Correlated Expression of Notch2 and ADAM17 in Primary Sjögren’s Syndrome Salivary Glands
Abstract
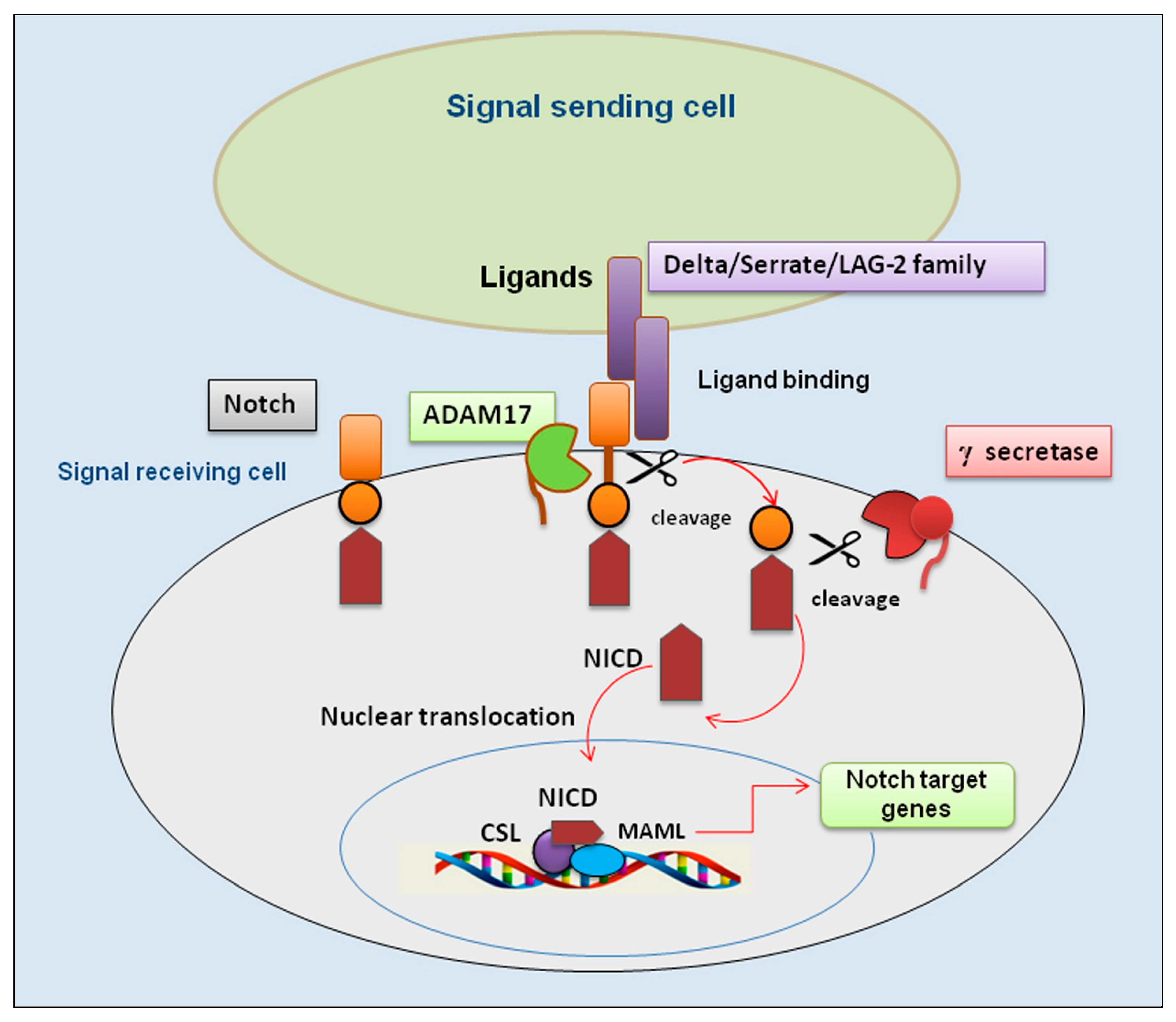
1. Introduction
2. Materials and Methods
2.1. Patients, Clinical Specimens
2.2. Notch2 mRNA ISH Assay
2.3. Notch2 Protein IHC
2.4. ADAM17 Protein IHC
2.5. IHC Analysis and Quantification
2.6. Aperio Digital ISH and IHC Analysis and Quantification
2.7. Statistic
3. Results
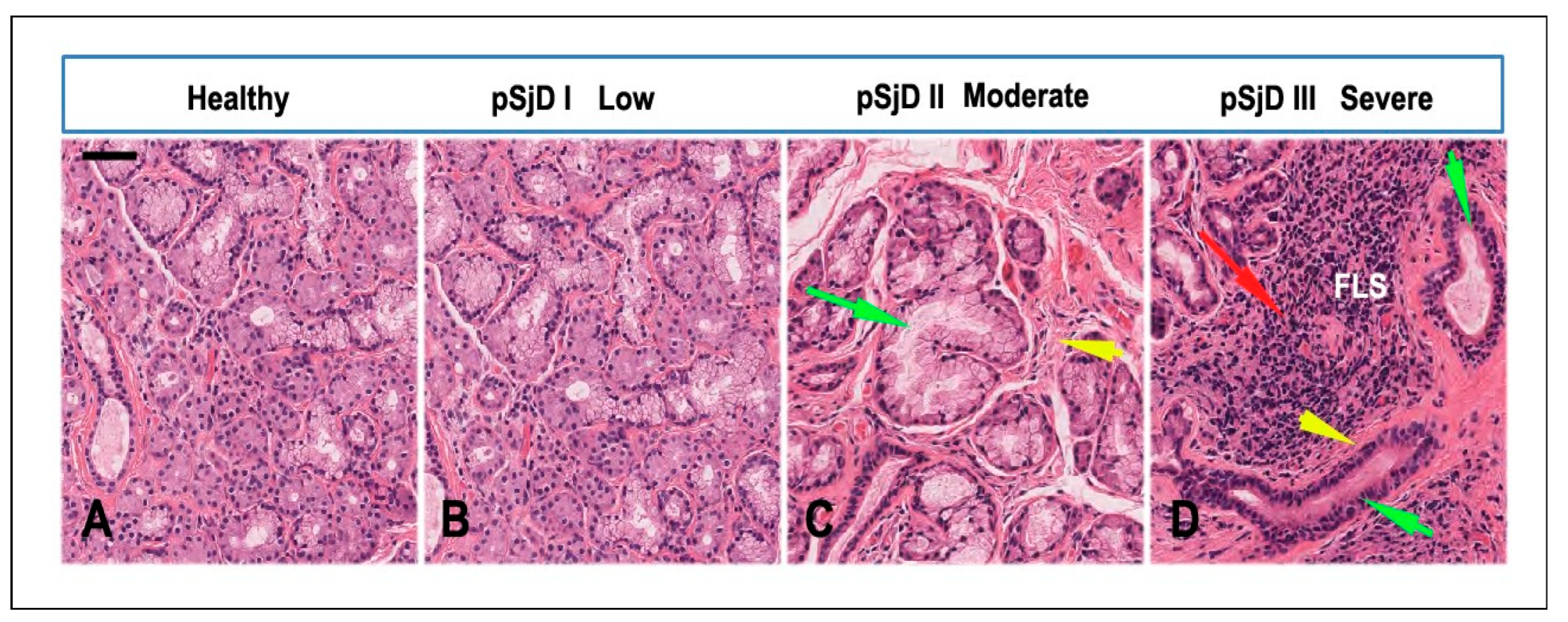
3.1. SjD Histopathology of the Bioptic SGs Specimens
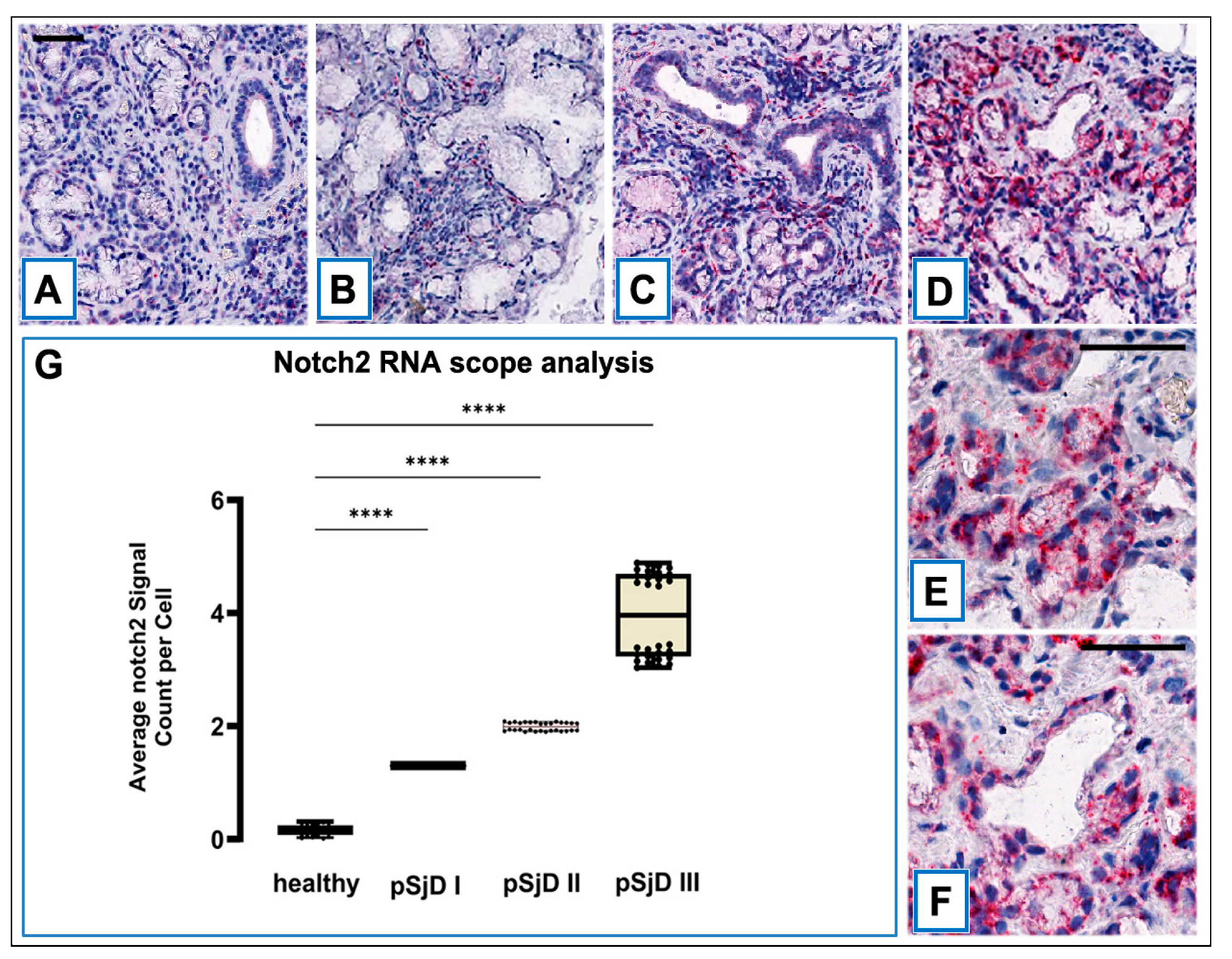
3.2. Notch2 mRNA Detection in SjD SGs by ISH
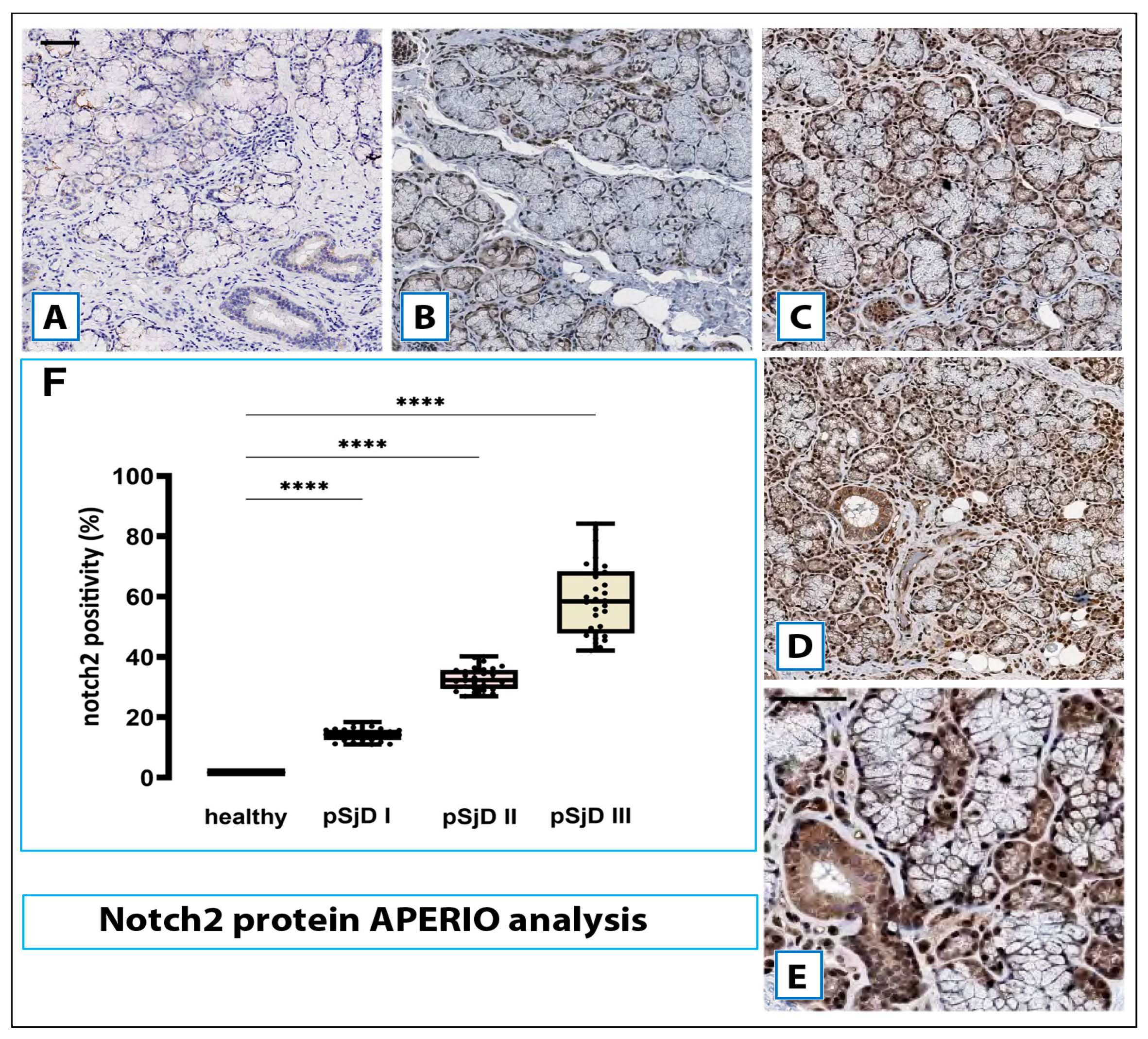
3.3. Notch2 Protein Expression in SjD SGs Biopsies Was Correlated with the Degree of Inflammation
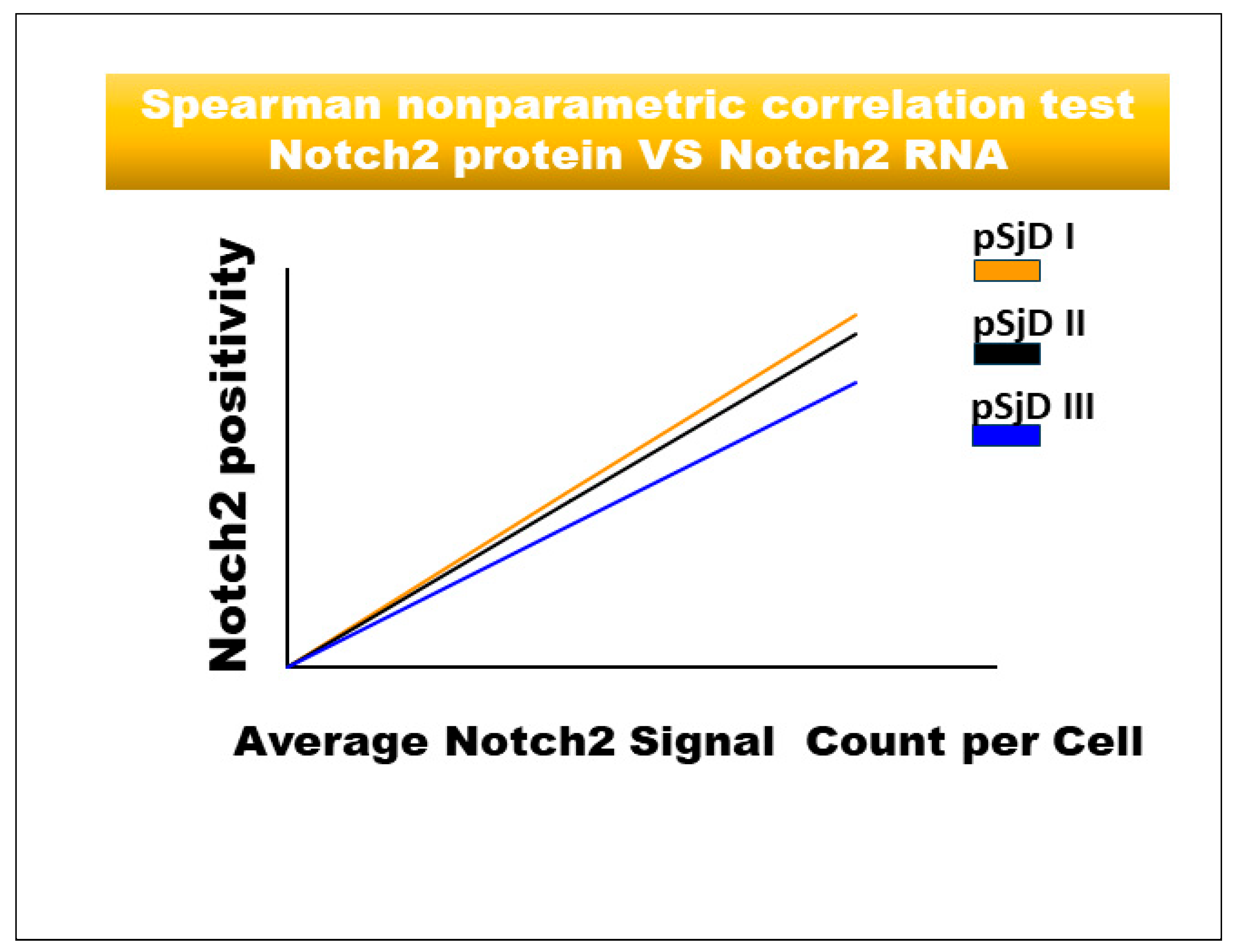
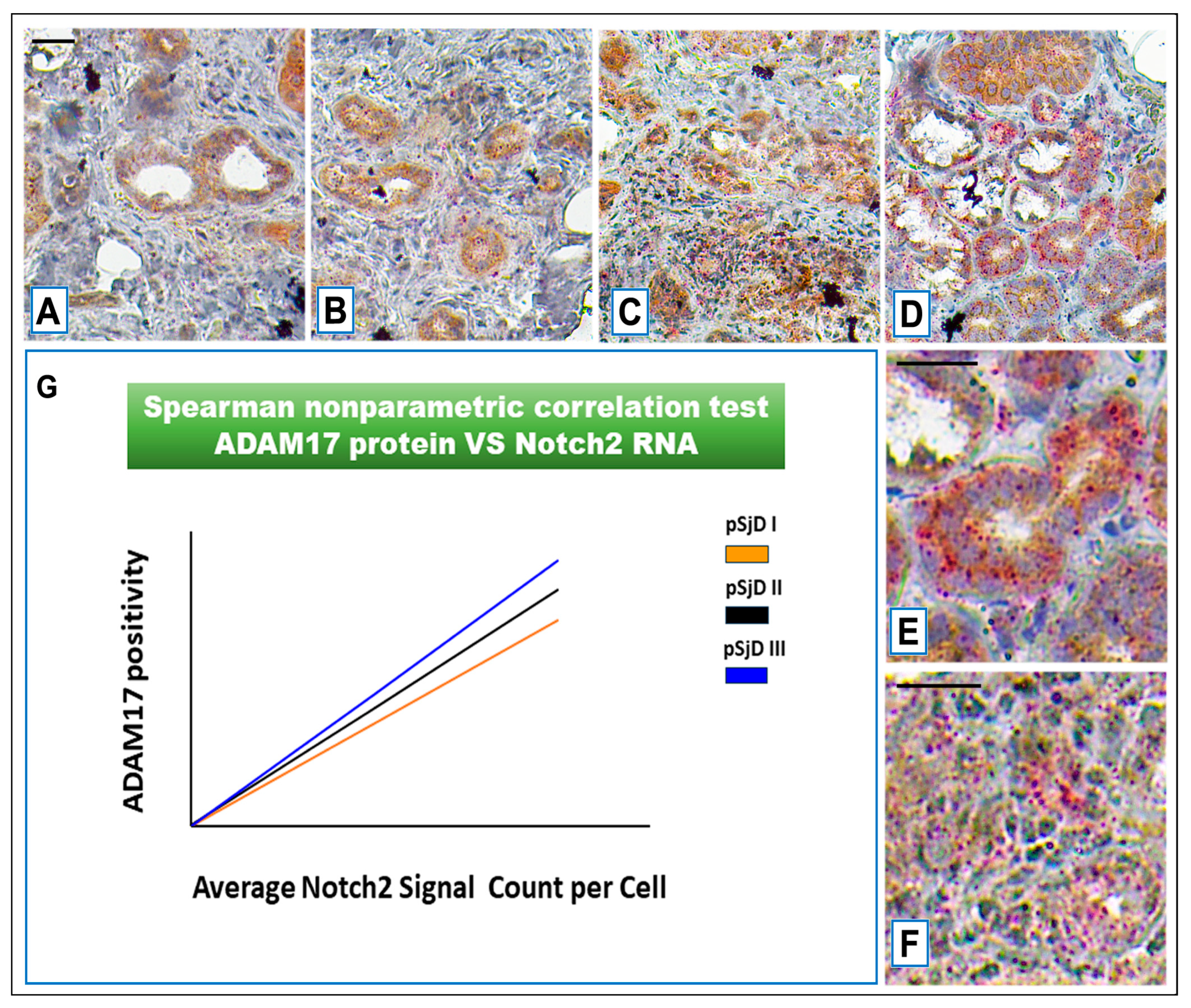
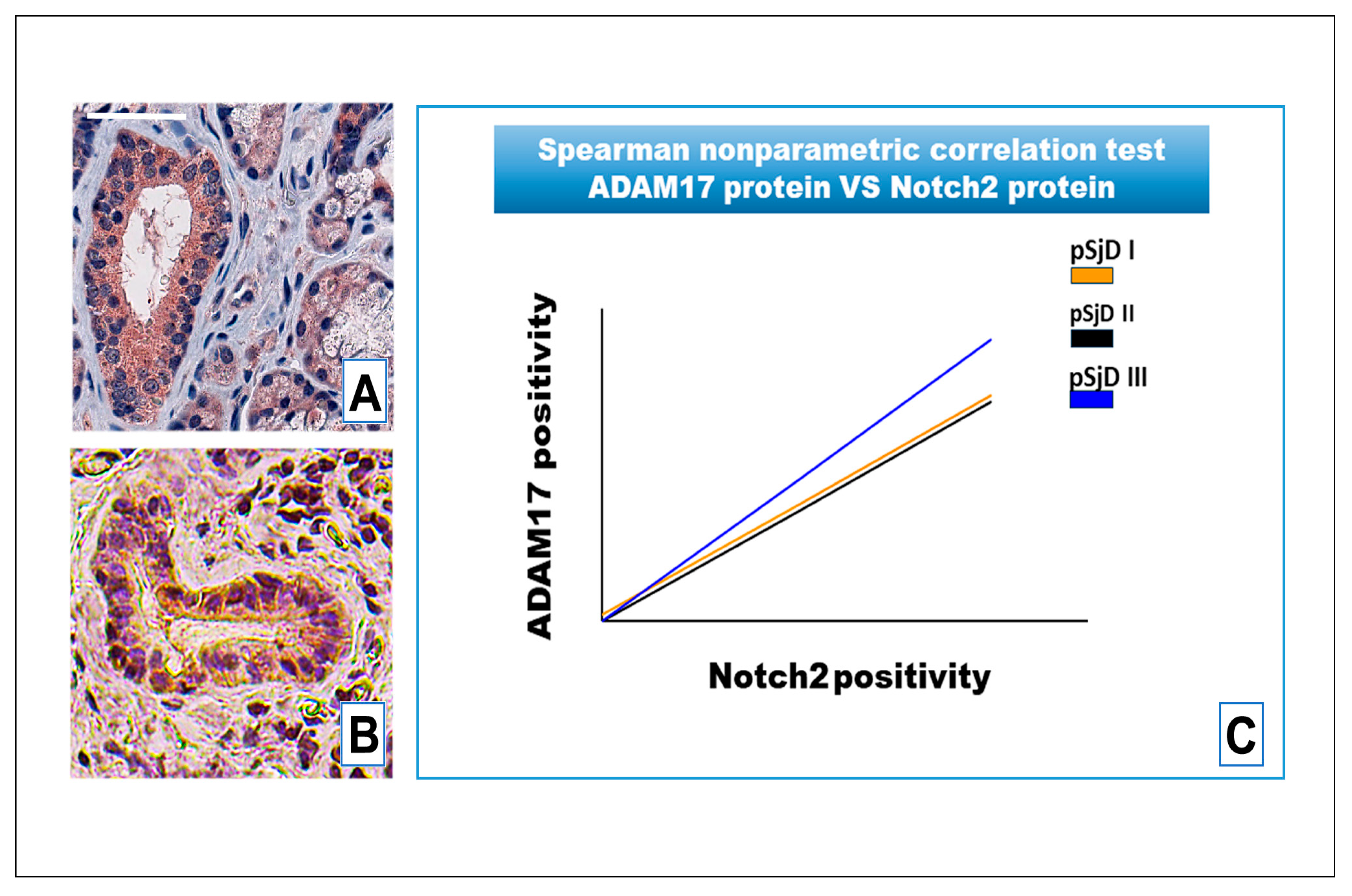
3.4. Notch2 Expression Correlates with ADAM17 Expression in SjD SGs Tissue
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parambath, S.; Selvraj, N.R.; Venugopal, P.; Aradhya, R. Notch Signaling: An Emerging Paradigm in the Pathogenesis of Reproductive Disorders and Diverse Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 5423. [Google Scholar] [CrossRef]
- Sachan, N.; Sharma, V.; Mutsuddi, M.; Mukherjee, A. Notch signalling: Multifaceted role in development and disease. FEBS J. 2024, 291, 3030–3059. [Google Scholar] [CrossRef] [PubMed]
- Bray, S.J. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef]
- Condorelli, A.G.; El Hachem, M.; Zambruno, G.; Nystrom, A.; Candi, E.; Castiglia, D. Notching up knowledge on molecular mechanisms of skin fibrosis: Focus on the multifaceted Notch signalling pathway. J. Biomed. Sci. 2021, 28, 36. [Google Scholar] [CrossRef] [PubMed]
- Tsao, P.N.; Matsuoka, C.; Wei, S.C.; Sato, A.; Sato, S.; Hasegawa, K.; Chen, H.K.; Ling, T.Y.; Mori, M.; Cardoso, W.V.; et al. Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proc. Natl. Acad. Sci. USA 2016, 113, 8242–8247. [Google Scholar] [CrossRef]
- Bae, S.E.; Park, S.H.; Kim, C.Y.; Lee, C.R.; Lee, C.; Payumo, R.M.; Kim, S.Y.; Sim, K.Y.; Kim, H.J.; Seo, H.; et al. Notch2-expressing CD4+ T cells attain immunoregulatory functions during autoimmune inflammation. Cell Mol. Immunol. 2025, 22, 1077–1092. [Google Scholar] [CrossRef]
- Fan, H.M.; Qiao, Y.L.; Liu, Y.; Xu, S.; Ni, H.F.; Jiao, W.E.; Tao, Z.Z.; Chen, S.M. Long-term consequences of regulatory T-cell-specific knockout of Notch2 in immune homeostasis. Int. Immunopharmacol. 2023, 124, 111069. [Google Scholar] [CrossRef]
- Quillard, T.; Devallière, J.; Coupel, S.; Charreau, B. Interestingly, Inflammation dysregulates Notch signaling in endothelial cells (implication of Notch2 and Notch4 to endothelial dysfunction. Biochem. Pharmacol. 2010, 80, 2032–2041. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S. Molecular cues for development and regeneration of salivary glands. Histol. Histopathol. 2014, 29, 305–312. [Google Scholar]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Ishii, H.; Nakamura, H.; Yoshino, S.I.; Fukamizu, A.; Nishioka, K.; Nakajima, T. NFkappaB2 (p52) promoter activation via Notch signaling pathway in rheumatoid synoviocytes. Int. J. Mol. Med. 2001, 7, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Kanazawa, S.; Tetsuka, T.; Ohta, S.; Jiang, X.; Tada, T.; Kobayashi, M.; Matsui, N.; Okamoto, T. Induction of Notch signaling by tumor necrosis factor in rheumatoid synovial fibroblasts. Oncogene 2003, 22, 7796–7803. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, W.; Guo, M.; Zhang, T.; Chen, L.; Wang, Y.; You, H.; Li, J. Expression analysis of Notch-related molecules in peripheral blood T helper cells of patients with rheumatoid arthritis. Scand. J. Rheumatol. 2010, 39, 26–32. [Google Scholar] [CrossRef]
- Yabe, Y.; Matsumoto, T.; Tsurumoto, T.; Shindo, H. Immunohistological localization of Notch receptors and their ligands Delta and Jagged in synovial tissues of rheumatoid arthritis. J. Orthop. Sci. 2005, 10, 589–594. [Google Scholar] [CrossRef]
- Ishii, H.; Nakazawa, M.; Yoshino, S.; Nakamura, H.; Nishioka, K.; Nakajima, T. Expression of notch homologues in the synovium of rheumatoid arthritis and osteoarthritis patients. Rheumatol. Int. 2001, 21, 10–14. [Google Scholar] [CrossRef]
- Nakazawa, M.; Ishii, H.; Aono, H.; Takai, M.; Honda, T.; Aratani, S.; Fukamizu, A.; Nakamura, H.; Yoshino, S.; Kobata, T.; et al. Role of Notch-1 intracellular domain in activation of rheumatoid synoviocytes. Arthritis Rheum. 2001, 44, 1545–1554. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.H.; Kim, K.; Jin, C.H.; Choi, K.Y.; Jang, J.; Choi, Y.; Gwon, A.R.; Baik, S.H.; Yun, U.J.; et al. Inhibition of notch signalling ameliorates experimental inflammatory arthritis. Ann. Rheum. Dis. 2015, 74, 267–274. [Google Scholar] [CrossRef]
- Murea, M.; Park, J.K.; Sharma, S.; Kato, H.; Gruenwald, A.; Niranjan, T.; Si, H.; Thomas, D.B.; Pullman, J.M.; Melamed, M.L.; et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int. 2010, 78, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, W.; Xiong, S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J. Immunol. 2010, 184, 6465–6478. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Tomcik, M.; Zerr, P.; Akhmetshina, A.; Horn, A.; Palumbo, K.; Beyer, C.; Zwerina, J.; Distler, O.; Schett, G.; et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann. Rheum. Dis. 2011, 70, 1304–1310. [Google Scholar] [CrossRef]
- Horai, Y.; Kurushima, S.; Shimizu, T.; Nakamura, H.; Kawakami, A. A Review of the Current Clinical Aspects of Sjögren’s Disease: Geographical Difference, Classification/Diagnostic Criteria, Recent Advancements in Diagnostic Methods, and Molecular Targeted Therapy. J. Clin. Med. 2025, 14, 5577. [Google Scholar] [CrossRef]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef]
- Sens, D.A.; Hintz, D.S.; Rudisill, M.T.; Sens, M.A.; Spicer, S.S. Explant culture of human submandibular gland epithelial cells: Evidence for ductal origin. Lab. Investig. 1985, 2, 559–567. [Google Scholar]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Kovall, R.A.; Blacklow, S.C. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr. Top. Dev. Biol. 2010, 92, 31–71. [Google Scholar]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Sig. Transduct. Target Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Yakovleva, S.; Knyazeva, A.; Yunusova, A.; Allayarova, E.; Lanshakov, D.; Malashicheva, A.; Shnaider, T. Functional Divergence of NOTCH1 and NOTCH2 in Human Cerebral Organoids Reveals Receptor-Specific Roles in Early Corticogenesis. Int. J. Mol. Sci. 2025, 26, 7309. [Google Scholar] [CrossRef]
- Lisi, S.; D’Amore, M.; Sisto, M. ADAM17 at the interface between inflammation and autoimmunity. Immunol. Lett. 2014, 162, 159–169. [Google Scholar] [CrossRef]
- Sisto, M.; Lisi, S.; Lofrumento, D.D.; D‘Amore, M.; Frassanito, M.A.; Ribatti, D. Sjögren’s syndrome pathological neovascularization is regulated by VEGF-A-stimulated TACE-dependent crosstalk between VEGFR2 and NF-κB. Genes Immun. 2012, 13, 411–420. [Google Scholar] [CrossRef]
- Le Pottier, L.; Devauchelle, V.; Fautrel, A.; Daridon, C.; Saraux, A.; Youinou, P.; Pers, J.O. Ectopic germinal centers are rare in Sjogren’s syndrome salivary glands and do not exclude autoreactive B cells. J. Immunol. 2009, 182, 3540–3547. [Google Scholar] [CrossRef]
- Guerrier, T.; Le Pottier, L.; Devauchelle, V.; Pers, J.-O.; Jamin, C.; Youinou, P. Role of Toll-like receptors in primary Sjögren’s syndrome with a special emphasis on B-cell maturation within exocrine tissues. J. Autoimmun. 2012, 39, 69–76. [Google Scholar] [CrossRef]
- Rossi, D.; Trifonov, V.; Fangazio, M.; Bruscaggin, A.; Rasi, S.; Spina, V.; Monti, S.; Vaisitti, T.; Arruga, F.; Famà, R.; et al. The coding genome of splenic marginal zone lymphoma: Activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 2012, 209, 1537–1551. [Google Scholar] [CrossRef]
- Kuksin, C.A.; Minter, L.M. The Link between Autoimmunity and Lymphoma: Does NOTCH Signaling Play a Contributing Role? Front. Oncol. 2015, 5, 51. [Google Scholar] [CrossRef]
- Gao, J.; Fan, L.; Zhao, L.; Su, Y. The interaction of Notch and Wnt signaling pathways in vertebrate regeneration. Cell Regen. 2021, 10, 11. [Google Scholar] [CrossRef]
- Varelas, X.; Wrana, J.L. Coordinating developmental signaling: Novel roles for the Hippo pathway. Trends Cell Biol. 2012, 22, 88–96. [Google Scholar] [CrossRef]
- Huang, J.; Tang, J.; Zhang, C.; Liu, T.; Deng, Z.; Liu, L. Single-cell transcriptomic analysis uncovers heterogeneity in the labial gland microenvironment of primary Sjögren’s syndrome. J. Transl. Autoimmun. 2024, 9, 100248. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Dolcino, M.; Del Papa, N.; Minniti, A.; Pignataro, F.; Maglione, W.; Lunardi, C.; Puccetti, A. Gene expression profiles in primary Sjögren’s Syndrome with and without systemic manifestations. ACR Open Rheumatol. 2019, 1, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Peck, A.B.; Nguyen, C.Q.; Ambrus, J.L., Jr. A MZB Cell Activation Profile Present in the Lacrimal Glands of Sjögren’s Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice Defined by Global RNA Transcriptomic Analyses. Int. J. Mol. Sci. 2022, 23, 6106. [Google Scholar] [CrossRef] [PubMed]
| Domain | Healthy | pSjD I | pSjD II | pSjD III |
|---|---|---|---|---|
| Age at diagnosis, years | 49.8 (range 21–66) | 57.7 (range 24.6–66.9) | 52.4 (range 24.8–66.7) | 52.9 (range 38.9–70.1) |
| Sex | female | female | female | female |
| Focus scores | negative | low lesions (FS ≤ 1) | moderate lesions (FS ≥ 2) | severe lesions FS ≥ 2 and GC+ |
| Anti-Ro positivity n (%) | negative | positive | positive | positive |
| Schirmer test ≤ 5 mm/5 min in at least 1 eye | negative | positive | positive | positive |
| Clinical parameters | ||||
| Unstimulated salivary flow (mL/5 min) | negative | mild hypofunction (>0.7 mL/min) | moderate hypofunction (0.1 to 0.7 mL/min) | severe hypofunction (>0.1 mL/min) |
| Rheumatoid factor | negative | positive | positive | positive |
| Anti-SSA antibody | negative | positive | positive | positive |
| Anti-SSB antibody | negative | positive | positive | positive |
| Anti-RNP antibody | negative | positive | positive | positive |
| Anti-centromere antibody | negative | negative | positive | positive |
| Anti-DNA antibody | negative | negative | negative | negative |
| Patient-reported outcomes | ||||
| Pain | negative | absence | presence | presence |
| Fatigue | negative | absence | presence | presence |
| Overall dryness | ocular and oral dryness | ocular and oral dryness | ocular and oral dryness | ocular and oral dryness |
| Systemic manifestations according to ESSDAI domains | ||||
| Glandular | normal salivary glands | unilateral salivary gland enlargement | swelling of mostly the parotid gland | swelling of mostly the parotid gland; diffuse sialectasis |
| Articular | absence of articular events | absence | arthralgia; arthritis | arthralgia; arthritis |
| Muscular | absence of muscular pains | muscle weakness | muscle weakness; myositis | myalgia; muscle weakness; myositis |
| Renal | absence | absence | absence | nephrogenic diabetes insipidus; proximal tubular acidosis; hypokalemia |
| Peripherical nervous system | absence | absence of neuronal pathological events | painful in the distal extremities; radiculoneuropathy; autonomic neuropathy | sensory ataxic neuropathy; cranial neuropathies; radiculoneuropathy; |
| Central nervous system | absence | absence of neuronal pathological events | motor or sensory deficits | spinal cord involvement cognitive dysfunction |
| Lymphadenopathy | absence | absence | splenomegaly | splenomegaly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sisto, M.; Lisi, S.; Tamma, R.; De Giorgis, M.; Ingravallo, G.; Della Mura, M.; Sorino, J.; Cascardi, E.; Ribatti, D. Correlated Expression of Notch2 and ADAM17 in Primary Sjögren’s Syndrome Salivary Glands. J. Clin. Med. 2026, 15, 182. https://doi.org/10.3390/jcm15010182
Sisto M, Lisi S, Tamma R, De Giorgis M, Ingravallo G, Della Mura M, Sorino J, Cascardi E, Ribatti D. Correlated Expression of Notch2 and ADAM17 in Primary Sjögren’s Syndrome Salivary Glands. Journal of Clinical Medicine. 2026; 15(1):182. https://doi.org/10.3390/jcm15010182
Chicago/Turabian StyleSisto, Margherita, Sabrina Lisi, Roberto Tamma, Michelina De Giorgis, Giuseppe Ingravallo, Mario Della Mura, Joana Sorino, Eliano Cascardi, and Domenico Ribatti. 2026. "Correlated Expression of Notch2 and ADAM17 in Primary Sjögren’s Syndrome Salivary Glands" Journal of Clinical Medicine 15, no. 1: 182. https://doi.org/10.3390/jcm15010182
APA StyleSisto, M., Lisi, S., Tamma, R., De Giorgis, M., Ingravallo, G., Della Mura, M., Sorino, J., Cascardi, E., & Ribatti, D. (2026). Correlated Expression of Notch2 and ADAM17 in Primary Sjögren’s Syndrome Salivary Glands. Journal of Clinical Medicine, 15(1), 182. https://doi.org/10.3390/jcm15010182











