Retinal Microvascular and Orbital Structural Alterations in Thyroid Eye Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Evaluation
2.2. OCTA Imaging
2.3. Orbital MRI Imaging
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| anti-TPO | anti-thyroid peroxidase antibodies |
| AUC | area under the curve |
| BCVA | best-corrected visual acuity |
| CAS | Clinical Activity Score |
| CI | confidence interval |
| DCP | deep capillary plexus |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| EUGOGO | European Society for Graves’ Orbitopathy |
| FAZ | foveal avascular zone |
| fT3 | free triiodothyronine |
| fT4 | free thyroxine |
| GH | Graves’ hyperthyroidism |
| HDL | high-density lipoprotein |
| ICC | interclass correlation |
| IOP | intraocular pressure |
| IQR | interquartile range |
| LDL | low-density lipoprotein |
| MRI | magnetic resonance imaging |
| OCTA | Optical coherence tomography angiography |
| ROC | receiver operating curve |
| SCP | superficial capillary plexus |
| TED | Thyroid eye disease |
| TRAb | thyrotropin receptor antibodies |
| TSH | thyroid-stimulating hormone |
| VD | vessel density |
| VIF | variance inflation factor |
References
- Alkhadrawi, A.M.; Lin, L.Y.; Langarica, S.A.; Kim, K.; Ha, S.K.; Lee, N.G.; Do, S. Deep-Learning Based Automated Segmentation and Quantitative Volumetric Analysis of Orbital Muscle and Fat for Diagnosis of Thyroid Eye Disease. Investig. Ophthalmol. Vis. Sci. 2024, 65, 6. [Google Scholar] [CrossRef]
- Kahaly, G.J. Management of Graves Thyroidal and Extrathyroidal Disease: An Update. J. Clin. Endocrinol. Metab. 2020, 105, 3704–3720. [Google Scholar] [CrossRef]
- Chin, Y.H.; Ng, C.H.; Lee, M.H.; Koh, J.W.H.; Kiew, J.; Yang, S.P.; Sundar, G.; Khoo, C.M. Prevalence of thyroid eye disease in Graves’ disease: A meta-analysis and systematic review. Clin. Endocrinol. 2020, 93, 363–374. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Perros, P.; Žarković, M.; Azzolini, C.; Ayvaz, G.; Baldeschi, L.; Bartalena, L.; Boschi, A.; Bournaud, C.; Brix, T.H.; Covelli, D.; et al. PREGO (presentation of Graves’ orbitopathy) study: Changes in referral patterns to European Group On Graves’ Orbitopathy (EUGOGO) centres over the period from 2000 to 2012. Br. J. Ophthalmol. 2015, 99, 1531–1535. [Google Scholar] [CrossRef]
- Bartalena, L.; Baldeschi, L.; Boboridis, K.; Eckstein, A.; Kahaly, G.J.; Marcocci, C.; Perros, P.; Salvi, M.; Wiersinga, W.M. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur. Thyroid. J. 2016, 5, 9–26. [Google Scholar] [CrossRef]
- Ippolito, S.; Cusini, C.; Lasalvia, P.; Gianfagna, F.; Veronesi, G.; Gallo, D.; Masiello, E.; Premoli, P.; Sabatino, J.; Mercuriali, A.; et al. Change in newly diagnosed Graves’ disease phenotype between the twentieth and the twenty-first centuries: Meta-analysis and meta-regression. J. Endocrinol. Investig. 2021, 44, 1707–1718. [Google Scholar] [CrossRef]
- Taylor, P.N.; Zhang, L.; Lee, R.W.J.; Muller, I.; Ezra, D.G.; Dayan, C.M.; Kahaly, G.J.; Ludgate, M. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat. Rev. Endocrinol. 2020, 16, 104–116. [Google Scholar] [CrossRef]
- Baltmr, A.; Lightman, S.; Tomkins-Netzer, O. Examining the choroid in ocular inflammation: A focus on enhanced depth imaging. J. Ophthalmol. 2014, 2014, 459136. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, T.J. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1735–1748. [Google Scholar] [CrossRef]
- Korkmaz, S.; Konuk, O. Surgical Treatment of Dysthyroid Optic Neuropathy: Long-Term Visual Outcomes with Comparison of 2-Wall versus 3-Wall Orbital Decompression. Curr. Eye Res. 2016, 41, 159–164. [Google Scholar] [CrossRef]
- Mourits, M.P.; Koornneef, L.; Wiersinga, W.M.; Prummel, M.F.; Berghout, A.; van der Gaag, R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: A novel approach. Br. J. Ophthalmol. 1989, 73, 639–644. [Google Scholar] [CrossRef]
- Čivrný, J.; Karhanová, M.; Hübnerová, P.; Schovánek, J.; Heřman, M. MRI in the assessment of thyroid-associated orbitopathy activity. Clin. Radiol. 2022, 77, 925–934. [Google Scholar] [CrossRef]
- Xu, B.; Wang, S.; Chen, L.; Tan, J. The early diagnostic value of optical coherence tomography (OCT) and OCT angiography in thyroid-associated ophthalmopathy. Ther. Adv. Chronic Dis. 2023, 14, 20406223231166802. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, J.; Kaushal, S.; Arora, P.; Wal, P.; Wal, A.; Gasmi, A. Molecular pathway and mechanism responsible for the progress of thyroid-associated orbitopathy. Int. Ophthalmol. 2025, 45, 197. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tu, Y.; Bao, L.; Wu, C.; Zheng, J.; Wang, J.; Lu, F.; Shen, M.; Chen, Q. Reduced Retinal Microvascular Density Related to Activity Status and Serum Antibodies in Patients with Graves’ Ophthalmopathy. Curr. Eye Res. 2020, 45, 576–584. [Google Scholar] [CrossRef]
- Gontarz-Nowak, K.; Szychlińska, M.; Matuszewski, W.; Stefanowicz-Rutkowska, M.; Bandurska-Stankiewicz, E. Current Knowledge on Graves’ Orbitopathy. J. Clin. Med. 2020, 10, 16. [Google Scholar] [CrossRef]
- Krassas, G.E.; Perros, P. Prevention of thyroid associated-ophthalmopathy in children and adults: Current views and management of preventable risk factors. Pediatr. Endocrinol. Rev. 2007, 4, 218–224. [Google Scholar]
- Ye, L.; Zhou, S.S.; Yang, W.L.; Bao, J.; Jiang, N.; Min, Y.L.; Yuan, Q.; Tan, G.; Shen, M.; Shao, Y. Retinal Microvasculature Alteration in Active Thyroid-Associated Ophthalmopathy. Endocr. Pr. 2018, 24, 658–667. [Google Scholar] [CrossRef]
- Liang, C.; Liu, J.; Hua, D.; Cao, T.; Meng, Y.; Xiao, D.; Zheng, H.; Chen, Z.; Chen, C.; Xu, Y. Retinal and choroidal microvascular analysis by swept-source optical coherence tomography angiography in thyroid-associated ophthalmopathy (TAO) and hyperthyroidism without clinical signs of TAO. Ann. Med. 2025, 57, 2478314. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jiao, Q.; Cheng, Y.; Zhu, Y.; Lin, Z.; Shen, X. Evaluation of retinal and choroidal variations in thyroid-associated ophthalmopathy using optical coherence tomography angiography. BMC Ophthalmol. 2020, 20, 421. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian Tehrani, M.; Mahdizad, Z.; Kasaei, A.; Fard, M.A. Early macular and peripapillary vasculature dropout in active thyroid eye disease. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2533–2540. [Google Scholar] [CrossRef]
- Mihailovic, N.; Lahme, L.; Rosenberger, F.; Hirscheider, M.; Termühlen, J.; Heiduschka, P.; Grenzebach, U.; Eter, N.; Alnawaiseh, M. Altered Retinal Perfusion in Patients with Inactive Graves Ophthalmopathy Using Optical Coherence Tomography Angiography. Endocr. Pr. 2020, 26, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Vingolo, E.M. Endothelin-1 role in human eye: A review. J. Ophthalmol. 2010, 2010, 354645. [Google Scholar] [CrossRef]
- Chu, C.H.; Lee, J.K.; Keng, H.M.; Chuang, M.J.; Lu, C.C.; Wang, M.C.; Sun, C.C.; Wei, M.C.; Lam, H.C. Hyperthyroidism is associated with higher plasma endothelin-1 concentrations. Exp. Biol. Med. 2006, 231, 1040–1043. [Google Scholar]
- Hiromatsu, Y.; Yang, D.; Bednarczuk, T.; Miyake, I.; Nonaka, K.; Inoue, Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 2000, 85, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Konuk, O.; Onaran, Z.; Ozhan Oktar, S.; Yucel, C.; Unal, M. Intraocular pressure and superior ophthalmic vein blood flow velocity in Graves’ orbitopathy: Relation with the clinical features. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1555–1559. [Google Scholar] [CrossRef]
- Kurioka, Y.; Inaba, M.; Kawagishi, T.; Emoto, M.; Kumeda, Y.; Inoue, Y.; Morii, H.; Nishizawa, Y. Increased retinal blood flow in patients with Graves’ disease: Influence of thyroid function and ophthalmopathy. Eur. J. Endocrinol. 2001, 144, 99–107. [Google Scholar] [CrossRef]
- Perri, P.; Campa, C.; Costagliola, C.; Incorvaia, C.; D’Angelo, S.; Sebastiani, A. Increased retinal blood flow in patients with active Graves’ ophthalmopathy. Curr. Eye Res. 2007, 32, 985–990. [Google Scholar] [CrossRef]
- Klein, I. Thyroid hormone and the cardiovascular system. Am. J. Med. 1990, 88, 631–637. [Google Scholar] [CrossRef]
- Dogan, M.E.; Basol, I.; Ilhan, H.D.; Ayaz, Y.; Ocal, O. Evaluation of macular choroidal and microvascular network changes by activity scores and serum antibodies in thyroid eye patients and healthy subjects. Int. J. Ophthalmol. 2023, 16, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Alp, M.N.; Ozgen, A.; Can, I.; Cakar, P.; Gunalp, I. Colour Doppler imaging of the orbital vasculature in Graves’ disease with computed tomographic correlation. Br. J. Ophthalmol. 2000, 84, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Akpolat, C.; Kurt, M.M.; Yılmaz, M.; Ordulu, F.; Evliyaoglu, F. Analysis of Foveal and Parafoveal Microvascular Density and Retinal Vessel Caliber Alteration in Inactive Graves’ Ophthalmopathy. J. Ophthalmol. 2020, 2020, 7643737. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.; Sabermoghaddam, A.; Salahi, Z.; Bakhtiari, E.; Motamed Shariati, M. Macular microvasculature in patients with thyroid-associated orbitopathy: A cross-sectional study. Thyroid. Res. 2023, 16, 31. [Google Scholar] [CrossRef]
- Fazil, K.; Ozturk Karabulut, G.; Alkin, Z. Evaluation of choroidal thickness and retinal vessel density in patients with inactive Graves’ orbitopathy. Photodiagnosis Photodyn. Ther. 2020, 32, 101898. [Google Scholar] [CrossRef]
- Wu, H.; Song, Q.; Zhang, Y.; Cheng, R.; Li, Y.; Su, M.; Zhang, X.; Sun, X. Optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) for assessing thyroid-associated ophthalmopathy activity. Photodiagnosis Photodyn. Ther. 2025, 53, 104578. [Google Scholar] [CrossRef]
- Lim, N.C.; Sundar, G.; Amrith, S.; Lee, K.O. Thyroid eye disease: A Southeast Asian experience. Br. J. Ophthalmol. 2015, 99, 512–518. [Google Scholar] [CrossRef]
- Dastiridou, A.; Kassos, I.; Samouilidou, M.; Koutali, D.; Mataftsi, A.; Androudi, S.; Ziakas, N. Age and signal strength-related changes in vessel density in the choroid and the retina: An OCT angiography study of the macula and optic disc. Acta Ophthalmol. 2022, 100, e1095–e1102. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, M.; Zhang, F.; Cao, J.; Xie, B.; Zhu, Z.; Xiong, W. Orbital decompression improves visual function and macular blood perfusion status in patients with thyroid-related eye disease. Front. Med. 2024, 11, 1455226. [Google Scholar] [CrossRef]
- Luccas, R.; Riguetto, C.M.; Alves, M.; Zantut-Wittmann, D.E.; Reis, F. Computed tomography and magnetic resonance imaging approaches to Graves’ ophthalmopathy: A narrative review. Front. Endocrinol. 2023, 14, 1277961. [Google Scholar] [CrossRef]
- Regensburg, N.I.; Wiersinga, W.M.; Berendschot, T.T.; Potgieser, P.; Mourits, M.P. Do subtypes of graves’ orbitopathy exist? Ophthalmology 2011, 118, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M.; Regensburg, N.I.; Mourits, M.P. Differential involvement of orbital fat and extraocular muscles in graves’ ophthalmopathy. Eur. Thyroid. J. 2013, 2, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Genere, N.; Stan, M.N. Current and Emerging Treatment Strategies for Graves’ Orbitopathy. Drugs 2019, 79, 109–124. [Google Scholar] [CrossRef]
- Hu, H.; Chen, L.; Zhou, J.; Chen, W.; Chen, H.H.; Zhang, J.L.; Hsu, Y.C.; Xu, X.Q.; Wu, F.Y. Multiparametric magnetic resonance imaging for differentiating active from inactive thyroid-associated ophthalmopathy: Added value from magnetization transfer imaging. Eur. J. Radiol. 2022, 151, 110295. [Google Scholar] [CrossRef]
- Zhou, M.; Shen, L.; Jiao, Q.; Ye, L.; Zhou, Y.; Zhu, W.; Wang, W.; Wang, S. Role of Magnetic Resonance Imaging in the Assessment of Active Thyroid-Associated Ophthalmopathy Patients with Long Disease Duration. Endocr. Pr. 2019, 25, 1268–1278. [Google Scholar] [CrossRef]
- Araujo, F.B.; Barbosa, D.S.; Hsin, C.Y.; Maranhão, R.C.; Abdalla, D.S. Evaluation of oxidative stress in patients with hyperlipidemia. Atherosclerosis 1995, 117, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Rotondo Dottore, G.; Ionni, I.; Menconi, F.; Casini, G.; Sellari-Franceschini, S.; Nardi, M.; Vitti, P.; Marcocci, C.; Marinò, M. Action of three bioavailable antioxidants in orbital fibroblasts from patients with Graves’ orbitopathy (GO): A new frontier for GO treatment? J. Endocrinol. Investig. 2018, 41, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.H.; Brix, T.H.; Leslie, R.G.; Hegedüs, L. A role for autoantibodies in enhancement of pro-inflammatory cytokine responses to a self-antigen, thyroid peroxidase. Clin. Immunol. 2009, 133, 218–227. [Google Scholar] [CrossRef]
- Ma, L.; Hui, S.; Li, Y.; Hou, Z.; Liu, Z.; Chang, Q.; Zhang, H.; Li, D. Different Characteristics of Orbital Soft Tissue Expansion in Graves Orbitopathy: Extraocular Muscle Expansion is Correlated to Disease Activity While Fat Tissue Volume with Duration. J. Craniofac Surg. 2022, 33, 2354–2359. [Google Scholar] [CrossRef]
- Cardo, C.; Bernardo Santos, R.; Pinotti Pedro Miklos, A.B.; Barbosa Jaconis, S.; Romaldini, J.H.; Villagelin, D. The relationship between cholesterol levels and thyroid eye disease. Eur. Thyroid. J. 2025, 14, e240133. [Google Scholar] [CrossRef] [PubMed]
- Dave, T.V.; Laghmisetty, S.; Krishnamurthy, G.; Bejjanki, K.; Ganguly, A.; Jonnadula, G.B.; Dave, V.P.; Reddy Pappuru, R. Retinal vascularity, nerve fiber, and ganglion cell layer thickness in thyroid eye disease on optical coherence tomography angiography. Orbit 2022, 41, 170–177. [Google Scholar] [CrossRef] [PubMed]
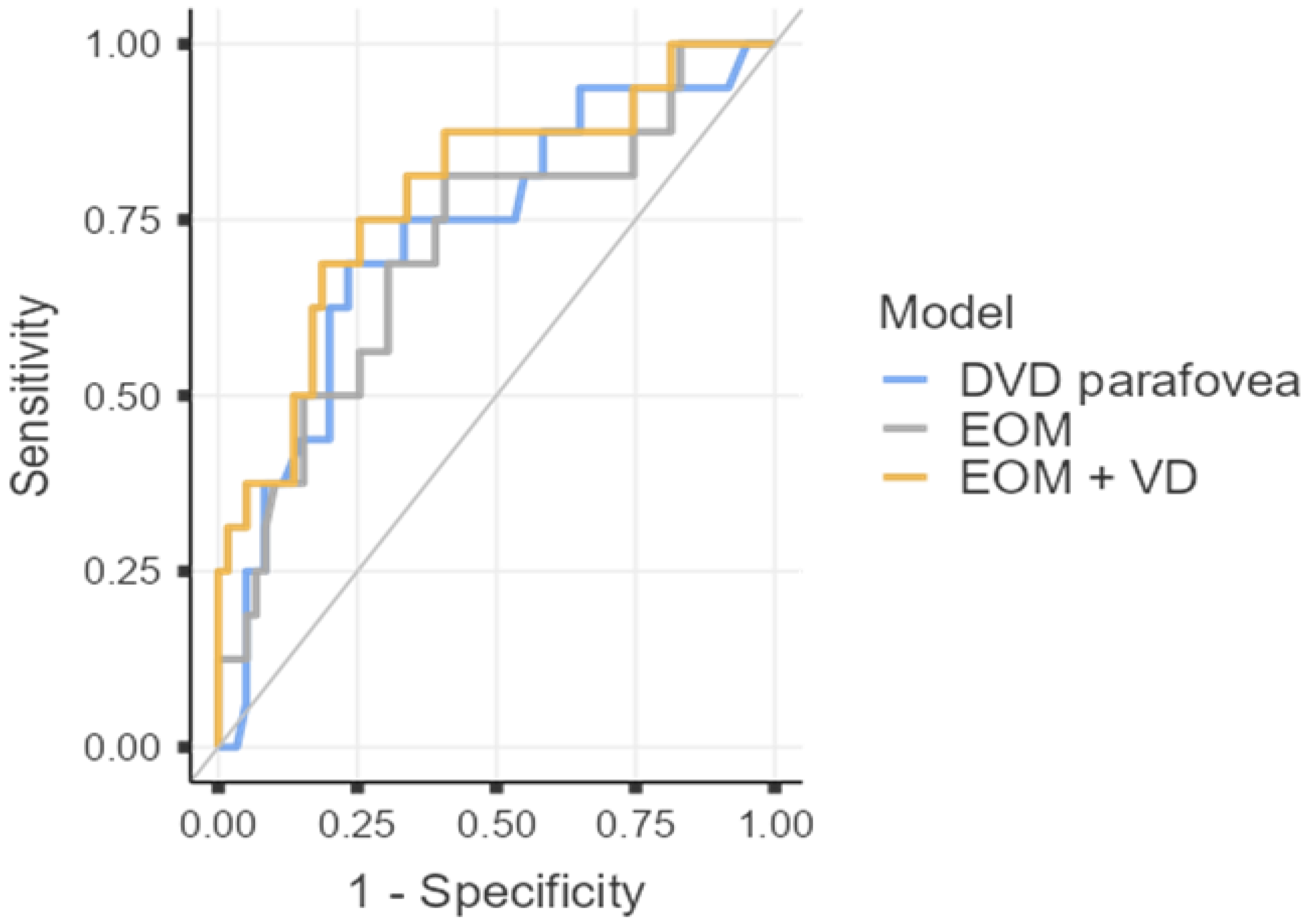
| Inactive TED (CAS < 3; n = 27) | Active TED (CAS ≥ 3; n = 11) | p-Value * | Hedges’ g/Hodges-Lehmann Estimate | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|---|
| Demographic and clinical parameters | ||||||
| Female gender | 18 (69.23) | 9 (75) | 0.606 | |||
| Age, years | 49.31 (9.67) | 51.75 (15.02) | 0.397 † | −0.21 | −0.69 | 0.28 |
| Smoking | 15 (57.69) | 5 (41.67) | 0.193 | |||
| Hyperlipidemia (n = 29) | 7 (35) | 6 (66.67) | 0.025 | |||
| Hypertension | 16 (31) | 6 (25) | 0.787 | |||
| TED duration, months | 32.5 (24, 90) | 16.5 (13.25, 25) | <0.001 | 18 | 11 | 39 |
| GH duration, months | 58 (32, 103) | 27.5 (16.75, 48.75) | 0.002 | 28 | 10 | 54 |
| Radioiodine therapy | 3 (11.54) | 1 (8.33) | 0.67 | |||
| Laboratory parameters | ||||||
| TSH, mIU/L | 0.89 (0.004, 1475.0) | 0.29 (0.04, 3779.5) | 0.201 | −0.04 | −560 | 0.21 |
| fT3, pmol/L | 4.79 (3.94, 9.55) | 4.61 (4.42, 6.55) | 0.708 | −0.13 | −0.78 | 0.87 |
| fT4, pmol/L | 14.25 (12.04, 20.51) | 11.85 (10.16, 14.37) | 0.003 | 3.22 | 1.02 | 6.49 |
| Anti-TPO, kIU/mL | 20.39 (2.07, 363.03) | 23.54 (0.61, 1000) | 0.968 | −6.9 | −34.37 | 5.55 |
| TRAb, IU/L | 6.25 (3.7, 9.6) | 13.45 (9.45, 21) | <0.001 | <0.001 | −11.3 | −3.2 |
| Total cholesterol, mmol/L | 4.59 (4.15, 5.68) | 5.31 (4.9, 6.77) | 0.048 | −0.77 | −1.47 | −0.0001 |
| HDL, mmol/L | 1.3 (1.1, 1.49) | 1.47 (1.4, 1.54) | 0.007 | −0.23 | −0.4 | −0.07 |
| LDL, mmol/L | 3.18 (2.68, 4.0) | 3.41 (3.02, 4.215) | 0.43 | −0.39 | −1 | 0.41 |
| Triglycerides, mmol/L | 1.37 (1.1, 1.8) | 2.28 (1.44, 3.5) | 0.003 | −0.87 | −1.75 | −0.31 |
| Inactive TED (CAS < 3; n = 50 Eyes) | Active TED (CAS ≥ 3; n = 16 Eyes) | p-Value * | Hedges’ g/Hodges-Lehmann Estimate | Lower 95% CI | Upper 95% CI | p-Value GMM | |
|---|---|---|---|---|---|---|---|
| Clinical parameters | |||||||
| BCVA, Snellen | 1 (1, 1) | 1 (0.9, 1.0) | 0.107 † | <0.001 | −0.0001 | 0.0001 | 0.334 |
| CAS | 1 (0, 2) | 3.5 (3, 4) | <0.001 † | −3.0 | −3.0 | −2.0 | <0.001 |
| Tonometry primary-gaze, mmHg, | 14.52 (2.87) | 15.25 (2.41) | 0.352 | −0.26 | −0.81 | 0.29 | 0.175 |
| Tonometry upward-gaze, mmHg | 16.92 (3.81) | 21.19 (4.93) | <0.001 | −1.04 | −1.61 | −0.46 | 0.035 |
| Hertel exophthalmometry, mm | 17.2 (3.86) | 20 (3.05) | 0.011 | −0.74 | −1.32 | −0.16 | 0.143 |
| OCTA parameters—deep capillary plexus vessel density | |||||||
| whole image | 46.81 (5.53) | 50.72 (5.09) | 0.013 | −0.71 | −1.27 | −0.15 | 0.016 |
| fovea | 38.03 (7.61) | 39.64 (5.24) | 0.444 | −0.22 | −0.79 | 0.35 | 0.192 |
| parafovea | 53.05 (4.76) | 56.4 (3.99) | 0.012 | −0.72 | −1.28 | −0.15 | 0.012 |
| perifovea | 47.88 (5.97) | 52.31 (5.64) | 0.009 | −0.74 | −1.31 | −0.18 | 0.03 |
| Foveal avascular zone | 0.26 (0.09) | 0.25 (0.07) | 0.789 | 0.08 | −0.48 | 0.63 | 0.215 |
| MRI parameters | |||||||
| Orbital fat-tissue thickness | 5.71 (0.97) | 6 (1.19) | 0.309 | −0.29 | −0.84 | 0.27 | 0.348 |
| Medial rectus muscle thickness | 4.5 (4.05, 5.15) | 4.85 (4.38, 6.2) | 0.103 † | −0.5 | −1.2 | 0.1 | 0.113 |
| Lateral rectus muscle thickness | 4.19 (0.76) | 4.58 (0.65) | 0.083 | −0.49 | −1.05 | 0.07 | 0.212 |
| Superior rectus muscle thickness | 4.0 (3.5, 4.75) | 4.9 (3.95, 5.85) | 0.037 † | −0.8 | −1.6 | 0.0001 | 0.042 |
| Inferior rectus muscle thickness | 5.79 (1.25) | 6.31 (1.41) | 0.148 | −0.41 | −0.96 | 0.15 | 0.117 |
| Exophthalmus | 18.92 (2.99) | 21.01 (4.04) | 0.024 | −0.64 | −1.2 | −0.078 | 0.098 |
| Model | Predictor | B | SE | β | t | p | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|---|---|
| M1 | Age | 0.05 | −0.11 | −2.12 | 0.042 | −0.21 | −0.01 | |
| Sex (male) | −2.48 | −1.93 | 0.061 | −5.03 | 0.08 | |||
| M2 | Age | 0.05 | −0.11 | −2.33 | 0.026 | −2.33 | −0.02 | |
| Sex (male) | −2.21 | −1.80 | 0.081 | −1.8 | 0.24 | |||
| CAS | 0.39 | 0.17 | 3.04 | 0.003 | 3.04 | 1.94 | ||
| M3 | Age | 5 | −0.12 | −2.7 | 0.011 | −0.21 | −0.03 | |
| CAS | 0.4 | 1.42 | 3.59 | <0.001 | 0.63 | 2.21 | ||
| Exophthalmos | 0.16 | −0.33 | −2.04 | 0.046 | −0.65 | −0.01 | ||
| Sex (male) | −1.97 | 5 | −1.97 | −1.71 | 0.096 | −4.27 | 0.33 | |
| Model | R2 | p | ||||||
| M1 | 0.12 | 0.016 | ||||||
| M2 | 0.22 | <0.001 | ||||||
| M3 | 0.26 | <0.001 |
| Model | Predictor | B | SE | β | t | p | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|---|---|
| M1 | Glucocorticosteroids (yes/no) | 1.06 | 9.45 | <0.001 | 1.41 | 2.17 | ||
| M2 | Glucocorticosteroids (yes/no) | 0.54 | 1.32 | 0.202 | −0.28 | 1.36 | ||
| TRAb | 0.01 | 0.03 | 2.77 | 0.011 | 0.01 | 0.05 | ||
| Triglycerides | 0.15 | 0.43 | 2.79 | 0.011 | 0.12 | 0.74 | ||
| M3 | Glucocorticosteroids (yes/no) | 0.33 | 0.82 | 0.42 | −0.47 | 1.12 | ||
| TRAb | 0.01 | 0.01 | 0.59 | 0.557 | −0.02 | 0.03 | ||
| Triglycerides | 0.14 | 0.47 | 3.30 | 0.003 | 0.18 | 0.76 | ||
| Medial rectus muscle thickness | 0.16 | 0.38 | 2.37 | 0.022 | 0.06 | 0.71 | ||
| DCP VD whole image | 0.03 | 0.06 | 2.30 | 0.027 | 0.01 | 0.12 | ||
| Model | R2 | p | ||||||
| M1 | 0.13 | 0.006 | ||||||
| M2 | 0.31 | <0.001 | ||||||
| M3 | 0.42 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Jelušić, V.; Maduna, I.; Biuk, D.; Krivdić Dupan, Z.; Barać, J.; Šilješ, N.; Jelušić, L.; Benašić, T.; Juri Mandić, J. Retinal Microvascular and Orbital Structural Alterations in Thyroid Eye Disease. J. Clin. Med. 2026, 15, 323. https://doi.org/10.3390/jcm15010323
Jelušić V, Maduna I, Biuk D, Krivdić Dupan Z, Barać J, Šilješ N, Jelušić L, Benašić T, Juri Mandić J. Retinal Microvascular and Orbital Structural Alterations in Thyroid Eye Disease. Journal of Clinical Medicine. 2026; 15(1):323. https://doi.org/10.3390/jcm15010323
Chicago/Turabian StyleJelušić, Vera, Ivanka Maduna, Dubravka Biuk, Zdravka Krivdić Dupan, Josip Barać, Nikolina Šilješ, Laura Jelušić, Tvrtka Benašić, and Jelena Juri Mandić. 2026. "Retinal Microvascular and Orbital Structural Alterations in Thyroid Eye Disease" Journal of Clinical Medicine 15, no. 1: 323. https://doi.org/10.3390/jcm15010323
APA StyleJelušić, V., Maduna, I., Biuk, D., Krivdić Dupan, Z., Barać, J., Šilješ, N., Jelušić, L., Benašić, T., & Juri Mandić, J. (2026). Retinal Microvascular and Orbital Structural Alterations in Thyroid Eye Disease. Journal of Clinical Medicine, 15(1), 323. https://doi.org/10.3390/jcm15010323






