Outcomes of Selective Versus Routine Gastric Tube Decompression After Gastrectomy for Gastric Cancer with Pyloric Obstruction: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
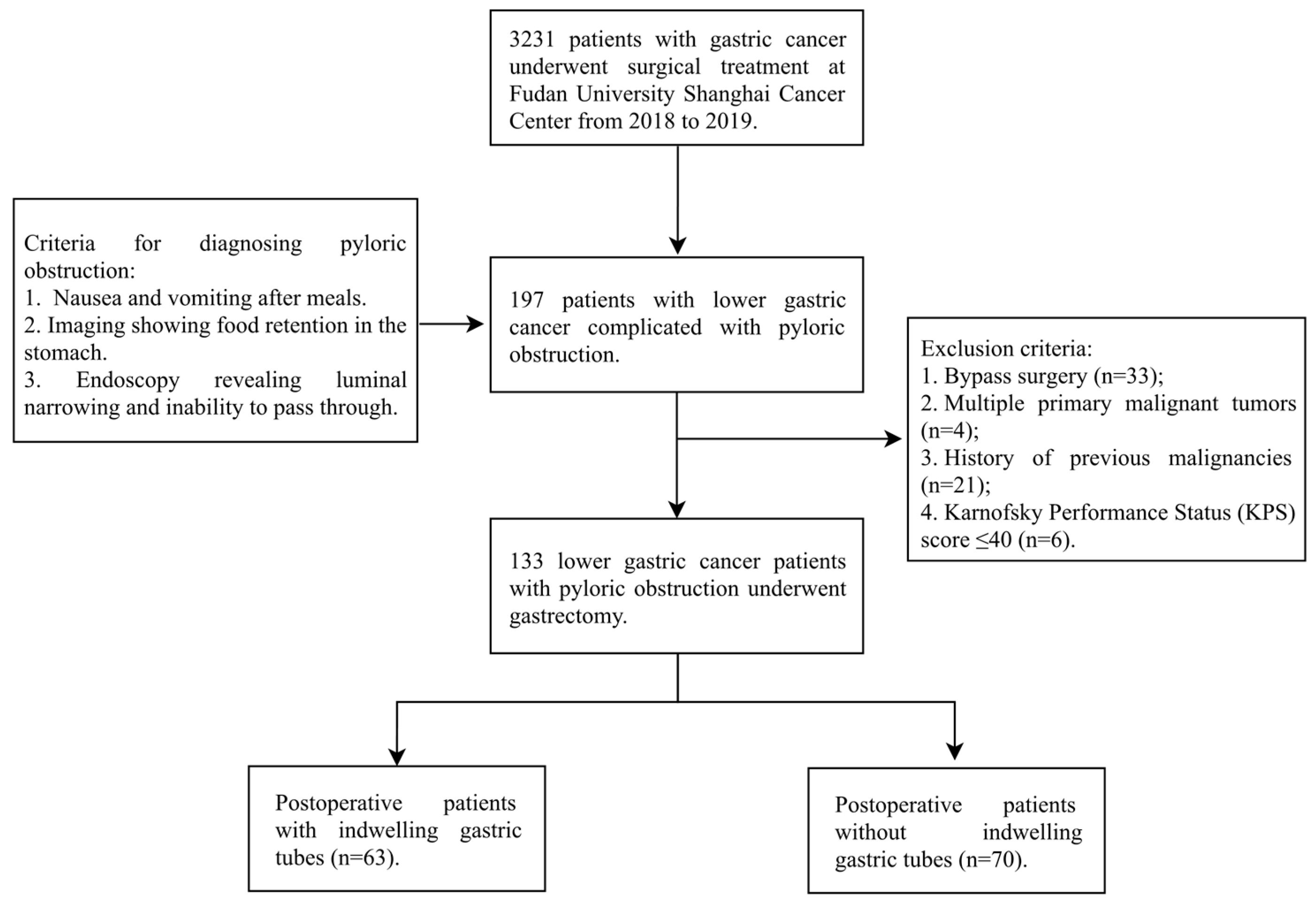
2.2. Patient Selection
2.3. Data Collection and Variables
- Preoperative variables: Demographic data (age, gender), body mass index (BMI), nutritional status assessed by the Nutritional Risk Screening 2002 (NRS-2002; high risk defined as score ≥ 3) [17], and the Global Leadership Initiative on Malnutrition (GLIM) criteria [18], preoperative fasting duration (calculated from the last oral intake to anesthesia induction), American Society of Anesthesiologists (ASA) physical status classification, and comorbidities. Preoperative dietary status was categorized based on the patient’s ability to tolerate oral intake in the week prior to surgery due to obstruction, as documented in physician and nursing notes: (1) Normal or soft diet: Ability to tolerate solid or soft foods. (2) Liquid diet only: ability to tolerate only liquids (e.g., soup, milk, nutritional supplements). (3) Nothing by mouth (NPO) or intravenous fluids only: inability to tolerate any oral intake, requiring intravenous hydration.
- Intraoperative variables: Surgical approach (open vs. laparoscopic), extent of lymphadenectomy (D1 vs. D2), estimated blood loss (mL), and reconstruction method (Billroth I, Billroth II, or Roux-en-Y).
- Postoperative outcomes: The primary outcomes were the duration of GT retention (days; removal criteria: drainage < 400 mL/24 h), total hospitalization costs (adjusted to 2023 Chinese Yuan using a national healthcare-specific inflation index), 30-day postoperative complications, and 90-day all-cause mortality. Laboratory parameters, including nutritional markers (hemoglobin, albumin, pre-albumin) and tumor markers (AFP, CEA, CA19-9, CA50, CA125, CA72-4, CA242), were recorded. Tumor stage was classified according to the 8th edition of the AJCC (American Joint Committee on Cancer) staging manual. Histopathological diagnosis was based on postoperative specimen examination according to standard clinical practice at our institution. The predominant reporting terminology in the included records was “poorly/moderately/well-differentiated adenocarcinoma.” In accordance with common clinicopathological practice. Specific subtypes such as signet-ring cell carcinoma were explicitly recorded when identified.
2.4. Standardized Perioperative Management
2.5. Surgical Technique
2.6. Statistical Analysis
3. Results
3.1. Patient Enrollment and Baseline Characteristics
3.2. Postoperative Complications and Mortality
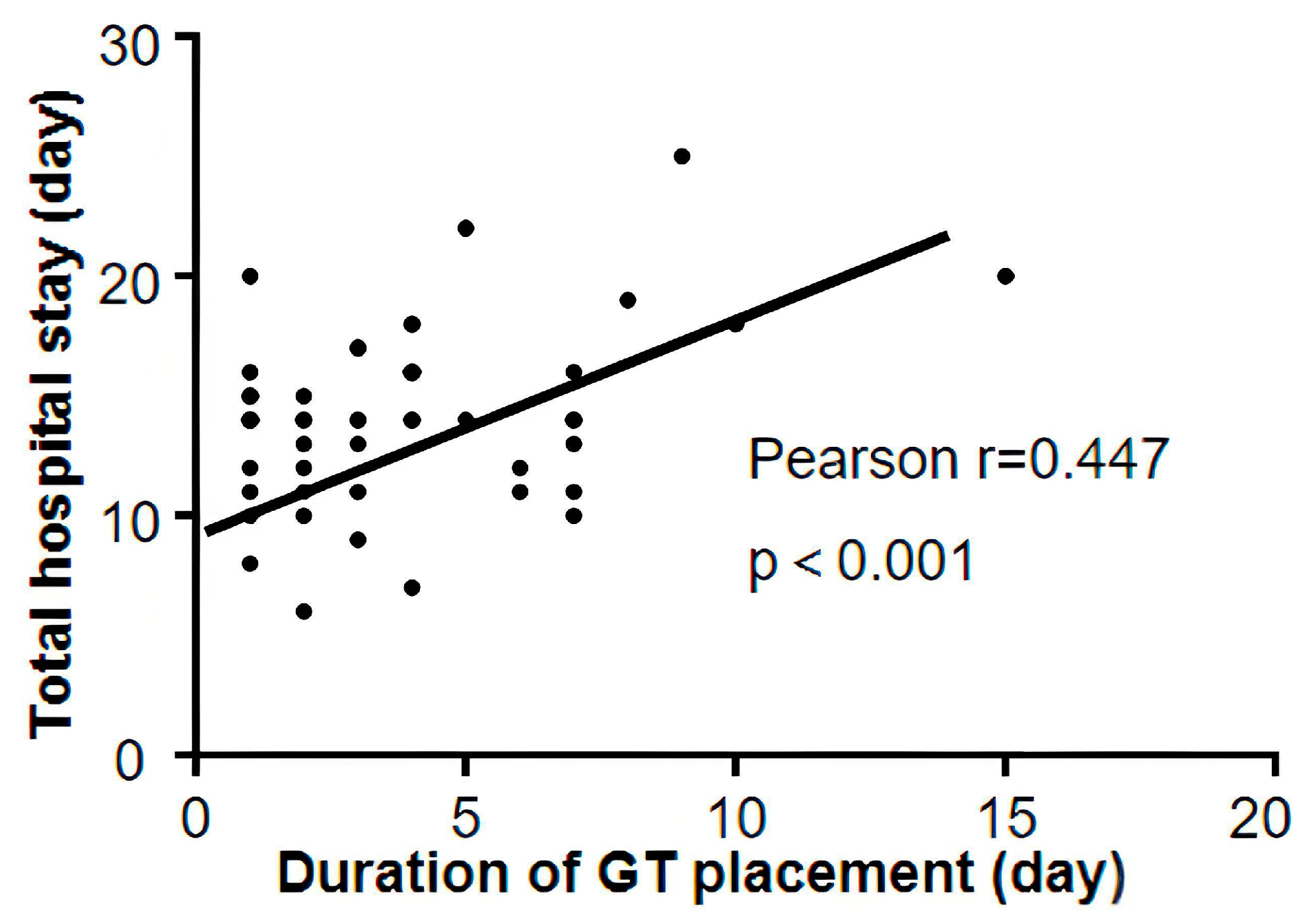
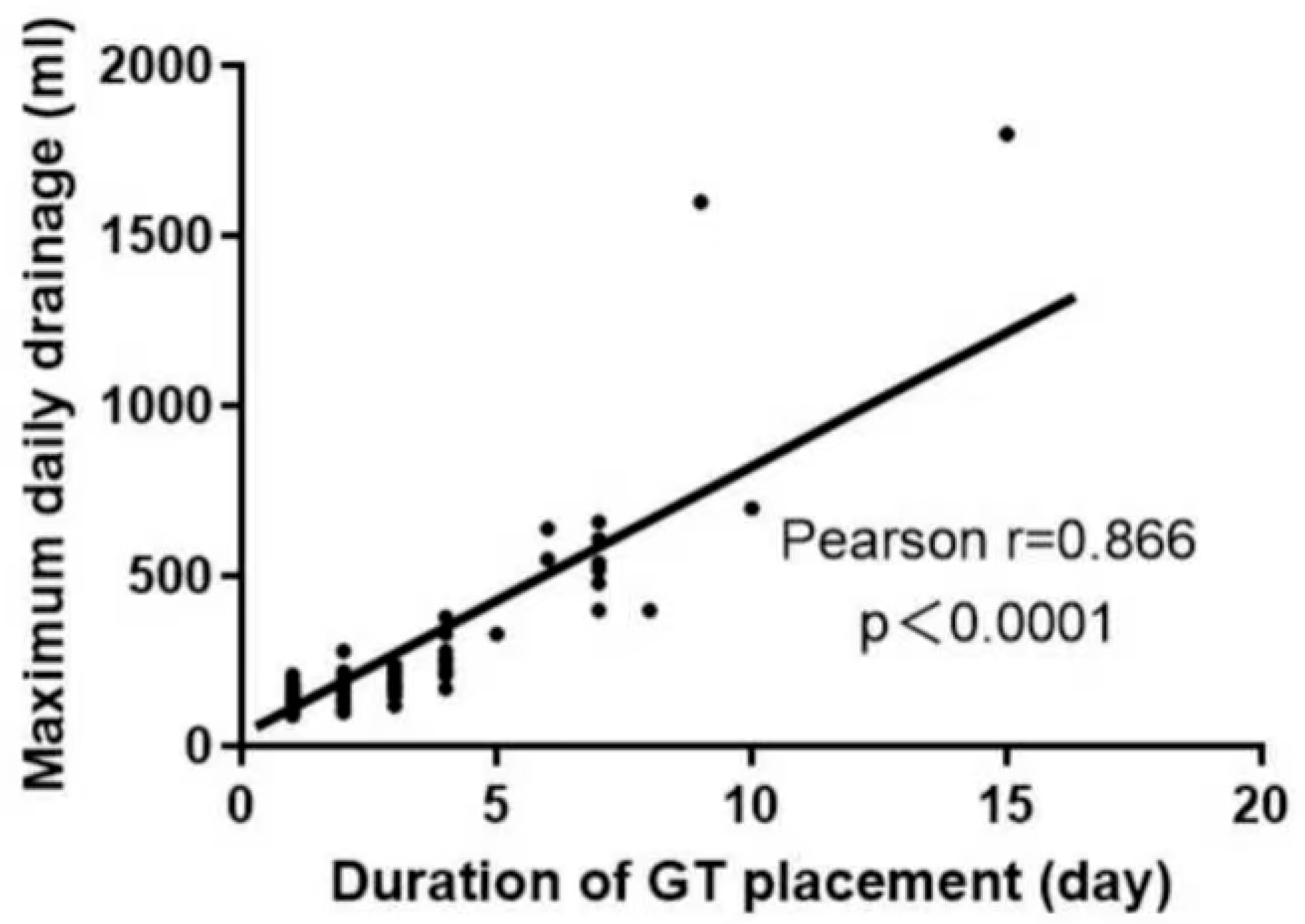
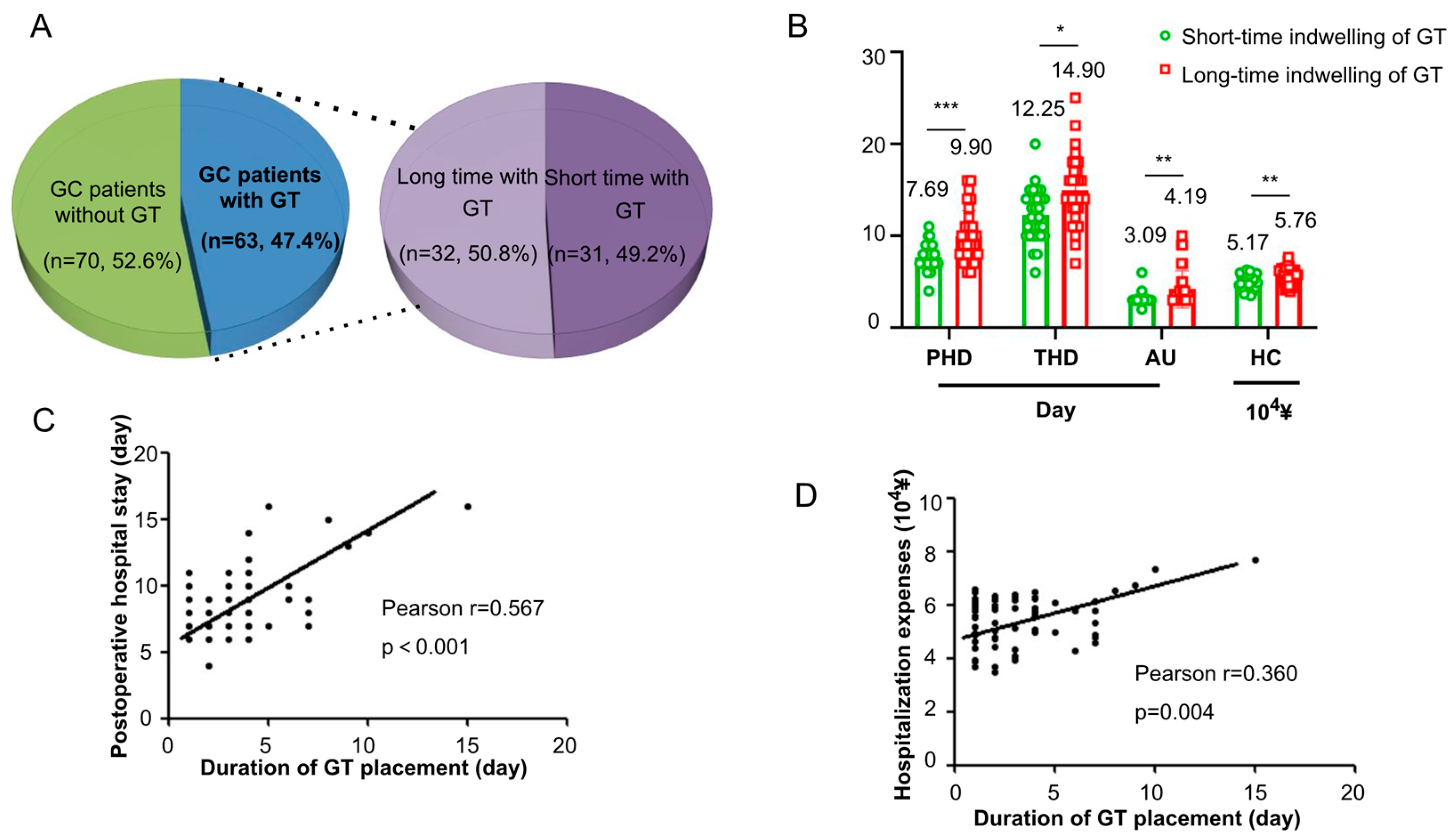
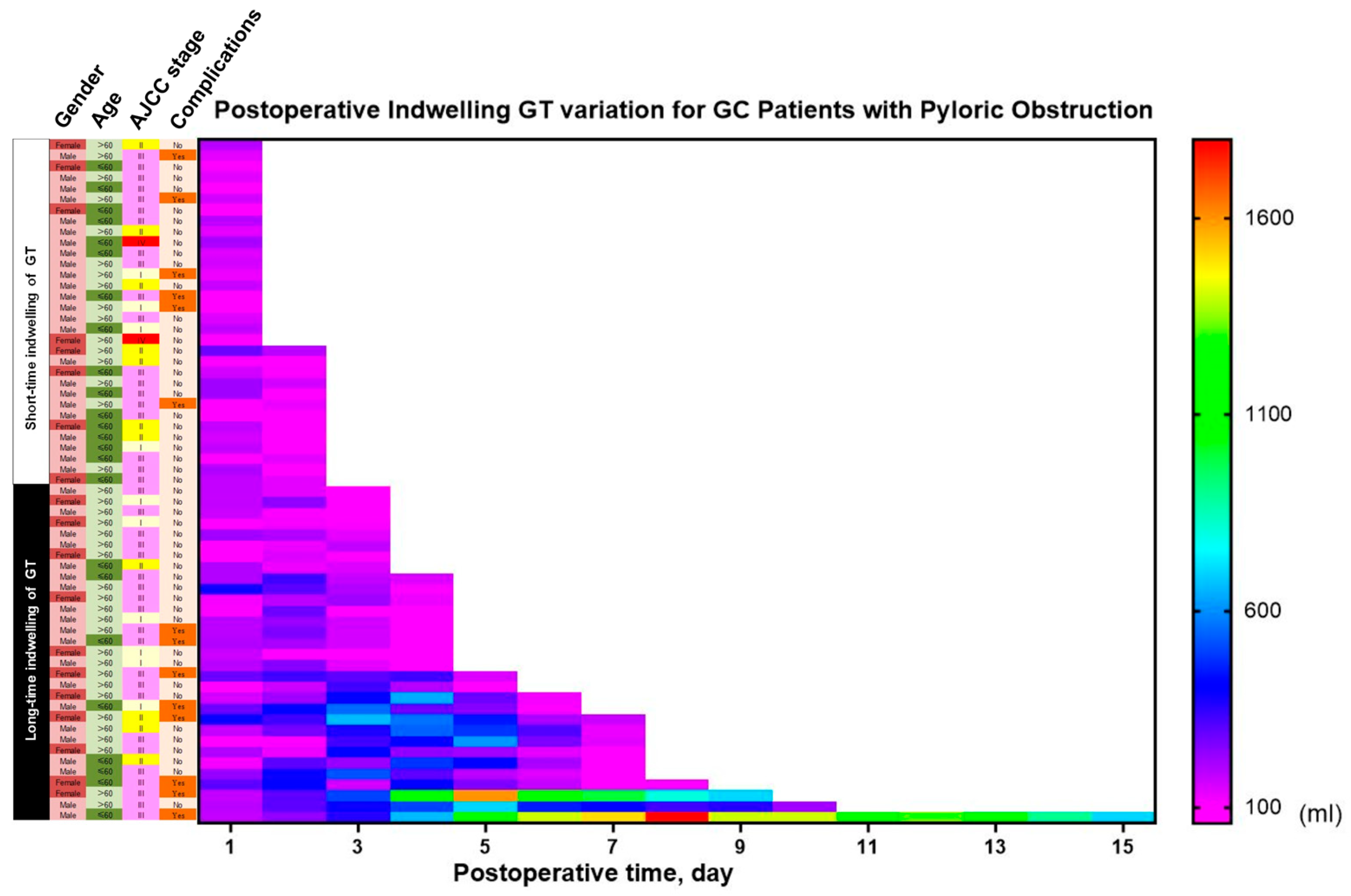
3.3. Impact of Gastric Tube Retention Duration on Resource Utilization
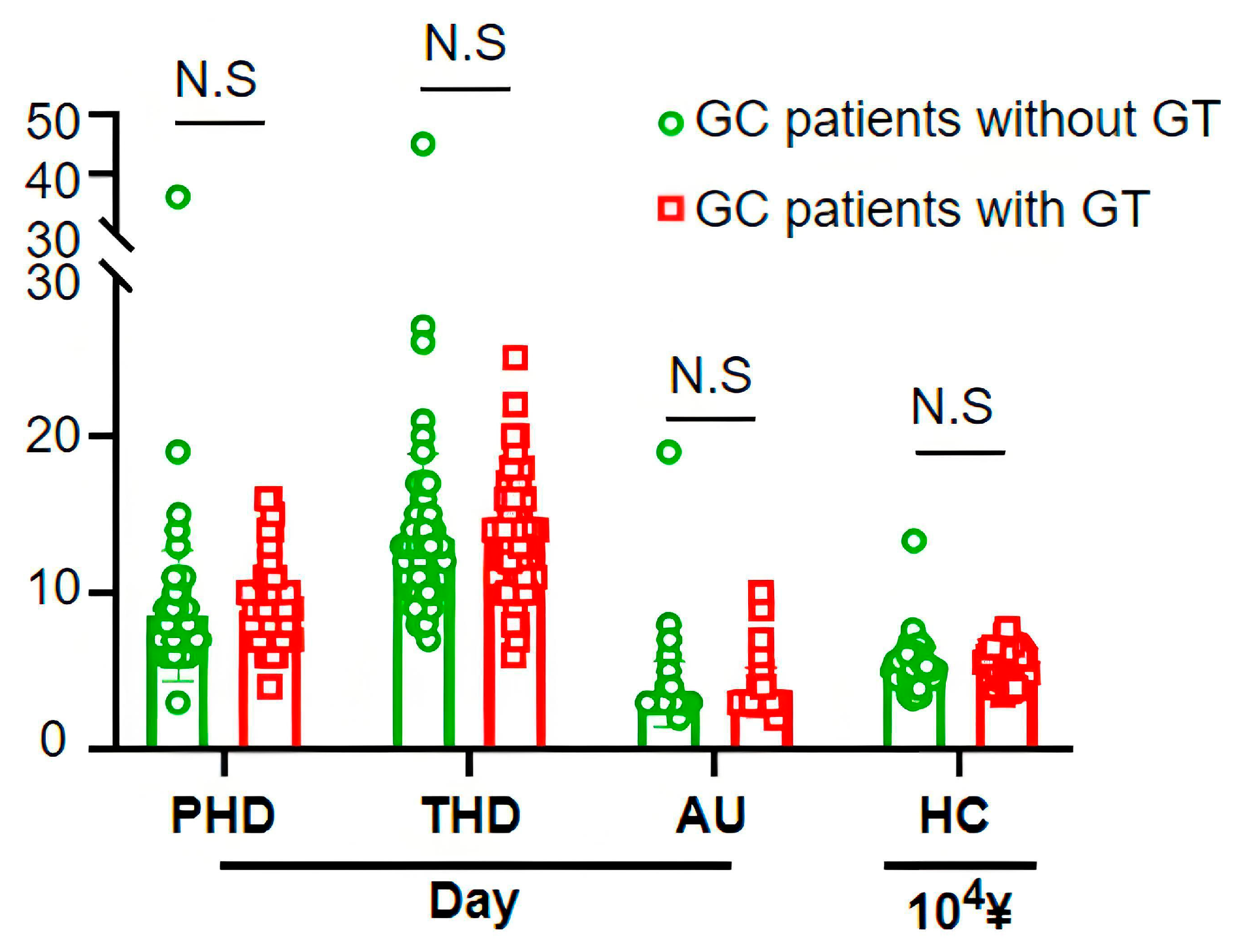
3.4. Economic Burden of Routine Gastric Tube Placement
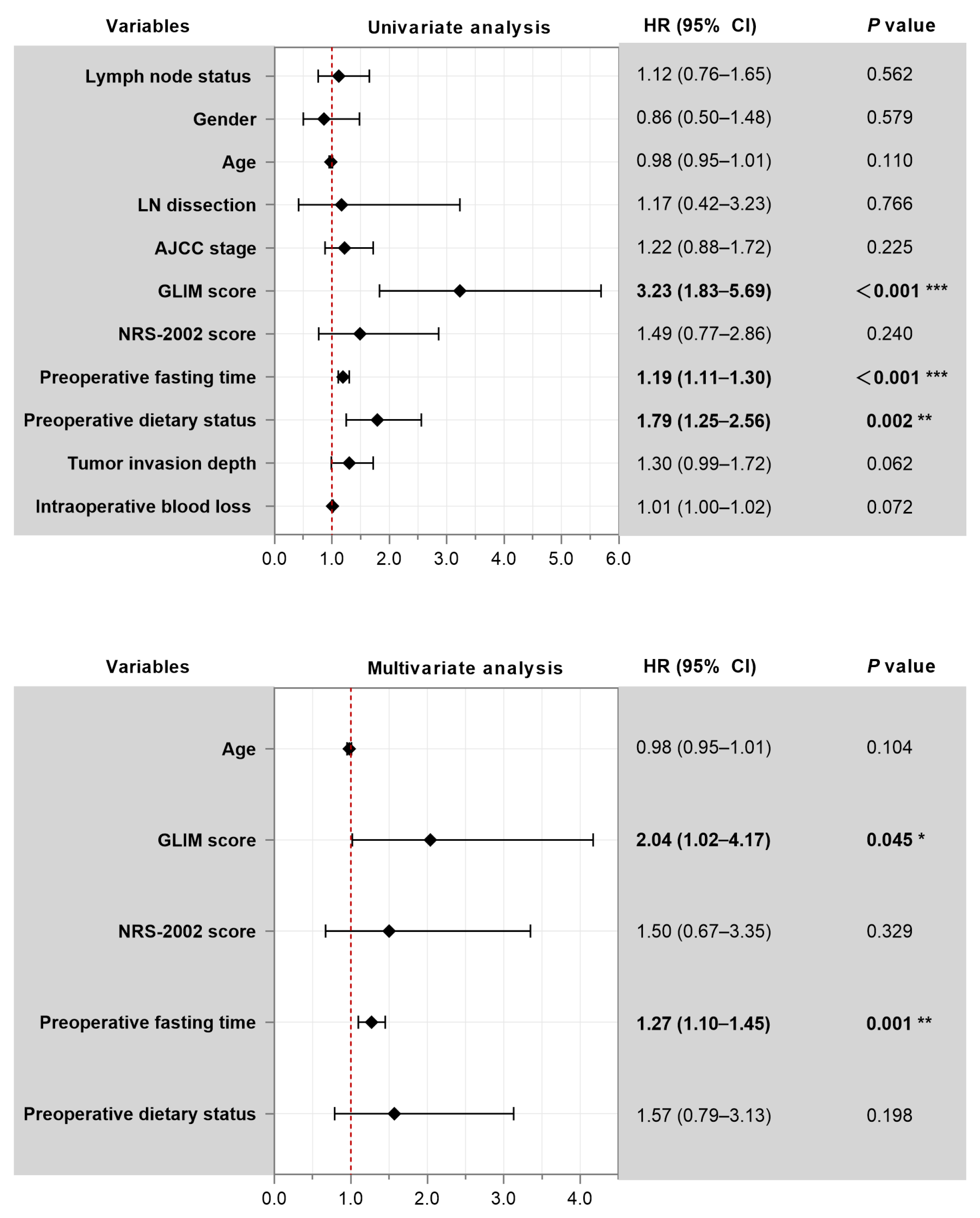
3.5. Predictors of Prolonged Gastric Tube Retention
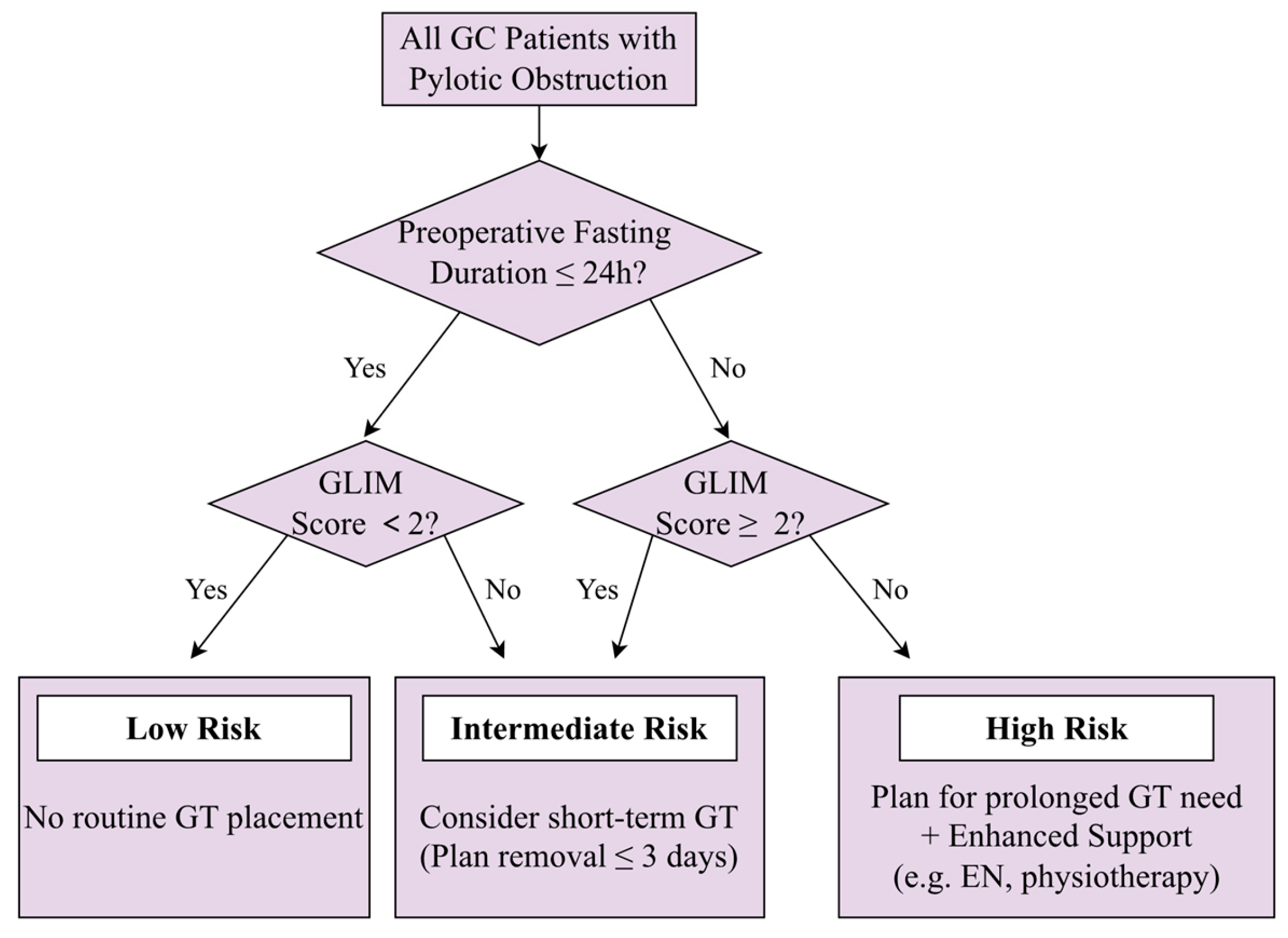
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GT | Gastric tube |
| GC | Gastric cancer |
| ERAS | Enhanced recovery after surgery |
| GLIM | Global leadership initiative on malnutrition |
| HR | Hazard ratio |
| CI | Confidence interval |
| FUSCC | Fudan University Shanghai Cancer Center |
| NRS-2002 | Nutritional Risk Screening 2002 |
| ASA | American Society of Anesthesiologists |
| NPO | Nil Per Os (Nothing by Mouth) |
| AJCC | American Joint Committee on Cancer |
| POD | Postoperative day |
| IQR | Interquartile range |
| GI | Gastrointestinal |
| EN | Enteral nutrition |
| RCT | Randomized controlled trial |
Appendix A
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Park, M.; Seo, K.W.; Min, J.S. Advances in Surgical Management of Malignant Gastric Outlet Obstruction. Cancers 2025, 17, 2567. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, I.S.; Siersema, P.D. Gastric Outlet Obstruction: Current Status and Future Directions. Gut Liver 2022, 16, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zeng, Y. Analysis of the safety and efficacy of laparoscopic gastrojejunostomy following neoadjuvant chemotherapy for gastric pyloric obstruction. Front. Oncol. 2025, 15, 1430761. [Google Scholar] [CrossRef]
- Rodríguez, J.I.; Kutscher, M.; Lemus, M.; Crovari, F.; Pimentel, F.; Briceño, E. Palliative gastrojejunostomy in unresectable cancer and gastric outlet obstruction: A retrospective cohort study. Ann. R. Coll. Surg. Engl. 2021, 103, 197–202. [Google Scholar] [CrossRef]
- Yasufuku, I.; Ohashi, M.; Makuuchi, R.; Hayami, M.; Ida, S.; Kumagai, K.; Sano, T.; Nunobe, S. High prevalence of peritoneal metastasis in gastric cancer presenting gastric outlet obstruction: A new candidate for consecutive diagnostic staging laparoscopy and laparoscopic gastrojejunostomy. Eur. J. Surg. Oncol. 2022, 48, 1746–1752. [Google Scholar] [CrossRef]
- Jang, S.; Stevens, T.; Lopez, R.; Bhatt, A.; Vargo, J.J. Superiority of gastrojejunostomy over endoscopic stenting for palliation of malignant gastric outlet obstruction. Clin. Gastroenterol. Hepatol. 2019, 17, 1295–1302.e1. [Google Scholar] [CrossRef]
- Kang, M.K.; Lee, H.J. Impact of malnutrition and nutritional support after gastrectomy in patients with gastric cancer. Ann. Gastroenterol. Surg. 2024, 8, 534–552. [Google Scholar] [CrossRef]
- Kosuga, T.; Tsujiura, M.; Nakashima, S.; Masuyama, M.; Otsuji, E. Current status of function-preserving gastrectomy for gastric cancer. Ann. Gastroenterol. Surg. 2021, 5, 278–286. [Google Scholar] [CrossRef]
- Carrère, N.; Seulin, P.; Julio, C.H.; Bloom, E.; Gouzi, J.L.; Pradère, B. Is nasogastric or nasojejunal decompression necessary after gastrectomy? A prospective randomized trial. World J. Surg. 2007, 31, 122–127. [Google Scholar] [CrossRef]
- Yoo, C.H.; Son, B.H.; Han, W.K.; Pae, W.K. Nasogastric decompression is not necessary in operations for gastric cancer: Prospective randomised trial. Eur. J. Surg. 2002, 168, 379–383. [Google Scholar] [CrossRef]
- Wei, Z.-W.; Li, J.-L.; Li, Z.-S.; Hao, Y.-T.; He, Y.-L.; Chen, W.; Zhang, C.-H. Systematic review of nasogastric or nasojejunal decompression after gastrectomy for gastric cancer. Eur. J. Surg. Oncol. 2014, 40, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, E.; Balducci, G.; Mercantini, P.; Giarnieri, E.; Giovagnoli, M.R.; Montagnini, M.; Proietti, A.; D’Urso, R.; Cavallini, M. Utility of nasogastric tube for medical and surgical oncology of gastric cancer: A prospective institutional study on a new and precious application of an old and economic device. Anticancer Res. 2018, 38, 433–439. [Google Scholar] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Takiguchi, S.; Takahashi, T.; Kurokawa, Y.; Makino, T.; Yamasaki, M.; Nakajima, K.; Mori, M.; Doki, Y. Treatment of gastric outlet obstruction that results from unresectable gastric cancer: Current evidence. World J. Gastrointest. Endosc. 2016, 8, 165–172. [Google Scholar] [CrossRef]
- Li, C.; Mei, J.W.; Yan, M.; Chen, M.M.; Yao, X.X.; Yang, Q.M.; Zhou, R.; Zhu, Z.G. Nasogastric decompression for radical gastrectomy for gastric cancer: A prospective randomized controlled study. Dig. Surg. 2011, 28, 167–172. [Google Scholar] [CrossRef]
- Wang, D.; Li, T.; Yu, J.; Hu, Y.; Liu, H.; Li, G. Is nasogastric or nasojejunal decompression necessary following gastrectomy for gastric cancer? A systematic review and meta-analysis of randomised controlled trials. J. Gastrointest. Surg. 2015, 19, 195–204. [Google Scholar] [CrossRef]
- Rossetti, G.; Fei, L.; Docimo, L.; Del Genio, G.; Micanti, F.; Belfiore, A.; Brusciano, L.; Moccia, F.; Cimmino, M.; Marra, T. Is nasogastric decompression useful in prevention of leaks after laparoscopic sleeve gastrectomy? A randomized trial. J. Investig. Surg. 2014, 27, 234–239. [Google Scholar] [CrossRef]
- Kimura, Y.; Yano, H.; Iwazawa, T.; Fujita, J.; Fujita, S. One-day nasogastric tube decompression after distal gastrectomy: A prospective randomized study. Surg. Today 2017, 47, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, W.G.; Vitale, G.C.; Mackie, C.R.; Cuschieri, A. Prophylactic postoperative nasogastric decompression a prospective study of its requirement and the influence of cimetidine in 200 patients. Ann. Surg. 1985, 202, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Nikniaz, Z.; Somi, M.H.; Naghashi, S. Malnutrition and weight loss as prognostic factors in the survival of patients with gastric cancer. Nutr. Cancer 2022, 74, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Z.; Zhang, X.Z.; Zhang, F.M.; Yu, D.Y.; Chen, W.H.; Lin, F.; Dong, Q.T.; Zhuang, C.L.; Yu, Z. Coexistence of GLIM-defined malnutrition and sarcopenia have negative effect on the clinical outcomes in the elderly gastric cancer patients after radical gastrectomy. Front. Nutr. 2022, 9, 960670. [Google Scholar] [CrossRef]
- Zheng, H.-L.; Lin, J.; Shen, L.-L.; Yang, H.-B.; Xu, B.-B.; Xue, Z.; Wu, D.; Huang, J.-B.; Lin, G.-S.; Zheng, C.-H.; et al. The GLIM criteria as an effective tool for survival prediction in gastric cancer patients. Eur. J. Surg. Oncol. 2023, 49, 964–973. [Google Scholar] [CrossRef]
- Deutz, N.E.; Ashurst, I.; Ballesteros, M.D.; Bear, D.E.; Cruz-Jentoft, A.J.; Genton, L.; Landi, F.; Laviano, A.; Norman, K.; Prado, C.M. The underappreciated role of low muscle mass in the management of malnutrition. J. Am. Med. Dir. Assoc. 2019, 20, 22–27. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Hajj, J.; Sizemore, B.; Singh, K. Impact of epigenetics, diet, and nutrition-related pathologies on wound healing. Int. J. Mol. Sci. 2024, 25, 10474. [Google Scholar] [CrossRef]
- Aldewachi, H.S.; Wright, N.A.; Appleton, D.R.; Watson, A.J. The effect of starvation and refeeding on cell population kinetics in the rat small bowel mucosa. J. Anat. 1975, 119, 105–121. [Google Scholar]
- Rao, W.; Zhang, X.; Zhang, J.; Yan, R.; Hu, Z.; Wang, Q. The role of nasogastric tube in decompression after elective colon and rectum surgery: A meta-analysis. Int. J. Color. Dis. 2011, 26, 423–429. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef]
| Characteristics | Value |
|---|---|
| Age (years, Mean ± SD) | 61.70 ± 10.92 |
| Gender (n, %) | |
| Male | 90 (67.67%) |
| Female | 43 (32.33%) |
| Lymph Node Metastasis (n, %) | |
| Present | 101 (75.9%) |
| Absent | 32 (24.1%) |
| Histopathological Type (n, %) | |
| Poorly differentiated adenocarcinoma | 103 (77.4%) |
| Moderately differentiated adenocarcinoma | 2 (1.5%) |
| Well-differentiated adenocarcinoma | 2 (1.5%) |
| Signet-ring cell carcinoma | 26 (19.6%) |
| AJCC 8th Stage (n, %) | |
| I | 19 (14.29%) |
| II | 28 (21.05%) |
| III | 78 (58.65%) |
| IV | 8 (6.02%) |
| Type of Surgery (n, %) | |
| Radical | 125 (93.98%) |
| Non-radical | 8 (6.02%) |
| LN Dissection (n, %) | |
| D1 | 10 (7.52%) |
| D2 | 123 (92.48%) |
| Postoperative GT Placement (n, %) | |
| Yes | 63 (47.37%) |
| No | 70 (52.63%) |
| Postoperative Hospitalization Duration (days, Mean ± SD) | 8.64 ± 3.45 |
| Total Hospitalization Cost (104 ¥, Mean ± SD) | 5.41 ± 1.17 |
| Characteristics | GT Group (n = 63) | Non-GT Group (n = 70) | p Value |
|---|---|---|---|
| Gender (n, %) | 0.611 | ||
| Male | 44 (69.84%) | 46 (65.71%) | |
| Female | 19 (30.16%) | 24 (34.29%) | |
| Age (n, %) | 0.502 | ||
| <60 years | 36 (57.14%) | 44 (62.86%) | |
| ≥60 years | 27 (42.86%) | 26 (37.14%) | |
| Lymph Node Metastasis | 0.199 | ||
| Present | 51 (81.0%) | 50 (71.4%) | |
| Absent | 12 (19.0%) | 20 (28.6%) | |
| ASA score (n, %) | 0.281 | ||
| 1 | 34 (53.97%) | 41 (58.57%) | |
| 2 | 19 (30.16%) | 24 (34.29%) | |
| 3 | 10 (15.87%) | 5 (7.14%) | |
| Comorbidities (n, %) | 0.886 | ||
| Yes | 26 (41.27%) | 24 (34.29%) | |
| No | 37 (58.73%) | 36 (65.71%) | |
| NRS-2002 score (n,%) | <0.001 | ||
| <3 | 0 (0%) | 20 (28.57%) | |
| ≥3 | 63 (100%) | 50 (71.43%) | |
| GLIM score (n, %) | |||
| <2 | 0 (0%) | 59 (84.29%) | <0.001 |
| ≥2 | 63 (100%) | 11 (15.71%) |
| Characteristics | GT Group (n = 63) | Non-GT Group (n = 70) | p Value |
|---|---|---|---|
| Hemoglobin (g/L) | 119.71 ± 27.22 | 122.81 ± 23.20 | 0.483 |
| Albumin (g/L) | 40.59 ± 4.41 | 41.15 ± 4.99 | 0.494 |
| Pre-Albumin (mg/dL) | 0.27 ± 0.16 | 0.24 ± 0.08 | 0.167 |
| AFP (μg/L) | 10.84 ± 48.84 | 7.62 ± 33.94 | 0.673 |
| CEA (μg/L) | 3.32 ± 2.85 | 9.78 ± 28.39 | 0.067 |
| CA199 (μ/mL) | 52.42 ± 137.09 | 72.32 ± 196.95 | 0.507 |
| CA50 (μ/mL) | 11.75 ± 19.63 | 31.55 ± 87.22 | 0.073 |
| CA125 (μ/mL) | 14.91 ± 12.01 | 15.39 ± 11.21 | 0.819 |
| CA72-4 (μ/mL) | 8.17 ± 12.87 | 12.19 ± 40.42 | 0.440 |
| CA242(μ/mL) | 19.36 ± 41.73 | 23.16 ± 49.48 | 0.640 |
| Characteristics | GT Group (n = 63) | Non-GT Group (n = 70) | p Value |
|---|---|---|---|
| Estimated Blood Loss (mL, Mean ± SD) | 126.56 ± 99.46 | 93.29 ± 45.45 | 0.019 |
| Lymphadenectomy Extent (n, %) | 0.627 | ||
| D1 | 4 (6.35%) | 6 (8.57%) | |
| D2 | 59 (93.65%) | 64 (91.43%) | |
| Resection Status (n, %) | 0.622 | ||
| 0 | 59 (93.65%) | 64 (91.43%) | |
| 1 | 0 (0.00%) | 1 (1.43%) | |
| 2 | 4 (6.35%) | 5 (7.14%) | |
| Reconstruction Method (n, %) | 0.160 | ||
| Billroth I | 7 (11.11%) | 3 (4.29%) | |
| Billroth II | 39 (61.90%) | 53 (75.71%) | |
| Roux-en-Y | 17 (26.98%) | 14 (20.00%) |
| Parameter | GT Group (n = 63) | Non-GT Group (n = 70) | p Value |
|---|---|---|---|
| Wound infection | 0 | 2 | |
| Intra-abdominal infection | 3 | 2 | |
| Ileus | 0 | 1 | |
| Chyle leakage | 1 | 0 | |
| Anastomotic leakage | 0 | 1 | |
| Gastroplegia | 4 | 2 | |
| Pulmonary complication | 2 | 3 | |
| Urinary tract infection | 2 | 2 | |
| Hepatic complication | 2 | 3 | |
| Total complications (n, %) | 14 (22.22%) | 16 (22.86%) | 0.945 |
| Mortality (n, %) | 1 (1.59%) | 0 (0%) | 0.290 |
| Resource Utilization Measure | Prolonged Retention (≥3 Days, n = 31) | Short-Term Retention (<3 Days, n = 32) | p Value |
|---|---|---|---|
| Preoperative Hospital Stay (days) | 5.00 ± 2.39 | 4.56 ± 1.98 | 0.434 |
| Postoperative Hospital Stay (days) | 9.90 ± 2.83 | 7.69 ± 1.38 | <0.001 |
| Total Hospital Stay (days) | 14.90 ± 3.79 | 12.25 ± 2.77 | 0.024 |
| Duration of antibiotic usage (days) | 4.19 ± 2.02 | 3.09 ± 0.59 | 0.006 |
| Total Hospitalization Cost (104 ¥) | 5.76 ± 0.82 | 5.17 ± 0.84 | 0.006 |
| Resource Utilization Measure | GT Group (n = 63) | Non-GT Group (n = 70) | p Value |
|---|---|---|---|
| Preoperative Hospital Stay (days) | 4.78 ± 2.19 | 4.99 ± 2.78 | 0.631 |
| Postoperative Hospital Stay (days) | 8.78 ± 2.47 | 8.01 ± 4.16 | 0.034 |
| Total Hospital Stay (days) | 13.56 ± 3.55 | 13.50 ± 5.38 | 0.442 |
| Duration of antibiotic usage (days) | 2.98 ± 0.14 | 2.98 ± 0.13 | 0.914 |
| Total Hospitalization Cost (104 ¥) | 5.46 ± 0.93 | 5.36 ± 1.36 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Xu, Y.; Liu, Y.; Kong, P.; Fang, Y.; Xu, D. Outcomes of Selective Versus Routine Gastric Tube Decompression After Gastrectomy for Gastric Cancer with Pyloric Obstruction: A Retrospective Cohort Study. J. Clin. Med. 2026, 15, 276. https://doi.org/10.3390/jcm15010276
Xu Y, Liu Y, Kong P, Fang Y, Xu D. Outcomes of Selective Versus Routine Gastric Tube Decompression After Gastrectomy for Gastric Cancer with Pyloric Obstruction: A Retrospective Cohort Study. Journal of Clinical Medicine. 2026; 15(1):276. https://doi.org/10.3390/jcm15010276
Chicago/Turabian StyleXu, Yonghu, Yushi Liu, Pengfei Kong, Yantian Fang, and Dazhi Xu. 2026. "Outcomes of Selective Versus Routine Gastric Tube Decompression After Gastrectomy for Gastric Cancer with Pyloric Obstruction: A Retrospective Cohort Study" Journal of Clinical Medicine 15, no. 1: 276. https://doi.org/10.3390/jcm15010276
APA StyleXu, Y., Liu, Y., Kong, P., Fang, Y., & Xu, D. (2026). Outcomes of Selective Versus Routine Gastric Tube Decompression After Gastrectomy for Gastric Cancer with Pyloric Obstruction: A Retrospective Cohort Study. Journal of Clinical Medicine, 15(1), 276. https://doi.org/10.3390/jcm15010276







