Cardio-Oncology Challenges in Elderly Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cardio-Oncologic Assessment
2.3. Clinical Characteristics of the Study Population
- Group 1: subjects with recent cancer diagnosis and naïve for anticancer regimens.
- Group 2: subjects undergoing antineoplastic protocols.
- Group 3: subjects who had already finished anticancer treatments.
2.4. Geriatric Evaluation
- -
- The Mini-Mental State Examination (MMSE) for the evaluation of cognitive impairment [27];
- The Geriatric Depression Scale (GDS) for the evaluation of depression [28];
- The Cumulative Illness Rating Scale C (CIRS-C) for comorbidity [29];
- The Cumulative Illness Rating Scale G (CIRS-G) for severity [29];
- The number of drugs used;
- A Mini Nutritional Assessment (MNA) for the evaluation of the risk of malnutrition [32];
- The Tinetti Scale for balance and gait evaluation [33];
- The Short Physical Performance Battery (SPPB) [34];
- A Physical Activity Scale for the Elderly (PASE) [35];
- A Social Support Assessment (SSA) [36];
- Subgroup A: survived patients;
- Subgroup B: patients who died during follow-up.
2.5. Follow-Up Visits
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. Cardiovascular Characteristics of Patients
3.2. Cancer Characteristics and Protocols
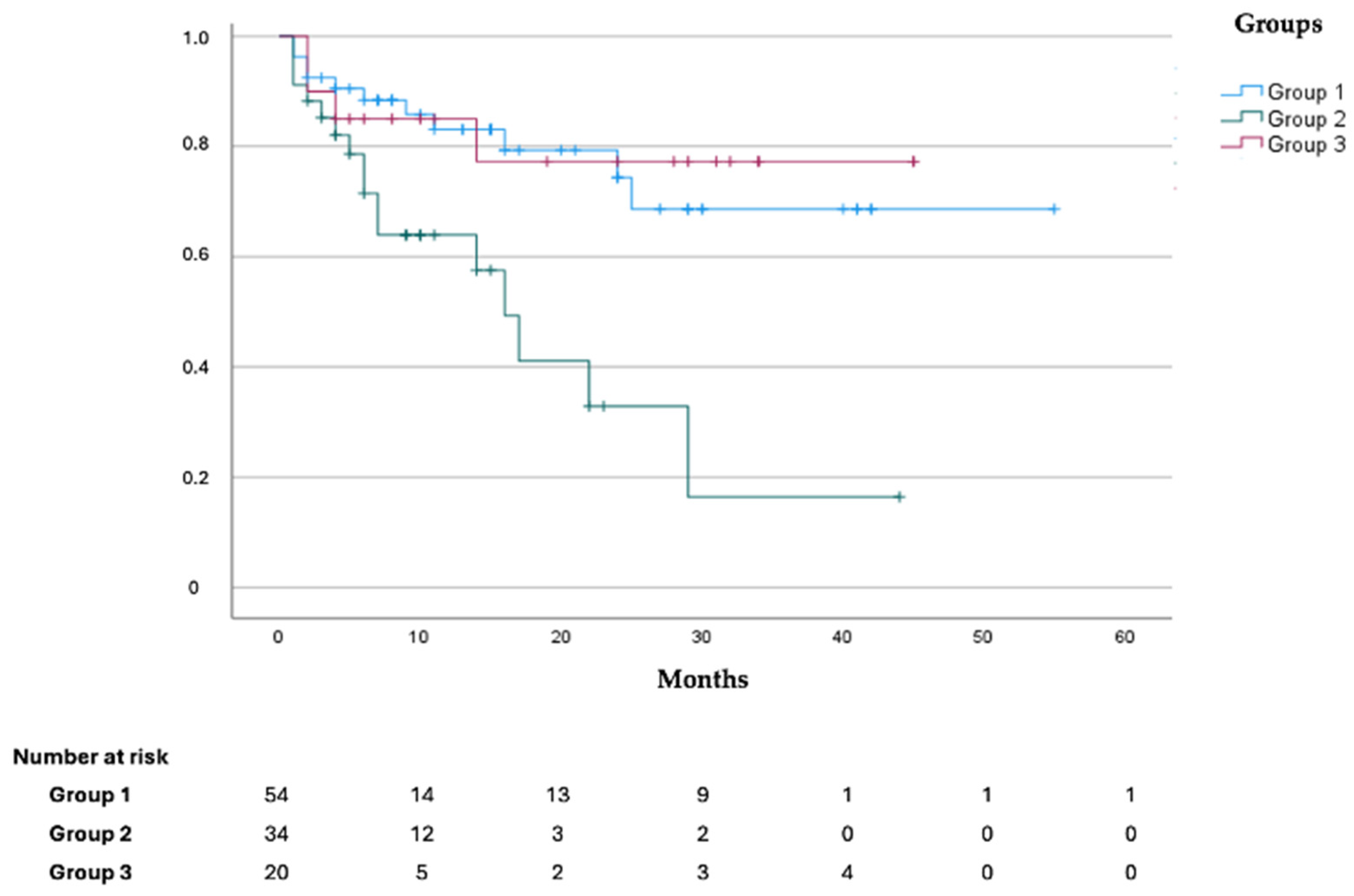
3.3. Outcomes
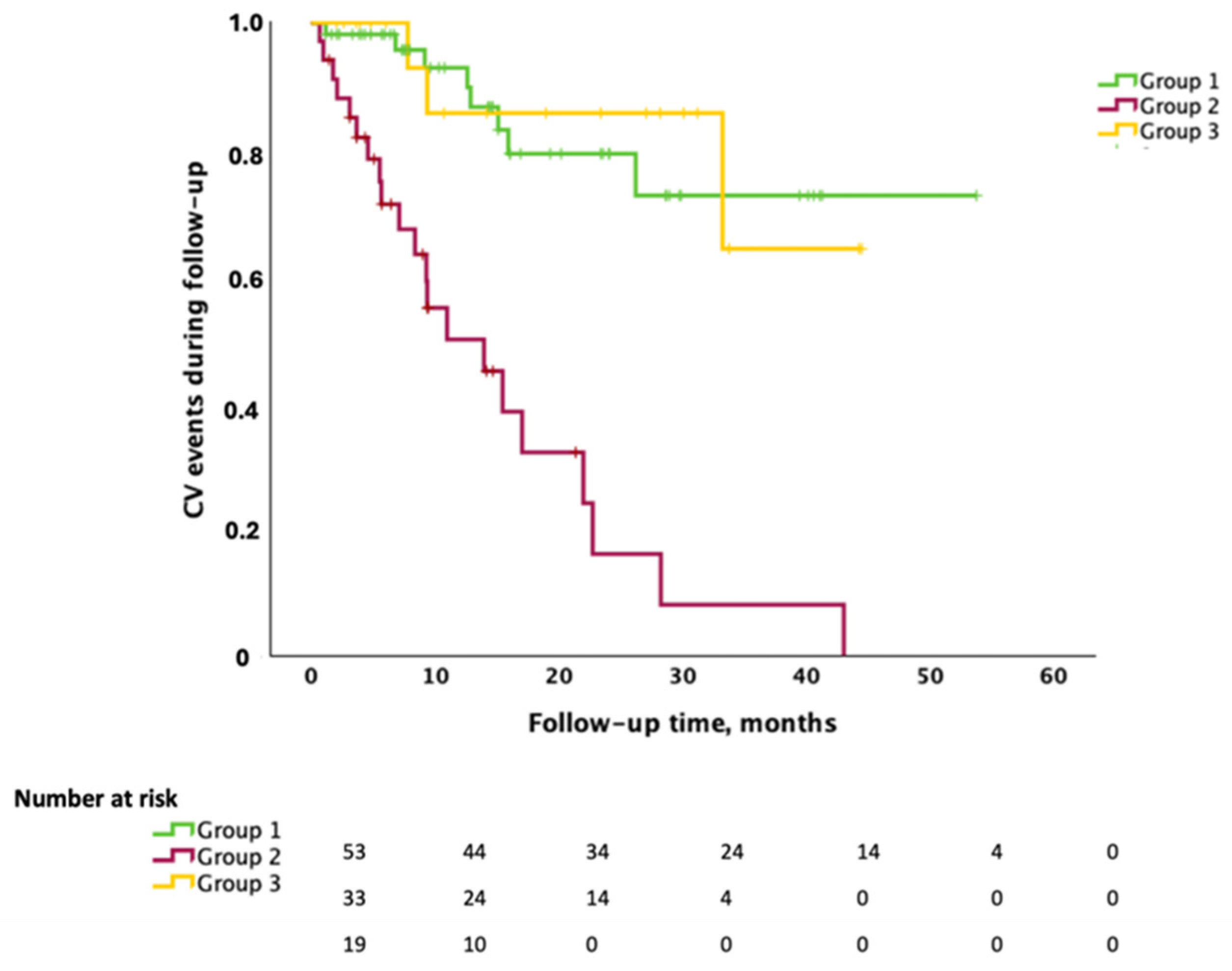
3.4. Cardiovascular Events During Follow-Up and Treatment Implementations
3.5. Comprehensive Geriatric Assessment
3.6. CGA and Echocardiographic Measurements
3.7. Correlation Between Cardiac Function Indicators and CGA Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BADL | Basic Activity of Daily Living; IADL |
| BMI | body mass index |
| CIRS-C | Cumulative Illness Rating Scale Cumulative—Comorbidities |
| CIRS-G | Cumulative Illness Rating Scale Cumulative—Geriatric |
| COPD | chronic obstructive pulmonary disease |
| Dec Time | deceleration time |
| GDS | Geriatric Depression Scale |
| IADL | Instrumental Activity of Daily Living |
| IDd | internal diameter during diastole |
| IFI | Italian fragility index |
| LAVi | left atrial volume indexed |
| LV | Left ventricle |
| LV-EDVi | left ventricular-end diastolic volume indexed |
| LV-EF | left ventricular ejection fraction |
| MMSE | Mini-Mental State Examination |
| MNA | Mini Nutritional Assessment |
| NYHA-FC | New York Association-Functional Class |
| PASE | Physical Activity Scale for the Elderly |
| PASP | pulmonary arterial systolic pressure |
| PWTd | posterior wall thickness during diastole |
| RWT | relative wall thickness |
| SPPB | Short Performance Physical Battery |
| SSA | Social Support Assessment |
| STd | septum thickness during diastole |
| TAPSE | Tricuspid annular plane systolic excursion |
| TINETTI | Tinetti Scale for balance and gait evaluation |
References
- Anker, M.S.; Von Haehling, S.; Landmesser, U.; Coats, A.J.S.; Anker, S.D. Cancer and heart failure-more than meets the eye: Common risk factors and co-morbidities. Eur. J. Heart Fail. 2018, 20, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- De Boer, R.A.; Meijers, W.C.; Van Der Meer, P.; Van Veldhuisen, D.J. Cancer and heart disease: Associations and relations. Eur. J. Heart Fail. 2019, 21, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Kobold, S. Inflammation: A common contributor to cancer, aging, and cardiovascular diseases-expanding the concept of cardio-oncology. Cardiovasc. Res. 2019, 115, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; De Boer, R.A. Common risk factors for heart failure and cancer. Cardiovasc. Res. 2019, 115, 844–853. [Google Scholar] [CrossRef]
- Scotte, F.; Bossi, P.; Carola, E.; Cudennec, T.; Dielenseger, P.; Gomes, F.; Knox, S.; Strasser, F. Addressing the quality of life needs of older patients with cancer: A SIOG consensus paper and practical guide. Ann. Oncol. 2018, 29, 1718–1726. [Google Scholar] [CrossRef]
- Russo, C.; Giannotti, C.; Signori, A.; Cea, M.; Murialdo, R.; Ballestrero, A.; Scabini, S.; Romairone, E.; Odetti, P.; Nencioni, A.; et al. Predictive values of two frailty screening tools in older patients with solid cancer: A comparison of SAOP2 and G8. Oncotarget 2018, 9, 35056–35068. [Google Scholar] [CrossRef]
- Abete, P.; Basile, C.; Bulli, G.; Curcio, F.; Liguori, I.; Della-Morte, D.; Gargiulo, G.; Langellotto, A.; Testa, G.; Galizia, G.; et al. The Italian version of the “frailty index” based on deficits in health: A validation study. Aging Clin. Exp. Res. 2017, 29, 913–926. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rickett, M.O.; Rockwood, K. Frailty in Elderly. Lancet 2014, 381, 752–762. [Google Scholar] [CrossRef]
- Handforth, C.; Clegg, A.; Young, A.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2014, 26, 1091–1101. [Google Scholar] [CrossRef]
- Denfeld, Q.E.; Winters-Stone, K.; Mudd, J.O.; Gelow, J.M.; Kurdi, S.; Lee, C.S. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 236, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jhund, P.S.; Petrie, M.C.; Claggett, B.L.; Barlera, S.; Cleland, J.G.F.; Dargie, H.J.; Granger, C.B.; Kjekshus, J.; Kober, L.; et al. Declining Risk of Sudden Death in Heart Failure. N. Engl. J. Med. 2017, 377, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Canoy, D.; Tran, J.; Pinho-Gomes, A.C.; Millett, E.R.C.; Salimi-Khorshidi, G.; Cleland, J.G.; Mcmurray, J.J.V.; Rahimi, K. Temporal Trends and Patterns in Mortality After Incident Heart Failure: A Longitudinal Analysis of 86000 Individuals. JAMA Cardiol. 2019, 4, 1102–1111. [Google Scholar] [CrossRef]
- Moliner, P.; Lupon, J.; De Antonio, M.; Domingo, M.; Santiago-Vacas, E.; Zamora, E.; Cediel, G.; Santesmases, J.; Diez-Quevedo, C.; Troya, M.I.; et al. Trends in modes of death in heart failure over the last two decades: Less sudden death but cancer deaths on the rise. Eur. J. Heart Fail. 2019, 21, 1259–1266. [Google Scholar] [CrossRef]
- Perez, I.E.; Taveras Alam, S.; Hernandez, G.A.; Sancassani, R. Cancer Therapy-Related Cardiac Dysfunction: An Overview for the Clinician. Clin. Med. Insights Cardiol. 2019, 13, 1179546819866445. [Google Scholar] [CrossRef]
- Bertero, E.; Ameri, P.; Maack, C. Bidirectional Relationship Between Cancer and Heart Failure: Old and New Issues in Cardio-oncology. Card. Fail. Rev. 2019, 5, 106–111. [Google Scholar] [CrossRef]
- Parker, S.G.; McCue, P.; Phelps, K.; McCleod, A.; Arora, S.; Nockels, K.; Kennedy, S.; Roberts, H.; Conroy, S. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing 2018, 47, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.C.; Oreto, L.; Qamar, R.; Paterick, T.E.; Carerj, S.; Khandheria, B.K. Cardioncology: State of the heart. Int. J. Cardiol. 2013, 168, 680–687. [Google Scholar] [CrossRef]
- Cuomo, A.; Mercurio, V.; Varricchi, G.; Galdiero, M.R.; Rossi, F.W.; Carannante, A.; Arpino, G.; Formisano, L.; Bianco, R.; Carlomagno, C.; et al. Impact of a cardio-oncology unit on prevention of cardiovascular events in cancer patients. ESC Heart Fail. 2022, 9, 1666–1676. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Čelutkienė, J.; Pudil, R.; López-Fernández, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar] [PubMed]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [PubMed]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 17, 412. [Google Scholar]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Ewer, M.S.; Lenihan, D.J. Left ventricular ejection fraction and cardiotoxicity: Is our ear really to the ground? J. Clin. Oncol. 2008, 26, 1201–1203. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychological function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Rämsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.S.; Charlton, K.E.; Maggio, M.; et al. Frequency of malnutrition in older adults: A multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Richman, D.; Powell, L. Falls efficacy as a measure of fear of falling. J. Gerontol. 1990, 45, P239–P243. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, F.; Cacciatore, F.; Galizia, G.; Della-Morte, D.; Rossetti, M.; Abbruzzese, R.; Langellotto, A.; Avolio, D.; Gargiulo, G.; Ferrara, N.; et al. Social support and long-term mortality in the elderly: Role of comorbidity. Arch. Gerontol. Geriatr. 2010, 51, 323–328. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- de Boer, R.A.; Hulot, J.S.; Tocchetti, C.G.; Aboumsallem, J.P.; Ameri, P.; Anker, S.D.; Bauersachs, J.; Bertero, E.; Coats, A.J.; Čelutkienė, J.; et al. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 2272–2289. [Google Scholar] [CrossRef]
- Cuomo, A.; Pirozzi, F.; Attanasio, U.; Franco, R.; Elia, F.; De Rosa, E.; Russo, M.; Ghigo, A.; Ameri, P.; Tocchetti, C.G.; et al. Cancer risk in the heart failure population: Epidemiology, mechanisms, and clinical implications. Curr. Oncol. Rep. 2020, 23, 7. [Google Scholar] [CrossRef]
- Mercurio, V.; Cuomo, A.; Cadeddu Dessalvi, C.; Deidda, M.; Di Lisi, D.; Novo, G.; Manganaro, R.; Zito, C.; Santoro, C.; Ameri, P.; et al. Redox imbalances in ageing and metabolic alterations: Implications in cancer and cardiac diseases. An overview from the Working Group of Cardiotoxicity and Cardioprotection of the Italian Society of Cardiology (SIC). Antioxidants 2020, 9, 641. [Google Scholar] [CrossRef]
- Narayan, H.K.; Finkelman, B.; French, B.; Plappert, T.; Hyman, D.; Smith, A.M.; Margulies, K.B.; Ky, B. Detailed echocardiographic phenotyping in breast cancer patients: Associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation 2017, 135, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.; Zhang, Q.; Moore, K.J. Crosstalk between the heart and cancer. Circulation 2020, 142, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Suter, T.M.; López-Fernández, T.; Galderisi, M.; Lyon, A.R.; Van der Meer, P.; Cohen Solal, A.; Zamorano, J.L.; Jerusalem, G.; Moonen, M.; et al. Cardio-Oncology Services: Rationale, organization, and implementation. Eur. Heart J. 2018, 40, 1–8. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Gottfridsson, C.; Asteggiano, R.; Atar, D.; Badimon, L.; Bax, J.J.; Cardinale, D.; Cardone, A.; Feijen, E.A.M.; Ferdinandy, P.; et al. The cancer patient and cardiology. Eur. J. Heart Fail. 2020, 22, 2290–2309. [Google Scholar] [CrossRef] [PubMed]
- Pareek, N.; Cevallos, J.; Moliner, P.; Shah, M.; Tan, L.L.; Chambers, V.; Baksi, A.J.; Khattar, R.S.; Sharma, R.; Rosen, S.D.; et al. Activity and outcomes of a cardio-oncology service in the United Kingdom—A five-year experience. Eur. J. Heart Fail. 2018, 20, 1721–1731. [Google Scholar] [CrossRef]
- Francis, S.A.; Asnani, A.; Neilan, T.; Scherrer-Crosbie, M. Optimizing cardio-oncology programs for cancer patients. Future Oncol. 2015, 11, 2011–2015. [Google Scholar] [CrossRef]
- Jung, H.; Kim, S.; Lee, C.S.; Byeon, S.H.; Kim, S.S.; Lee, S.W.; Kim, Y.J. Real-World Incidence of Incident Noninfectious Uveitis in Patients Treated with BRAF Inhibitors: A Nationwide Clinical Cohort Study. Am. J. Ophthalmol. 2024, 267, 142–152. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Russo, A.; Capoluongo, E.; Barillaro, C.; Pahor, M.; Bernabei, R.; Onder, G. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. J. Clin. Epidemiol. 2010, 63, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Chiarantini, D.; Volpato, S.; Sioulis, F.; Bartalucci, F.; Del Bianco, L.; Mangani, I.; Pepe, G.; Tarantini, F.; Berni, A.; Marchionni, N.; et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J. Card. Fail. 2010, 16, 390–395. [Google Scholar] [CrossRef]
- Caccialanza, R.; Pedrazzoli, P.; Cereda, E.; Gavazzi, C.; Pinto, C.; Paccagnella, A.; Beretta, G.D.; Nardi, M.; Laviano, A.; Zagonel, V. Nutritional Support in Cancer Patients: A Position Paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J. Cancer 2016, 7, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, G.; Skonecki, E.; Boparai, M.K. The Impact of Polypharmacy on Patient Outcomes in Older Adults with Cancer. Cancer J. 2017, 23, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- van Nieuwenhuizen, A.J.; Buffart, L.M.; van Uden-Kraan, C.F.; van der Velden, L.A.; Lacko, M.; Brug, J.; Leemans, C.R.; Verdonck-de Leeuw, I.M. Patient-reported physical activity and the association with health-related quality of life in head and neck cancer survivors. Support. Care Cancer 2018, 26, 1087–1095. [Google Scholar] [CrossRef]
- Holmes, M.D.; Chen, W.Y.; Feskanich, D.; Kroenke, C.H.; Colditz, G.A. Physical activity and survival after breast cancer diagnosis. JAMA 2005, 293, 2479–2486. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J.M. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol. 2011, 29, 726–732. [Google Scholar] [CrossRef]
- Pardal, E.; Díez Baeza, E.; Salas, Q.; García, T.; Sancho, J.M.; Monzón, E.; Moraleda, J.M.; Córdoba, R.; de la Cruz, F.; Queizán, J.A.; et al. A new prognostic model identifies patients aged 80 years and older with diffuse large B-cell lymphoma who may benefit from curative treatment: A multicenter, retrospective analysis by the Spanish GELTAMO group. Am. J. Hematol. 2018, 93, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, C.; Smith, S.M.; Helenowski, I.; Ramsdale, E.; Parsons, B.; Karmali, R.; Feliciano, J.; Hanson, B.; Smith, S.; McKoy, J.; et al. Analysis of very elderly (≥80 years) non-hodgkin lymphoma: Impact of functional status and co-morbidities on outcome. Br. J. Haematol. 2012, 156, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, A.; Boslooper, K.; Hoogendoorn, M.; Joosten, P.; Beerden, T.; Storm, H.; Kibbelaar, R.E.; Veldhuis, G.J.; van Kamp, H.; van Rees, B.; et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: A population-based cohort study. Br. J. Haematol. 2014, 165, 489–496. [Google Scholar] [CrossRef]
- Saygin, C.; Jia, X.; Hill, B.; Dean, R.; Pohlman, B.; Smith, M.R.; Jagadeesh, D. Impact of comorbidities on outcomes of elderly patients with diffuse large B-cell lymphoma. Am. J. Hematol. 2017, 92, 989–996. [Google Scholar] [CrossRef]
- Tucci, A.; Martelli, M.; Rigacci, L.; Riccomagno, P.; Cabras, M.G.; Salvi, F.; Stelitano, C.; Fabbri, A.; Storti, S.; Fogazzi, S.; et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: A prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk. Lymphoma 2015, 56, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Nadruz Jr, W.; Kitzman, D.; Windham, B.G.; Kucharska-Newton, A.; Butler, K.; Palta, P.; Griswold, M.E.; Wagenknecht, L.E.; Heiss, G.; Solomon, S.D.; et al. Cardiovascular Dysfunction and Frailty Among Older Adults in the Community: The ARIC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Okushi, Y.; Yamada, H.; Nishio, S.; Torii, Y.; Hirata, Y.; Saijo, Y.; Ise, T.; Yamaguchi, K.; Yagi, S.; et al. Prognostic Value of Frailty and Diastolic Dysfunction in Elderly Patients. Circ. J. 2018, 82, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Abdar Esfahani, M.; Mokarian, F.; Karimipanah, M. Alterations in the echocardiographic variables of the right ventricle in asymptomatic patients with breast cancer during anthracycline chemotherapy. Postgrad. Med J. 2017, 93, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Skyttä, T.; Tuohinen, S.; Virtanen, V.; Raatikainen, P.; Kellokumpu-Lehtinen, P.L. The concurrent use of aromatase inhibitors and radiotherapy induces echocardiographic changes in patients with breast cancer. Anticancer Res. 2015, 35, 1559–1566. [Google Scholar]
- Rahmati, M.; Lee, S.; Yon, D.K.; Lee, S.W.; Udeh, R.; McEvoy, M.; Oh, H.; Butler, L.; Keyes, H.; Barnett, Y.; et al. Physical activity and prevention of mental health complications: An umbrella review. Neurosci. Biobehav. Rev. 2024, 160, 105641. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Hashmi, M.S.A.; Raza, A.; Ibrahim, M.; Fitriyani, N.L.; Syafrudin, M.; Lee, S.W. Novel Ensemble Learning Algorithm for Early Detection of Lower Back Pain Using Spinal Anomalies. Mathematics 2024, 12, 1955. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Naqvi, R.A.; Alizadehsani, R.; Hussain, S.; Moqurrab, S.A.; Lee, S.W. An adaptive ensemble deep learning framework for reliable detection of pandemic patients. Comput. Biol. Med. 2024, 168, 107836. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Ebner, N.; Dos Santos, M.R.; Ishida, J.; Hasenfuss, G.; von Haehling, S. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur. J. Heart Fail. 2020, 22, 2314–2326. [Google Scholar] [CrossRef]
| Variables | All (n = 108) | Group 1 (n = 54) | Group 2 (n = 34) | Group 3 (n = 20) | p-Value |
|---|---|---|---|---|---|
| Age | 73.55 ± 5.43 | 73.32 ± 5.40 | 73.46 ± 5.09 | 74.34 ± 6.23 | 1 |
| Female, n (%) | 44 (40.7) | 18 (33.3) | 15 (44.1) | 11 (55) | 0.211 |
| Comorbidities | |||||
| Diabetes mellitus, n (%) | 30 (27.7) | 16 (29.6) | 8 (23.5) | 6 (30) | 0.844 |
| Hypertension, n (%) | 83 (76.8) | 44 (81.4) | 25 (73.5) | 14 (70) | 0.466 |
| Dyslipidemia, n (%) | 49 (45.3) | 25 (46.2) | 16 (47) | 8 (40) | 0.903 |
| Dysthyroidism, n (%) | 9 (8.3) | 4 (7.4) | 3 (8.8) | 2 (10) | 0.903 |
| Active smoking, n (%) | 16 (14.8) | 7 (12.9) | 7 (20.5) | 2 (10) | 0.585 |
| Previous smoking, n (%) | 37 (34.2) | 23 (42.5) | 8 (23.5) | 6 (30) | 0.188 |
| LV dysfunction, n (%) | 24 (22.2) | 8 (14.8) | 12 (35.2) | 4 (20) | 0.087 |
| Atrial fibrillation, n (%) | 17 (15.7) | 9 (16.6) | 5 (14.7) | 3 (15) | 1 |
| COPD, n (%) | 15 (13.8) | 11 (20.3) | 2 (5.8) | 2 (10) | 0.164 |
| Carotid atherosclerosis, n (%) | 29 (26.8) | 16 (29.6) | 8 (23,5) | 5 (25) | 0.843 |
| BMI 25–29.9 overweight, n (%) | 47 (43.5) | 27 (50) | 11 (32.3) | 9 (45) | 0.286 |
| BMI ≥ 30, obese, n (%) | 14 (12.9) | 7 (12.9) | 4 (11.7) | 3 (15) | 1 |
| Heart failure, n (%) | 24 (22) | 8 (15) | 12 (35) | 4 (20) | 0.077 |
| Previous MI, n (%) | 20 (19) | 13 (24) | 5 (15) | 2 (10) | 0.302 |
| Arrhythmias, n (%) | 23 (21) | 12 (22) | 7 (21) | 3 (15) | 0.790 |
| Cancer type | |||||
| Colon, n (%) | 42 (38.9) | 25 (46.3) | 14 (41.2) | 3 (15) | 0.038 |
| Gastric, n (%) | 7 (6.5) | 3 (5.6) | 1 (2.9) | 3 (15) | 0.242 |
| Genital, n (%) | 1 (0.9) | 1 (1.9) | 0 (0) | 0 (0) | 1 |
| Larynx, n (%) | 3 (2.8) | 2 (3.7) | 1 (2.9) | 0 (0) | 1 |
| Hodgkin’s lymphoma, n (%) | 4 (3.7) | 3 (5.6) | 0 (0) | 1 (5) | 0.398 |
| Non-Hodgkin’s lymphoma, n (%) | 10 (9.3) | 7 (13.0) | 0 (0) | 3 (15) | 0.049 |
| Tongue, n (%) | 2 (1.9) | 1 (1.9) | 1 (2.9) | 0 (0) | 1 |
| Breast, n (%) | 13 (12.0) | 2 (3.7) | 6 (17.6) | 5 (25) | 0.015 |
| Melanoma, n (%) | 4 (3.7) | 3 (5.6) | 1 (2.9) | 0 (0) | 0.818 |
| Ovarian, n (%) | 1 (0.9) | 0 (0) | 1 (2.9) | 0 (0) | 0.5 |
| Pancreas, n (%) | 3 (2.8) | 0 (0) | 2 (5.9) | 1 (5) | 0.172 |
| Parotid, n (%) | 1 (0.9) | 1 (1.9) | 0 (0) | 0 (0) | 1 |
| Pleura, n (%) | 1 (0.9) | 0 (0) | 1 (2.9) | 0 (0) | 0.5 |
| Polycythemia vera, n (%) | 1 (0.9) | 0 (0) | 1 (2.9) | 0 (0) | 0.5 |
| Lung, n (%) | 7 (6.5) | 2 (3.7) | 3 (8.8) | 2 (10) | 0.390 |
| Prostate, n (%) | 4 (3.7) | 2 (3.7) | 1 (2.9) | 1 (5) | 1 |
| Kidney, n (%) | 1 (0.9) | 0 (0) | 0 (0) | 1 (5) | 0.185 |
| Sarcoma, n (%) | 1 (0.9) | 1 (1.9) | 0 (0) | 0 (0) | 1 |
| Thyroid, n (%) | 1 (0.9) | 1 (1.9) | 0 (0) | 0 (0) | 1 |
| Biliary tract, n (%) | 1 (0.9) | 0 (0) | 1 (2.9) | 0 (0) | 0.5 |
| Anticancer drugs (ongoing or previously taken), n (%) | |||||
| VEGF-based and/or EGFR-based protocols (± PA ± PDC ± other chemotherapeutic agents) | 29 (26.8) | 12 (22.2) | 16 (47) | 1 (5) | 0.002 |
| PA-based and/or PDC-based protocols (± other chemotherapeutic agents) | 33 (30.5) | 21 (38.8) | 8 (23.5) | 4 (20) | 0.184 |
| Anthracyclines-based protocols | 9 (8.3) | 2 (3.7) | 5 (14.7) | 2 (10) | 0.160 |
| Non-anthracyclines-based schemes for lymphomas | 8 (7.4) | 7 (12.9) | 0 | 1 (5) | 0.056 |
| MEKi ± BRAFi | 1 (0.9) | 0 | 1 (2.9) | 0 | 0.500 |
| Immunotherapy | 7 (6.4) | 5 (9.2) | 0 | 2 (10) | 0.144 |
| Hormone-based protocols | 3 (2.7) | 0 | 0 | 3 (15) | 0.006 |
| Others | 18 (16.6) | 7 (12.9) | 4 (11.7) | 7 (35) | 0.072 |
| All (n = 108) | Group 1 (n = 54) | Group 2 (n = 34) | Group 3 (n = 20) | p-Value | |
|---|---|---|---|---|---|
| CVEs during follow-up, n (%) | 32 (29.6) | 8 (14.8) | 21 (61.8) | 3 (15) | <0.001 |
| Cancer treatment modification or temporary suspension, n (%) | 12 (11.1) | 2 (3.7) | 10 (29.4) | NA | <0.001 |
| Cancer treatment withdrawal, n (%) | 3 (2.8) | 0 (0) | 3 (8.8) | NA | 0.035 |
| Death for all causes, n (%) | 32 (29.6) | 12 (22.2) | 16 (47.1) | 4 (20) | 0.034 |
| All (n = 108) | Group 1 (n = 54) | Group 2 (n = 34) | Group 3 (n = 20) | p-Value | |
|---|---|---|---|---|---|
| Cardiovascular treatment optimization | |||||
| Beta-blockers, n (%) | 19 (17.6) | 9 (16.7) | 7 (20.6) | 3 (15) | 0.892 |
| ACE-inhibitors, n (%) | 8 (7.4) | 4 (7.4) | 2 (5.9) | 2 (10) | 0.792 |
| ARBs, n (%) | 6 (5.6) | 2 (3.7) | 2 (5.9) | 2 (10) | 0.792 |
| ARNIs, n (%) | 1 (0.9) | 0 (0) | 1 (2.9) | 0 (0) | 0.5 |
| Diuretics, n (%) | 6 (5.6) | 5 (9.3) | 0 (0) | 1 (5) | 0.228 |
| Calcium channel blockers, n (%) | 5 (4.6) | 4 (7.4) | 1 (2.9) | 0 (0) | 0.580 |
| Statin, n (%) | 13 (12) | 9 (16.7) | 3 (8.8) | 1 (5) | 0.356 |
| Antiplatelet, n (%) | 3 (2.8) | 1 (1.9) | 2 (5.9) | 0 (0) | 0.582 |
| Cardiovascular treatment initiation | |||||
| Beta-blockers, n (%) | 31 (28.7) | 15 (27.8) | 11 (32.4) | 5 (25) | 0.883 |
| ACE-inhibitors, n (%) | 7 (6.5) | 2 (3.7) | 2 (5.9) | 3 (15) | 0.520 |
| ARBs, n (%) | 5 (4.6) | 3 (5.6) | 2 (5.9) | 0 (0) | 0.705 |
| ARNIs, n (%) | 2 (1.9) | 0 (0) | 2 (5.9) | 0 (0) | 0.130 |
| Diuretics, n (%) | 22 (20.4) | 9 (16.7) | 7 (20.6) | 6 (30) | 0.436 |
| Calcium channel blockers, n (%) | 8 (7.4) | 5 (9.3) | 0 (0) | 3 (15) | 0.067 |
| Statin, n (%) | 27 (25) | 15 (27.8) | 8 (23.5) | 4 (20) | 0.837 |
| Antiplatelet, n (%) | 33 (30.6) | 16 (29.6) | 13 (38.2) | 4 (20) | 0.396 |
| Anticoagulation, n (%) | 12 (11.1) | 7 (13) | 3 (8.8) | 2 (10) | 0.918 |
| With CGA (n = 62) | No CGA (n = 46) | p-Value | |
|---|---|---|---|
| Age, years | 73 ± 5 | 74 ± 6 | 0.809 |
| Female, n (%) | 24 (39) | 20 (43) | 0.844 |
| SBP, mmHg | 135 ± 19 | 134 ± 16 | 0.847 |
| DBP, mmHg | 80 ± 11 | 81 ± 10 | 0.725 |
| HR, bpm | 72 ± 17 | 72 ± 18 | 0.881 |
| LVEF, % | 53 ± 8 | 52 ± 9 | 0.648 |
| Hypertension, n (%) | 47 (76) | 36 (78) | 0.765 |
| Diabetes, n (%) | 20 (33) | 10 (21) | 0.202 |
| Obese, n (%) | 7 (12) | 7 (15) | 0.774 |
| Death, n (%) | 19 (31) | 13 (28) | 0.832 |
| Dyslipidemia, n (%) | 30 (49) | 19 (40) | 0.437 |
| HF, n (%) | 13 (21) | 11 (23) | 0.819 |
| Group 1, n (%) | 30 (49) | 24 (51) | 0.352 |
| Group 2, n (%) | 17 (28) | 17 (36) | |
| Group 3, n (%) | 14 (23) | 6 (13) | |
| Beta blockers, n (%) | 31 (51) | 23 (49) | 1.000 |
| ARBs, n (%) | 14 (23) | 19 (40) | 0.060 |
| ACE-I, n (%) | 28 (46) | 15 (32) | 0.168 |
| Statins, n (%) | 22 (38) | 15 (33) | 0.681 |
| diuretics, n (%) | 18 (30) | 19 (40) | 0.307 |
| CCBs, n (%) | 17 (28) | 10 (21) | 0.505 |
| Variables | All (n = 62) | Group 1 (n = 30) | Group 2 (n = 18) | Group 3 (n = 14) | p-Value |
|---|---|---|---|---|---|
| Age, yrs | 73.55 ± 5.41 | 73.63 ± 4.86 | 73.69 ± 5.41 | 73.19 ± 6.81 | 0.962 |
| Females, n (%) | 25 (40) | 11 (37) | 8 (44) | 6 (43) | 0.847 |
| NYHA-FC III-V, n (%) | 2 (3.2) | 1 (3.3) | 0 | 1 (7.1) | 0.618 |
| MMSE | 26.4 ± 3.4 | 26.4 ± 3.6 | 25.8 ± 2.7 | 27.3 ± 3.4 | 0.445 |
| GDS | 3.9 ± 3.6 | 3.4 ± 3.2 | 5.2 ± 4.4 | 3.4 ± 2.9 | 0.219 |
| BADL | 0.7 ± 1.1 | 0.5 ± 0.9 | 0.9 ± 1.2 | 0.7 ± 1.5 | 0.554 |
| IADL | 1.9 ± 2.4 | 1.4 ± 2.1 | 2.7 ± 2.8 | 2.2 ± 2.5 | 0.181 |
| TINETTI | 24.5 ± 4.1 | 25.2 ± 3.4 | 23 ± 5 | 25 ± 3.7 | 0.164 |
| SPPB | 7 ± 3.4 | 7.6 ± 3.2 | 6.2 ± 3.7 | 6.7 ± 3.5 | 0.361 |
| MNA | 23.5 ± 3.7 | 23.43 ± 3.8 | 23.3 ± 3.9 | 24.1 ± 3.5 | 0.814 |
| CIRS-C | 4.5 ± 1.9 | 5 ± 1.9 | 4.1 ± 1.8 | 4.2 ± 2 | 0.234 |
| CIRS-G | 2.1 ± 0.7 | 2.2 ± 1 | 2 ± 0.4 | 1.8 ± 0.3 | 0.278 |
| Drug number, n | 6.7 ± 3.3 | 6.7 ± 3 | 7.1 ± 3.1 | 6.5 ± 4.4 | 0.876 |
| PASE | 78.6 ± 64.7 | 89.9 ± 65.8 | 46.2 ± 55.3 | 93.3 ± 63.1 | 0.060 |
| SSA | 5.6 ± 2.6 | 5.2 ± 2.3 | 6.4 ± 3 | 5.5 ± 2.5 | 0.292 |
| IFI | 12.2 ± 7 | 10.7 ± 5.75 | 14.1 ± 7.9 | 12.7 ± 8 | 0.255 |
| All (n = 62) | Group 1 (n = 30) | Group 2 (n = 18) | Group 3 (n = 14) | p-Value | |
|---|---|---|---|---|---|
| LV-IDd, mm | 49 ± 5.7 | 48.5 ± 5.1 | 49.6 ± 7 | 50.2 ± 5.2 | 0.615 |
| LV-STd, mm | 11 ± 1.5 | 10.7 ± 1.4 | 10.7 ± 1.4 | 10.9 ± 1.7 | 0.94 |
| LV-PWTd, mm | 9 ± 1.1 | 9.6 ± 1.1 | 9 ± 1.3 | 9.3 ± 1 | 0.313 |
| RWT | 0 ± 0.05 | 0.3 ± 0.05 | 0.3 ± 0.06 | 0.3 ± 0.05 | 0.339 |
| LV mass i, g/m2 | 103 ± 23.2 | 102.8 ± 21.2 | 100 ± 27.4 | 104.1 ± 24.4 | 0.884 |
| LV-EDVi, mL/m2 | 56 ± 14.7 | 53.2 ± 12.5 | 56.7 ± 17.1 | 60.8 ± 15.3 | 0.275 |
| LV-EF, % | 53 ± 7.7 | 54.7 ± 5.5 | 49.7 ± 9.3 | 51.8 ± 8.4 | 0.079 |
| E wave, cm/s | 65 ± 21.9 | 71.1 ± 22.8 | 61.1 ± 19.22 | 56.9 ± 20.9 | 0.089 |
| A wave, cm/s | 83 ± 26 | 83.8 ± 24.2 | 81.7 ± 30.8 | 82.8 ± 24.3 | 0.964 |
| Dec Time, ms | 241 ± 72.1 | 244.9 ± 73.6 | 225.2 ± 78.5 | 247.3 ± 66 | 0.615 |
| E/A | 1 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.6 ± 0.2 | 0.167 |
| E/e’ | 19 ± 4.2 | 9.4 ± 3 | 10.9 ± 5.1 | 10.6 ± 4.8 | 0.447 |
| LAVi, mL/m2 | 37 ± 10.8 | 38 ± 11.5 | 34.6 ± 8 | 39.1 ± 12.5 | 0.458 |
| TAPSE, mm | 21 ± 3.4 | 21.2 ± 3.4 | 21.9 ± 4.2 | 20.3 ± 2 | 0.443 |
| S’, cm/s | 12 ± 2.7 | 12.1 ± 2.6 | 12.8 ± 3.5 | 12.7 ± 1.8 | 0.652 |
| PASP, mmHg | 32 ± 10.1 | 31.4 ± 9.6 | 30.5 ± 10 | 34.1 ± 11.7 | 0.598 |
| Variables | Subgroup A (n = 43) | Subgroup B (n = 19) | p-Value |
|---|---|---|---|
| MMSE | 26.1 ± 3.6 | 27.2 ± 2.6 | 0.263 |
| GDS | 3.6 ± 3.6 | 4.7 ± 3.5 | 0.268 |
| BADL | 0.4 ± 0.7 | 1.2 ± 1.7 | 0.074 |
| IADL | 1.6 ± 2.3 | 2.8 ± 2.7 | 0.071 |
| TINETTI | 25.1 ± 3.6 | 23.2 ± 4.8 | 0.103 |
| SPPB | 7.5 ± 3.2 | 4.7 ± 3.6 | 0.060 |
| MNA | 24.1 ± 3.3 | 22.2 ± 4.3 | 0.073 |
| CIRS-C | 4.4 ± 2 | 4.8 ± 1.7 | 0.379 |
| CIRS-G | 2.1 ± 0.9 | 2.1 ± 0.4 | 0.988 |
| Drugs number, n | 6 ± 2.6 | 8.4 ± 4.2 | 0.035 |
| PASE | 93.8 ± 62.3 | 41.8 ± 53.3 | 0.004 |
| SSA | 5.5 ± 2.7 | 5.9 ± 2.4 | 0.555 |
| IFI | 11.6 ± 6.6 | 13.5 ± 7.8 | 0.338 |
| Subgroup A (n = 43) | Subgroup B (n = 19) | p-Value | |
|---|---|---|---|
| LV-IDd, mm | 48.8 ± 5.6 | 50.1 ± 5.9 | 0.435 |
| LV-STd, mm | 10.4 ± 1.4 | 11.5 ± 1.4 | 0.009 |
| LV-PWTd, mm | 9.3 ± 1.2 | 9.5 ± 1.1 | 0.404 |
| RWT | 0.3 ± 0.06 | 0.3 ± 0.04 | 0.627 |
| LV mass i, g/m2 | 97.2 ± 19.1 | 113.2 ± 28.5 | 0.036 |
| LV-EDVi, mL/m2 | 56.6 ± 15.6 | 54.4 ± 12.6 | 0.578 |
| LV-EF, % | 52.5 ± 8.2 | 52.8 ± 6.4 | 0.895 |
| E wave, cm/sec | 67.4 ± 22.3 | 59.5 ± 20.5 | 0.197 |
| A wave, cm/sec | 80.6 ± 21.8 | 87.6 ± 33.2 | 0.341 |
| Dec Time, ms | 232.9 ± 78.1 | 254 ± 59.6 | 0.302 |
| E/A | 0.8 ± 0.3 | 0.7 ± 0.1 | 0.099 |
| E/e’ | 10.1 ± 4.4 | 10.1 ± 3.6 | 0.973 |
| LAVi, mL/m2 | 38.5 ± 11.8 | 34.5 ± 7.8 | 0.192 |
| TAPSE, mm | 21.1 ± 3.4 | 21.4 ± 3.6 | 0.808 |
| S’, cm/sec | 12 ± 2.7 | 13.3 ± 2.6 | 0.111 |
| PASP, mmHg | 31.3 ± 9.4 | 32.7 ± 11.9 | 0.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topa, E.; De Rosa, E.; Cuomo, A.; Curcio, F.; Rizza, M.; Elia, F.; Flocco, V.; Attanasio, U.; Iengo, M.; Fiore, F.; et al. Cardio-Oncology Challenges in Elderly Patients. J. Clin. Med. 2025, 14, 3257. https://doi.org/10.3390/jcm14093257
Topa E, De Rosa E, Cuomo A, Curcio F, Rizza M, Elia F, Flocco V, Attanasio U, Iengo M, Fiore F, et al. Cardio-Oncology Challenges in Elderly Patients. Journal of Clinical Medicine. 2025; 14(9):3257. https://doi.org/10.3390/jcm14093257
Chicago/Turabian StyleTopa, Ester, Eliana De Rosa, Alessandra Cuomo, Francesco Curcio, Marika Rizza, Francesco Elia, Veronica Flocco, Umberto Attanasio, Martina Iengo, Francesco Fiore, and et al. 2025. "Cardio-Oncology Challenges in Elderly Patients" Journal of Clinical Medicine 14, no. 9: 3257. https://doi.org/10.3390/jcm14093257
APA StyleTopa, E., De Rosa, E., Cuomo, A., Curcio, F., Rizza, M., Elia, F., Flocco, V., Attanasio, U., Iengo, M., Fiore, F., Luise, M. C., Arpino, G., Bianco, R., Carlomagno, C., Giuliano, M., Formisano, L., Picardi, M., Della Corte, C. M., Morgillo, F., ... Tocchetti, C. G. (2025). Cardio-Oncology Challenges in Elderly Patients. Journal of Clinical Medicine, 14(9), 3257. https://doi.org/10.3390/jcm14093257













