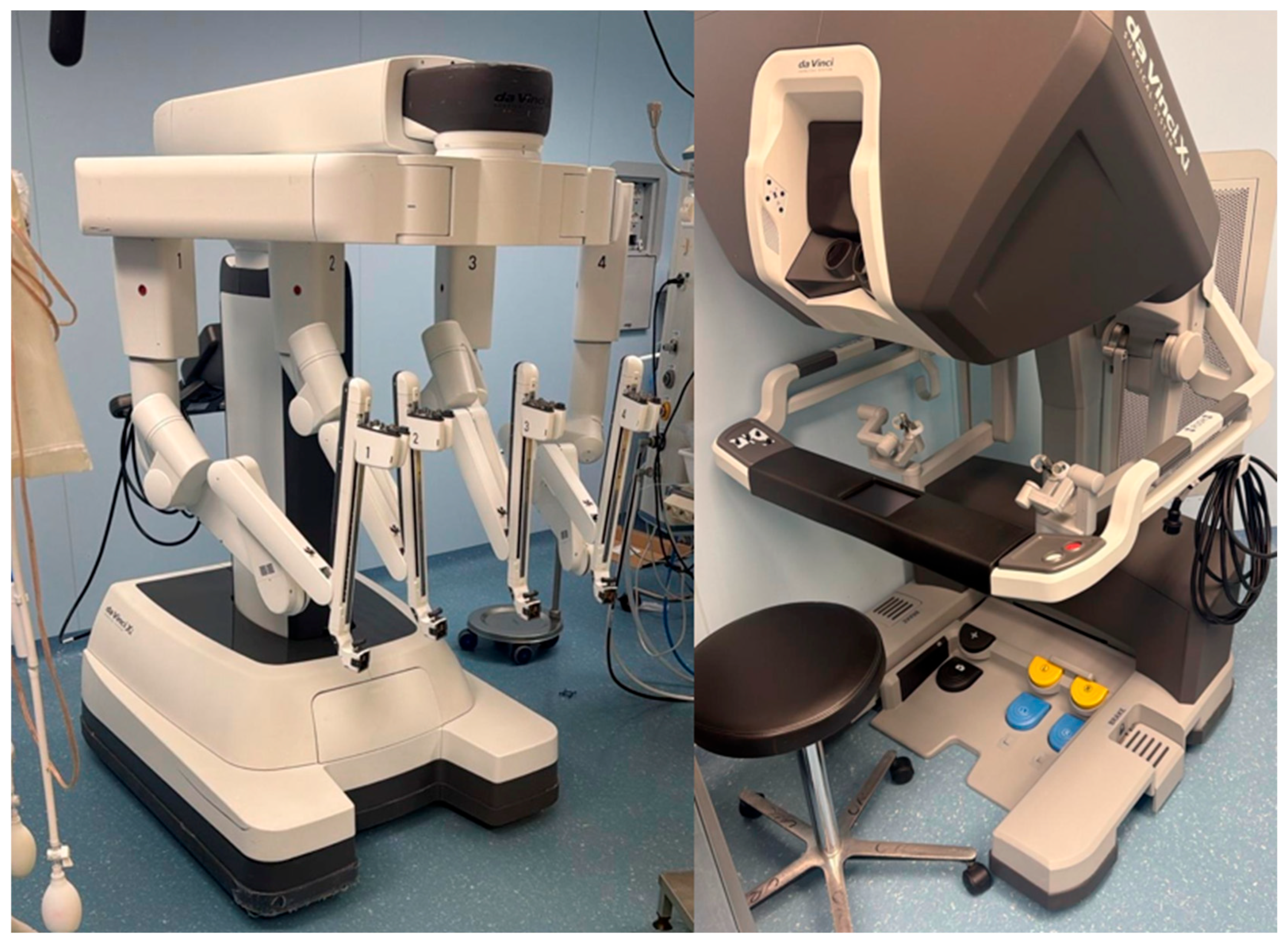
4.5. Robotic Technique
5. Discussion and Future Directions
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAR | Ascending Aorta Replacement |
| AVR | Aortic Valve Replacement |
| CABG | Coronary Artery Bypass Graft |
| CPB | Cardio-Pulmonary Bypass |
| ICU | Intensive Care Unit |
| MICS | Minimally Invasive Cardiac Surgery |
| MIDCAB | Minimally Invasive Direct Coronary Artery Bypass |
| MS | Mini-Sternotomy |
| MV | Mitral Valve |
| MVr | Mitral Valve Repair |
| PCI | Percutaneous Coronary Intervention |
| QoL | Quality of Life |
| RMT | Right Mini-Thoracotomy |
| SotA | State-of-the-Art |
| TA TAVI | Trans-Apical Trans-Catheter Aortic Valve Implantation |
| TE | Totally Endoscopic |
| TECAB | (robotic) Totally Endoscopic Coronary Artery Bypass |
| TF | Trans-Femoral (TAVI) |
| TV | Tricuspid Valve |
| RT | Robotic |
References
- Schmitto, J.D.; Mokashi, S.A.; Cohn, L.H. Minimally-invasive valve surgery. J. Am. Coll. Cardiol. 2010, 56, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, W.R., Jr. Historical evolution of robot-assisted cardiac surgery: A 25-year journey. Ann. Cardiothorac. Surg. 2022, 11, 564–582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holubec, T.; Dahle, G.; Bonaros, N. Editorial: Minimally invasive cardiac surgery: State of the art and current challenges. Front. Cardiovasc. Med. 2023, 10, 1286868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillinov, A.M.; Mihaljevic, T.; Javadikasgari, H.; Suri, R.M.; Mick, S.L.; Navia, J.L.; Desai, M.Y.; Bonatti, J.; Khosravi, M.; Idrees, J.J.; et al. Early results of robotically assisted mitral valve surgery: Analysis of the first 1000 cases. J. Thorac. Cardiovasc. Surg. 2018, 155, 82–91.e2. [Google Scholar] [CrossRef] [PubMed]
- Arghami, A.; Jahanian, S.; Daly, R.C.; Hemmati, P.; Lahr, B.D.; Rowse, P.G.; Crestanello, J.A.; Dearani, J.A. Robotic Mitral Valve Repair: A Decade of Experience With Echocardiographic Follow-up. Ann. Thorac. Surg. 2022, 114, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.S.; Merkebu, J.; Varpio, L. State-of-the-art literature review methodology: A six-step approach for knowledge synthesis. Perspect. Med. Educ. 2022, 11, 281–288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barry, E.S.; Merkebu, J.; Varpio, L. How to Conduct a State-of-the-Art Literature Review. J. Grad. Med. Educ. 2022, 14, 663–665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barry, E.S.; Merkebu, J.; Varpio, L. Understanding State-of-the-Art Literature Reviews. J. Grad. Med. Educ. 2022, 14, 659–662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Huang, L.C.; Chen, D.Z.; Chen, L.W.; Zheng, Z.H.; Dai, X.F. Totally endoscopic mitral valve surgery: Early experience in 188 patients. J. Cardiothorac. Surg. 2021, 16, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yilmaz, A.; Van Genechten, S.; Claessens, J.; Packlé, L.; Maessen, J.; Kaya, A. A totally endoscopic approach for aortic valve surgery. Eur. J. Cardiothorac. Surg. 2022, 62, ezac467. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Dearani, J.A.; Mihaljevic, T.; Chitwood, W.R., Jr.; Murphy, D.A.; Trento, A.; Javadikasgari, H.; Burkhart, H.M.; Nifong, W.L.; Daly, R.C.; et al. Mitral valve repair using robotic technology: Safe, effective, and durable. J. Thorac. Cardiovasc. Surg. 2016, 151, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Dumani, S.; Likaj, E.; Dibra, L.; Kuci, S.; Rruci, E.; Ibrahimi, A.; Zaimi Petrela, E.; Mehmeti, A.; Beca, V.; Pellumbi, D.; et al. Ministernotomy Versus Standard Sternotomy for Aortic Valve Replacement. Cureus 2024, 16, e76652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Telyuk, P.; Hancock, H.; Maier, R.; Batty, J.A.; Goodwin, A.; Owens, W.A.; Ogundimu, E.; Akowuah, E. Long-term outcomes of mini-sternotomy versus conventional sternotomy for aortic valve replacement: A randomized controlled trial. Eur. J. Cardiothorac. Surg. 2022, 63, ezac540. [Google Scholar] [CrossRef] [PubMed]
- Bonacchi, M.; Dokollari, A.; Parise, O.; Sani, G.; Prifti, E.; Bisleri, G.; Gelsomino, S. Ministernotomy compared with right anterior minithoracotomy for aortic valve surgery. J. Thorac. Cardiovasc. Surg. 2023, 165, 1022–1032.e2. [Google Scholar] [CrossRef] [PubMed]
- Kulacoglu, U.K.; Kaya, M. Ministernotomy in Aortic Root and Arch Surgery: Early Outcomes. Braz. J. Cardiovasc. Surg. 2023, 38, 15–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reser, D.; Holubec, T.; Yilmaz, M.; Guidotti, A.; Maisano, F. Right lateral mini-thoracotomy for mitral valve surgery. Multimed. Man. Cardiothorac. Surg. 2015, 2015, mmv031. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, J.; Alnajar, A. Comparing Outcomes of Sternal Sparing Aortic Valve Replacement With and Without Concomitant Ascending Aortic Replacement. Ann. Thorac. Surg. 2025, 121, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Alnajar, A.; Chatterjee, S.; Olive, J.K.; Kaymakci, M.S.; Gray, L.; Gray, Z.; Breda, J.R.; Lamelas, J. Outcomes of minimally invasive isolated tricuspid valve repair and replacement through right mini-thoracotomy. JTCVS Open 2023, 17, 98–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, R.; Xie, M.; Yim, W.Y.; Wu, W.; Jiang, W.; Wang, Y.; Hu, X. Dose approach matter? A meta-analysis of outcomes following transfemoral versus transapical transcatheter aortic valve replacement. BMC Cardiovasc. Disord. 2021, 21, 358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maeda, K.; Shimamura, K.; Mizote, I.; Nakamura, D.; Yamashita, K.; Kawamura, A.; Yoshioka, D.; Sakata, Y.; Miyagawa, S. Long-term outcomes of transapical-transcatheter aortic valve replacement. Gen. Thorac. Cardiovasc. Surg. 2025, 73, 472–478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.; Li, Y.; Bao, W.; Qiu, S. MIDCAB versus off-pump CABG: Comparative study. Hell. J. Cardiol. 2020, 61, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Manuel, L.; Fong, L.S.; Betts, K.; Bassin, L.; Wolfenden, H. LIMA to LAD grafting returns patient survival to age-matched population: 20-year outcomes of MIDCAB surgery. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Praetere, H.; Verbrugghe, P.; Rega, F.; Meuris, B.; Herijgers, P. Starting minimally invasive valve surgery using endoclamp technology: Safety and results of a starting surgeon. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Casselman, F.P.; Van Slycke, S.; Dom, H.; Lambrechts, D.L.; Vermeulen, Y.; Vanermen, H. Endoscopic mitral valve repair: Feasible, reproducible, and durable. J. Thorac. Cardiovasc. Surg. 2003, 125, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhou, K.; Wang, Z.; Zang, X.; Guo, H.; Gao, Q.; Teng, Y.; Liu, J.; He, B.; Guo, H.; et al. Totally endoscopic aortic valve replacement: Techniques and early results. Front. Cardiovasc. Med. 2023, 9, 1106845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zoni, D.; Cresce, G.D.; Hinna-Danesi, T.; Benvegnù, L.; Poddi, S.; Gallo, M.; Sella, M.; Salvador, L. Endoscopic aortic valve surgery in isolated and concomitant procedures. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claessens, J.; Packlé, L.; Oosterbos, H.; Smeets, E.; Geens, J.; Gielen, J.; Van Genechten, S.; Heuts, S.; Maessen, J.G.; Yilmaz, A. Totally endoscopic coronary artery bypass grafting: Experience in 1500 patients. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 39, ivae159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvador, L.; Benvegnù, L.; Zoni, D.; Hinna Danesi, T.; Al Jaber, E.; Rasovic, O.; Poddi, S.; Cresce, G.D. Pushing the Boundaries in Totally Endoscopic Cardiac Surgery: Combined Triple Valve Surgery and Ascending Aorta Replacement. Innovations 2024, 19, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Roach, A.; Trento, A.; Emerson, D.; Gill, G.; Rowe, G.; Peiris, A.; Hussaini, A.; Cheng, W.; Ramzy, D.; Egorova, N.; et al. Durable Robotic Mitral Repair of Degenerative Primary Regurgitation With Long-Term Follow-Up. Ann. Thorac. Surg. 2022, 114, 84–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waldron, C.; Mori, M.; LaLonde, M.; Geirsson, A. Concomitant Procedures in Robotic Mitral Valve Surgery. Semin. Thorac. Cardiovasc. Surg. 2025, 37, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Wei, L.M.; Cook, C.C.; Hayanga, J.W.A.; Daggubati, R.; Sengupta, P.P.; Rankin, J.S. Robotic aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2021, 161, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Pereda, D.; Khaliel, F.H.; Poffo, R.; Darehzereshki, A.; Mehaffey, J.H.; Yan, T.D.; Melnitchouk, S.; Geirsson, A.; Arghami, A.; et al. Outcomes following initial multicenter experience with robotic aortic valve replacement: Defining a path forward. J. Thorac. Cardiovasc. Surg. 2024, 167, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Raikar, G.V.; Darehzereshki, A.; Mehaffey, J.H.; Daggubati, R.; Wei, L.M. Robotic-Assisted Aortic Valve Replacement and Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2025, 119, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Nisivaco, S.; Kitahara, H.; Bhasin, R.; Patel, B.; Coleman, C.; Balkhy, H.H. A decade of robotic beating-heart totally endoscopic coronary bypass (TECAB) at a single institution: Outcomes with 10-year follow-up. J. Thorac. Cardiovasc. Surg. 2025, 169, 1753–1760.e3. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.H.; Chen, J.H.; Dai, X.F. Learning Curve from 100 Cases of Totally Thoracoscopic Mitral Valve Replacement. Heart Surg. Forum 2021, 24, E882–E886. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.J.P.; Wierup, P.; Gillinov, M. Minimally Invasive Mitral Surgery: Patient Selection and Technique. Cardiol. Clin. 2021, 39, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Berretta, P.; Kempfert, J.; Van Praet, F.; Salvador, L.; Lamelas, J.; Nguyen, T.C.; Wilbring, M.; Gerdisch, M.; Rinaldi, M.; Bonaros, N.; et al. Risk-related clinical outcomes after minimally invasive mitral valve surgery: Insights from the Mini-Mitral International Registry. Eur. J. Cardiothorac. Surg. 2023, 63, ezad090. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Hadjinikolaou, L.; Sogliani, F.; Stanbridge, R.D. Mini-sternotomy for coronary artery bypass grafting. Ann. Thorac. Surg. 1996, 62, 1884–1885. [Google Scholar] [PubMed]
- Arom, K.V.; Emery, R.W.; Nicoloff, D.M. Mini-sternotomy for coronary artery bypass grafting. Ann. Thorac. Surg. 1996, 61, 1271–1272. [Google Scholar] [CrossRef] [PubMed]
- Szerafin, T.; Jagamos, E.; Jaber, O.; Horváth, A.; Horváth, G.; Olin, C.; Péterffy, A. Mini-sternotomy for aortic valve surgery. Acta Chir. Hung. 1997, 36, 352–355. [Google Scholar] [PubMed]
- Byrne, J.G.; Adams, D.H.; Couper, G.S.; Rizzo, R.J.; Cohn, L.H.; Aranki, S.F. Minimally-invasive aortic root replacement. Heart Surg. Forum 1999, 2, 326–329. [Google Scholar] [PubMed]
- Masiello, P.; Coscioni, E.; Panza, A.; Triumbari, F.; Preziosi, G.; Di Benedetto, G. Surgical results of aortic valve replacement via partial upper sternotomy: Comparison with median sternotomy. Cardiovasc. Surg. 2002, 10, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, E.; Prayaga, S.; Kinsella, J.; Sutherland, F.W. Mini-sternotomy for aortic valve replacement reduces the length of stay in the cardiac intensive care unit: Meta-analysis of randomised controlled trials. BMJ Open 2011, 1, e000266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furukawa, N.; Kuss, O.; Aboud, A.; Schönbrodt, M.; Renner, A.; Hakim Meibodi, K.; Becker, T.; Zittermann, A.; Gummert, J.F.; Börgermann, J. Ministernotomy versus conventional sternotomy for aortic valve replacement: Matched propensity score analysis of 808 patients. Eur. J. Cardiothorac. Surg. 2014, 46, 221–226; discussion 226–227. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Loulmet, D.; Carpentier, A.; Le Bret, E.; Haugades, B.; Dassier, P.; Guibourt, P. Chirurgie à coeur ouvert par vidéo-chirurgie et mini-thoracotomie. Premier cas (valvuloplastie mitrale) opéré avec succès [Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success]. C R Acad. Sci. III 1996, 319, 219–223. (In French) [Google Scholar] [PubMed]
- Chitwood, W.R., Jr.; Wixon, C.L.; Elbeery, J.R.; Moran, J.F.; Chapman, W.H.; Lust, R.M. Video-assisted minimally invasive mitral valve surgery. J. Thorac. Cardiovasc. Surg. 1997, 114, 773–780; discussion 780–782. [Google Scholar] [CrossRef] [PubMed]
- Karimov, J.H.; Bevilacqua, S.; Solinas, M.; Glauber, M. Triple heart valve surgery through a right antero-lateral minithoracotomy. Interact. Cardiovasc. Thorac. Surg. 2009, 9, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Glauber, M.; Miceli, A.; Gilmanov, D.; Ferrarini, M.; Bevilacqua, S.; Farneti, P.A.; Solinas, M. Right anterior minithoracotomy versus conventional aortic valve replacement: A propensity score matched study. J. Thorac. Cardiovasc. Surg. 2013, 145, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.F.; Grossi, E.A.; Galloway, A.C. Minimally invasive mitral surgery through right mini-thoracotomy under direct vision. J. Thorac. Dis. 2013, 5, S673–S679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sündermann, S.H.; Sromicki, J.; Rodriguez Cetina Biefer, H.; Seifert, B.; Holubec, T.; Falk, V.; Jacobs, S. Mitral valve surgery: Right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1989–1995.e4. [Google Scholar] [CrossRef] [PubMed]
- Mihos, C.G.; Santana, O.; Pineda, A.M.; Lamas, G.A.; Lamelas, J. Right anterior minithoracotomy versus median sternotomy surgery for native mitral valve infective endocarditis. J. Heart Valve Dis. 2014, 23, 343–349. [Google Scholar] [PubMed]
- Mihos, C.G.; Santana, O.; Pineda, A.M.; La Pietra, A.; Lamelas, J. Aortic valve replacement and concomitant right coronary artery bypass grafting performed via a right minithoracotomy approach. Innovations 2014, 9, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.O.; Saint, L.L.; Leidenfrost, J.E.; Damiano, R.J., Jr. Illustrated techniques for performing the Cox-Maze IV procedure through a right mini-thoracotomy. Ann. Cardiothorac. Surg. 2014, 3, 105–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lichtenstein, S.V.; Cheung, A.; Ye, J.; Thompson, C.R.; Carere, R.G.; Pasupati, S.; Webb, J.G. Transapical transcatheter aortic valve implantation in humans: Initial clinical experience. Circulation 2006, 114, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.J.; Ballester, C. Use of thoracoscopy and a minimal thoracotomy, in mammary-coronary bypass to left anterior descending artery, without extracorporeal circulation. Experience in 2 cases. J. Cardiovasc. Surg. 1995, 36, 159–161. [Google Scholar] [PubMed]
- Holubkov, R.; Zenati, M.; Akin, J.J.; Erb, L.; Courcoulas, A. MIDCAB characteristics and results: The CardioThoracic Systems (CTS) registry. Eur. J. Cardiothorac. Surg. 1998, 14, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Kettering, K. Minimally invasive direct coronary artery bypass grafting: A meta-analysis. J. Cardiovasc. Surg. 2008, 49, 793–800. [Google Scholar] [PubMed]
- Karpuzoglu, O.E.; Ozay, B.; Sener, T.; Aydin, N.B.; Ketenci, B.; Aksu, T.; Gercekoglu, H.; Demirtas, M. Comparison of minimally invasive direct coronary artery bypass and off-pump coronary artery bypass in single-vessel disease. Heart Surg. Forum 2009, 12, E39–E43. [Google Scholar] [CrossRef] [PubMed]
- Reser, D.; Hemelrijck, M.v.; Pavicevic, J.; Tolboom, H.; Holubec, T.; Falk, V.; Jacobs, S. Mid-Term Outcomes of Minimally Invasive Direct Coronary Artery Bypass Grafting. Thorac. Cardiovasc. Surg. 2015, 63, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, W.R., Jr.; Elbeery, J.R.; Chapman, W.H.; Moran, J.M.; Lust, R.L.; Wooden, W.A.; Deaton, D.H. Video-assisted minimally invasive mitral valve surgery: The “micro-mitral” operation. J. Thorac. Cardiovasc. Surg. 1997, 113, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Glower, D.D.; Landolfo, K.P.; Clements, F.; Debruijn, N.P.; Stafford-Smith, M.; Smith, P.K.; Duhaylongsod, F. Mitral valve operation via Port Access versus median sternotomy. Eur. J. Cardiothorac. Surg. 1998, 14, S143–S147. [Google Scholar] [CrossRef] [PubMed]
- Mohr, F.W.; Falk, V.; Diegeler, A.; Walther, T.; van Son, J.A.; Autschbach, R. Minimally invasive port-access mitral valve surgery. J. Thorac. Cardiovasc. Surg. 1998, 115, 567–574; discussion 574–576. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Khanna, S.N.; Wasir, H.; Sharma, K.K.; Mehta, Y.; Trehan, N. Port-access approach for cardiac surgical procedures: Our experience in 776 patients. Indian Heart J. 2005, 57, 688–693. [Google Scholar] [PubMed]
- Gersak, B.; Sostaric, M.; Kalisnik, J.M. Endoscopic aortic valve replacement. Heart Surg. Forum 2003, 6, E197–E199. [Google Scholar] [CrossRef] [PubMed]
- Reichenspurner, H.; Detter, C.; Deuse, T.; Boehm, D.H.; Treede, H.; Reichart, B. Video and robotic-assisted minimally invasive mitral valve surgery: A comparison of the Port-Access and transthoracic clamp techniques. Ann. Thorac. Surg. 2005, 79, 485–490; discussion 490–491. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Aybek, T.; Risteski, P.S.; Detho, F.; Rapp, A.; Wimmer-Greinecker, G.; Moritz, A. Minimally invasive port access versus conventional mitral valve surgery: Prospective randomized study. Ann. Thorac. Surg. 2005, 79, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Yozu, R.; Okamoto, K.; Kudo, M.; Nonaka, H.; Adams, D.H. New innovative instruments facilitate both direct-vision and endoscopic-assisted mini-mitral valve surgery. J. Thorac. Cardiovasc. Surg. 2012, 143, S82–S85. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Loulmet, D.; Aupècle, B.; Kieffer, J.P.; Tournay, D.; Guibourt, P.; Fiemeyer, A.; Méléard, D.; Richomme, P.; Cardon, C. Chirurgie à coeur ouvert assistée par ordinateur. Premier cas opéré avec succès [Computer assisted open heart surgery. First case operated on with success]. C R Acad. Sci. III 1998, 321, 437–442. (In French) [Google Scholar] [CrossRef] [PubMed]
- Loulmet, D.; Carpentier, A.; d’Attellis, N.; Berrebi, A.; Cardon, C.; Ponzio, O.; Aupècle, B.; Relland, J.Y. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J. Thorac. Cardiovasc. Surg. 1999, 118, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, R.; Onnasch, J.F.; Falk, V.; Walther, T.; Krüger, M.; Schilling, L.O.; Mohr, F.W. The Leipzig experience with robotic valve surgery. J. Card. Surg. 2000, 15, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.A.; Lapietra, A.; Applebaum, R.M.; Ribakove, G.H.; Galloway, A.C.; Baumann, F.G.; Ursomanno, P.; Steinberg, B.M.; Colvin, S.B. Case report of robotic instrument-enhanced mitral valve surgery. J. Thorac. Cardiovasc. Surg. 2000, 120, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, W.R., Jr.; Nifong, L.W.; Elbeery, J.E.; Chapman, W.H.; Albrecht, R.; Kim, V.; Young, J.A. Robotic mitral valve repair: Trapezoidal resection and prosthetic annuloplasty with the da vinci surgical system. J. Thorac. Cardiovasc. Surg. 2000, 120, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Folliguet, T.A.; Vanhuyse, F.; Magnano, D.; Laborde, F. Robotic aortic valve replacement: Case report. Heart Surg. Forum 2004, 7, E551–E553. [Google Scholar] [CrossRef] [PubMed]
- Nifong, L.W.; Chitwood, W.R.; Pappas, P.S.; Smith, C.R.; Argenziano, M.; Starnes, V.A.; Shah, P.M. Robotic mitral valve surgery: A United States multicenter trial. J. Thorac. Cardiovasc. Surg. 2005, 129, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, T.; Jarrett, C.M.; Gillinov, A.M.; Blackstone, E.H. A novel running annuloplasty suture technique for robotically assisted mitral valve repair. J. Thorac. Cardiovasc. Surg. 2010, 139, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Burkhart, H.M.; Daly, R.C.; Dearani, J.A.; Park, S.J.; Sundt, T.M., 3rd; Li, Z.; Enriquez-Sarano, M.; Schaff, H.V. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: Establishing the benchmark against which percutaneous interventions should be judged. J. Thorac. Cardiovasc. Surg. 2011, 142, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Antiel, R.M.; Burkhart, H.M.; Huebner, M.; Li, Z.; Eton, D.T.; Topilsky, T.; Sarano, M.E.; Schaff, H.V. Quality of life after early mitral valve repair using conventional and robotic approaches. Ann. Thorac. Surg. 2012, 93, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Thompson, J.E.; Burkhart, H.M.; Huebner, M.; Borah, B.J.; Li, Z.; Michelena, H.I.; Visscher, S.L.; Roger, V.L.; Daly, R.C.; et al. Improving affordability through innovation in the surgical treatment of mitral valve disease. Mayo Clin. Proc. 2013, 88, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Kim, J.B.; Jung, S.H.; Kim, D.H.; Choo, S.J.; Chung, C.H.; Lee, J.W. Mitral durability after robotic mitral valve repair: Analysis of 200 consecutive mitral regurgitation repairs. J. Thorac. Cardiovasc. Surg. 2014, 148, 2773–2779. [Google Scholar] [CrossRef] [PubMed]
- Halkos, M.E.; Liberman, H.A.; Devireddy, C.; Walker, P.; Finn, A.V.; Jaber, W.; Guyton, R.A.; Puskas, J.D. Early clinical and angiographic outcomes after robotic-assisted coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2014, 147, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, M.; Calafiore, A.M.; Lorusso, R. All roads lead to Rome, but some are safer. J. Card. Surg. 2021, 36, 4320–4321. [Google Scholar] [CrossRef] [PubMed]
- Gofus, J.; Cerny, S.; Shahin, Y.; Sorm, Z.; Vobornik, M.; Smolak, P.; Sethi, A.; Marcinov, S.; Karalko, M.; Chek, J.; et al. Robot-assisted vs. conventional MIDCAB: A propensity-matched analysis. Front. Cardiovasc. Med. 2022, 9, 943076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raja, S.G.; Uzzaman, M.; Garg, S.; Santhirakumaran, G.; Lee, M.; Soni, M.K.; Khan, H. Comparison of minimally invasive direct coronary artery bypass and drug-eluting stents for management of isolated left anterior descending artery disease: A systematic review and meta-analysis of 7710 patients. Ann. Cardiothorac. Surg. 2018, 7, 567–576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khaliel, F.H.; Haq, M.I.; Alsulbud, A.K.; Altwaijri, Y.A.; Alenazy, A.B.; Almustafa, A.R.; Aly, A.A.; Mehra, M.R. “First-in-human” totally robotic orthotopic heart transplant. J. Heart Lung Transplant. 2025, 44, 1000–1003. [Google Scholar] [CrossRef] [PubMed]


| Right Mini-Thoracotomy | Totally Endoscopic | Robotic | |
|---|---|---|---|
| Skin Incision | 5–6 cm | 3–4 cm | 3–4 cm |
| Visualization | Direct vision Video-assisted | Video-guided | Video-guided |
| Surgeries | Usually MV also AVR, AAR, CABG | Usually MV also AVR, AAR, CABG | Usually MV also AVR, CABG |
| Technical Advantages | Sternal sparing Magnified vision when video-assisted | Sternal sparing Smaller incision than RMT Magnified vision | Sternal sparing Smaller incision than RMT 3D Magnified vision Tremor filtration Enhanced dexterity |
| Technical Disadvantages | Bigger incision than TE Learning curve for long-shafted instruments No magnified vision if direct vision | Learning curve for long-shafted instruments | Learning curve for robotic instruments |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Poddi, S.; Rungatscher, A. Minimally Invasive Cardiac Surgery: A State-of-the-Art Review. J. Clin. Med. 2026, 15, 371. https://doi.org/10.3390/jcm15010371
Poddi S, Rungatscher A. Minimally Invasive Cardiac Surgery: A State-of-the-Art Review. Journal of Clinical Medicine. 2026; 15(1):371. https://doi.org/10.3390/jcm15010371
Chicago/Turabian StylePoddi, Salvatore, and Alessio Rungatscher. 2026. "Minimally Invasive Cardiac Surgery: A State-of-the-Art Review" Journal of Clinical Medicine 15, no. 1: 371. https://doi.org/10.3390/jcm15010371
APA StylePoddi, S., & Rungatscher, A. (2026). Minimally Invasive Cardiac Surgery: A State-of-the-Art Review. Journal of Clinical Medicine, 15(1), 371. https://doi.org/10.3390/jcm15010371






