Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts
Abstract
1. Introduction
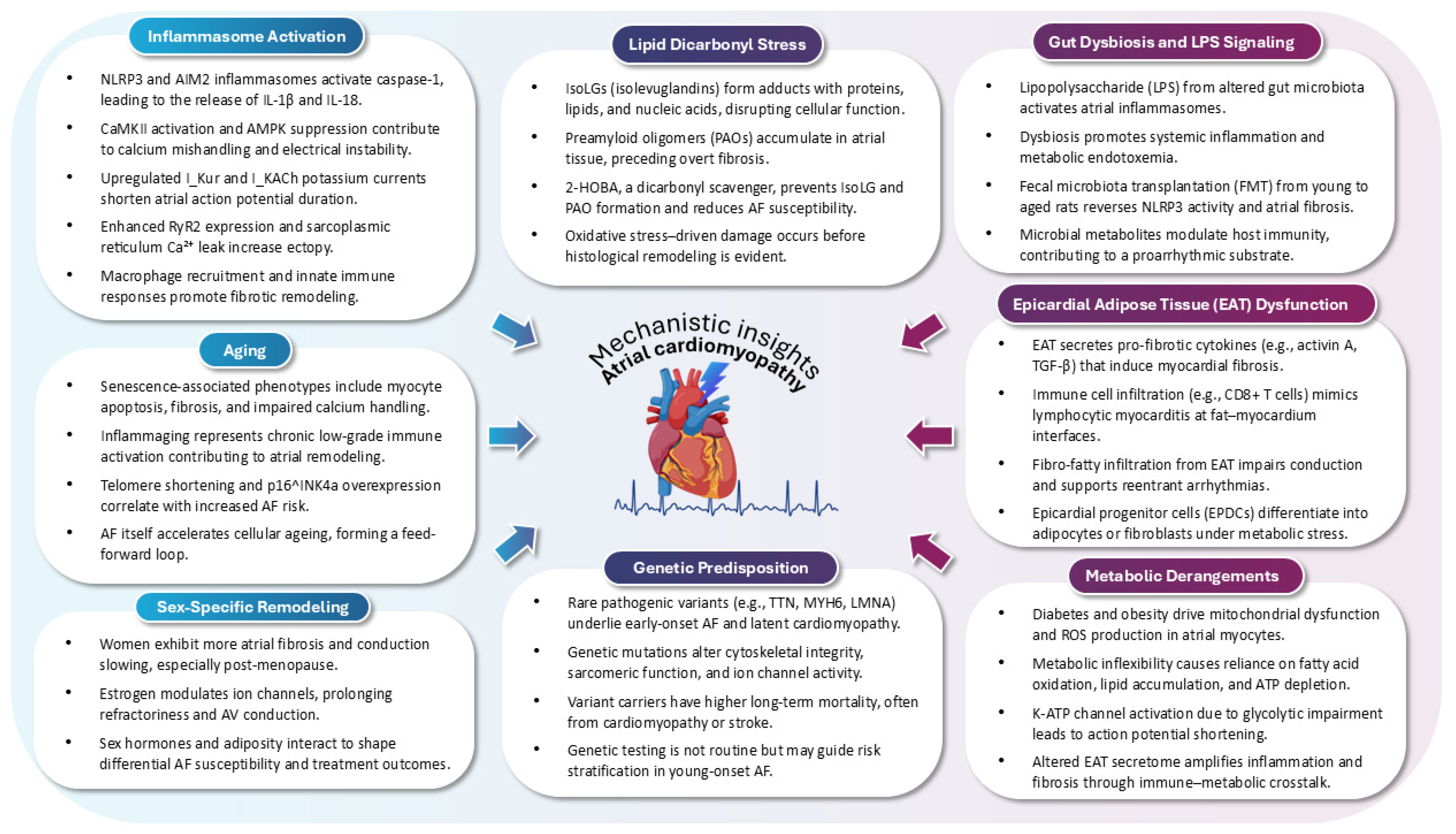
2. Pathophysiology of AtCM
2.1. Inflammasome Activation as a Pathophysiological Driver of AtCM in AF
2.2. Gut Microbiota–Inflammasome Crosstalk in AtCM and AF
2.3. Lipid Dicarbonyl Stress and Preamyloidogenic Injury in AtCM
2.4. Epicardial Adipose Tissue as a Modulator of Atrial Substrate and Arrhythmogenesis in AtCM
2.5. Functional Mitral and Tricuspid Regurgitation and AtCM
2.6. Ageing as a Central Driver of AtCM in AF
2.7. Sex-Based Differences in AtCM
2.8. Metabolic Dysregulation as a Modifiable Determinant of AtCM and AF
2.9. Fibrosis as a Key Driver of AtCM in AF
2.10. Endocardial Remodeling: A Molecular Substrate for Thrombogenesis in AtCM
2.11. Genetic Underpinnings of Early-Onset AF and AtCM
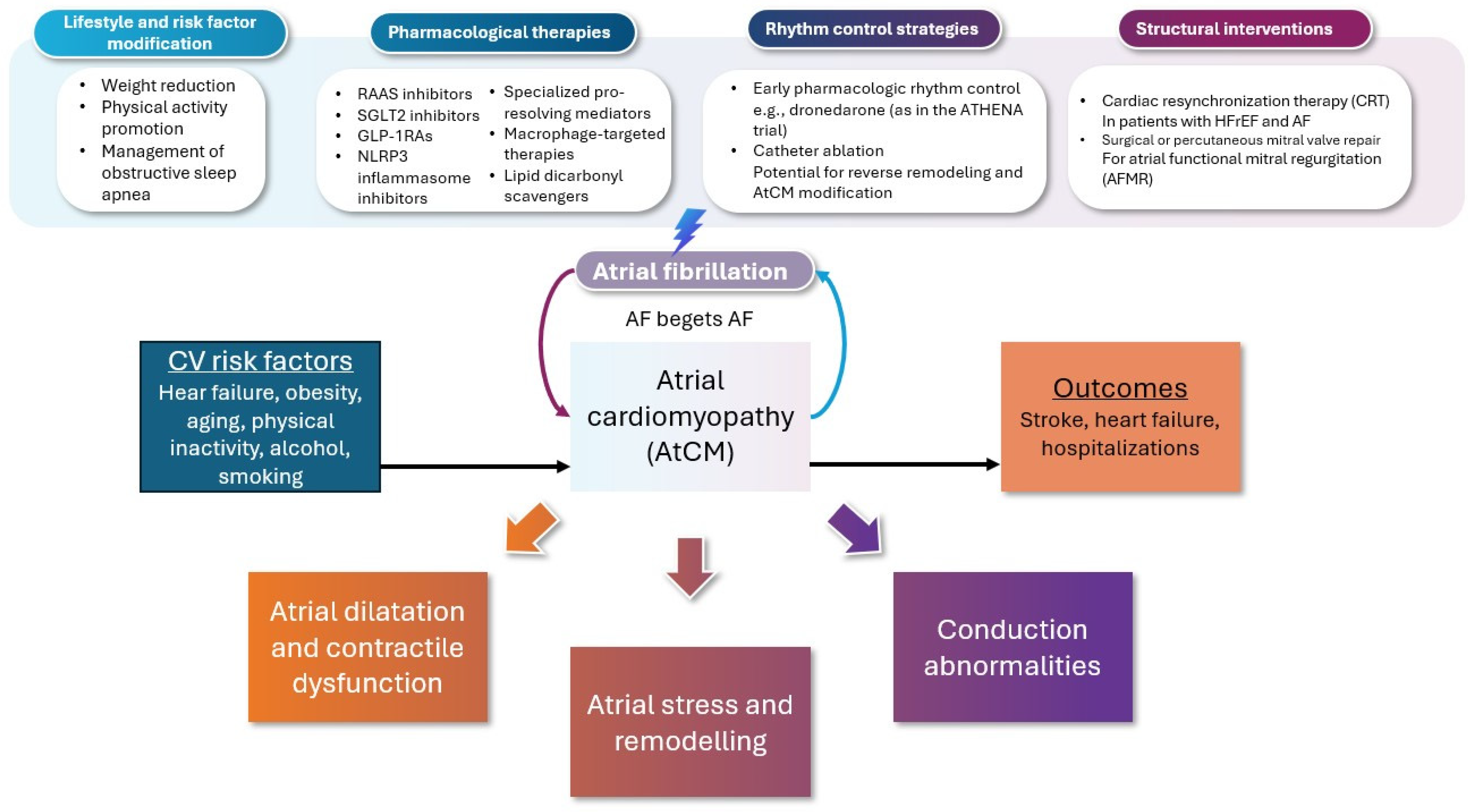
3. Management Approach
3.1. Preventive Strategies Targeting AtCM
3.2. Neurohormonal and Metabolic Modulation in Slowing Progression of AtCM in AF
3.3. Rhythm Control and Reversal of AtCM
3.4. Interventional Reversal of Atrial Remodeling
3.4.1. Device Therapy
3.4.2. Ablation Therapy
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, S.; Zhou, J.; Veang, T.; Lin, Q.; Liu, Q. Global, Regional, and National Burden of Atrial Fibrillation and Atrial Flutter from 1990 to 2021: Sex Differences and Global Burden Projections to 2046—A Systematic Analysis of the Global Burden of Disease Study 2021. Europace 2025, 27, euaf027. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial Fibrillation: Epidemiology, Screening and Digital Health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Bengtson, L.G.S. A Rising Tide: The Global Epidemic of Atrial Fibrillation. Circulation 2014, 129, 829–830. [Google Scholar] [CrossRef]
- Linz, D.; Andrade, J.G.; Arbelo, E.; Boriani, G.; Breithardt, G.; Camm, A.J.; Caso, V.; Nielsen, J.C.; De Melis, M.; De Potter, T.; et al. Longer and Better Lives for Patients with Atrial Fibrillation: The 9th AFNET/EHRA Consensus Conference. Europace 2024, 26, euae070. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Siontis, K.C.; Theofilis, P.; Samaras, A.; Patoulias, D.; Stachteas, P.; Karagiannidis, E.; Stavropoulos, G.; Tzikas, A.; et al. Major Clinical Outcomes in Symptomatic vs. Asymptomatic Atrial Fibrillation: A Meta-Analysis. Eur. Heart J. 2025, 46, 1189–1202. [Google Scholar] [CrossRef]
- Pamporis, K.; Karakasis, P.; Sagris, M.; Theofilis, P.; Milaras, N.; Pantelidaki, A.; Mourouzis, I.; Fragakis, N.; Vlachos, K.; Kordalis, A.; et al. Prevalence of Asymptomatic Atrial Fibrillation and Risk Factors Associated with Asymptomatic Status: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2025, zwaf138. [Google Scholar] [CrossRef]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.-A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial Cardiomyopathy Revisited-Evolution of a Concept: A Clinical Consensus Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Hear. Europace 2024, 26, euae204. [Google Scholar] [CrossRef]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC. Basic to Transl. Sci. 2019, 4, 640–654. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Korantzopoulos, P.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 26, 209. [Google Scholar] [CrossRef]
- Ruperez, C.; Madeo, F.; de Cabo, R.; Kroemer, G.; Abdellatif, M. Obesity Accelerates Cardiovascular Ageing. Eur. Heart J. 2025, ehaf216. [Google Scholar] [CrossRef]
- Su, K.N.; Ma, Y.; Cacheux, M.; Ilkan, Z.; Raad, N.; Muller, G.K.; Wu, X.; Guerrera, N.; Thorn, S.L.; Sinusas, A.J.; et al. Atrial AMP-Activated Protein Kinase Is Critical for Prevention of Dysregulation of Electrical Excitability and Atrial Fibrillation. JCI Insight 2022, 7, e141213. [Google Scholar] [CrossRef] [PubMed]
- Perike, S.; Gonzalez-Gonzalez, F.J.; Abu-Taha, I.; Damen, F.W.; Hanft, L.M.; Lizama, K.S.; Aboonabi, A.; Capote, A.E.; Aguilar-Sanchez, Y.; Levin, B.; et al. PPP1R12C Promotes Atrial Hypocontractility in Atrial Fibrillation. Circ. Res. 2023, 133, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Lefkou, E.; Antoniadis, A.P.; Patoulias, D.; Korantzopoulos, P.; Fragakis, N. Clonal Hematopoiesis of Indeterminate Potential and Atrial Fibrillation: Insights into Pathophysiology and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 2739. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Gerra, L.; Mantovani, M.; Tartaglia, E.; Mei, D.A.; Imberti, J.F.; Vitolo, M.; Bonini, N. Atrial Cardiomyopathy: An Entity of Emerging Interest in the Clinical Setting. Eur. J. Intern. Med. 2023, 118, 14–21. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L.J.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.D.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Song, J.; Wu, J.; Robichaux, D.J.; Li, T.; Wang, S.; Arredondo Sancristobal, M.J.; Dong, B.; Dobrev, D.; Karch, J.; Thomas, S.S.; et al. A High-Protein Diet Promotes Atrial Arrhythmogenesis via Absent-in-Melanoma 2 Inflammasome. Cells 2024, 13, 108. [Google Scholar] [CrossRef]
- Hulsmans, M.; Schloss, M.J.; Lee, I.-H.; Bapat, A.; Iwamoto, Y.; Vinegoni, C.; Paccalet, A.; Yamazoe, M.; Grune, J.; Pabel, S.; et al. Recruited Macrophages Elicit Atrial Fibrillation. Science 2023, 381, 231–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Li, B.; Luo, Y.; Gong, Y.; Jin, X.; Zhang, J.; Zhou, Y.; Zhuo, X.; Wang, Z.; et al. Gut Microbiota Dysbiosis Promotes Age-Related Atrial Fibrillation by Lipopolysaccharide and Glucose-Induced Activation of NLRP3-Inflammasome. Cardiovasc. Res. 2022, 118, 785–797. [Google Scholar] [CrossRef]
- Ishii, Y.; Abe, I.; Kira, S.; Harada, T.; Takano, M.; Oniki, T.; Kondo, H.; Teshima, Y.; Yufu, K.; Shuto, T.; et al. Detection of Fibrotic Remodeling of Epicardial Adipose Tissue in Patients with Atrial Fibrillation: Imaging Approach Based on Histological Observation. Heart Rhythm O2 2021, 2, 311–323. [Google Scholar] [CrossRef]
- Adili, A.; Zhu, X.; Cao, H.; Tang, X.; Wang, Y.; Wang, J.; Shi, J.; Zhou, Q.; Wang, D. Atrial Fibrillation Underlies Cardiomyocyte Senescence and Contributes to Deleterious Atrial Remodeling during Disease Progression. Aging Dis. 2022, 13, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Chun, K.R.J.; Metzner, A.; Ouyang, F.; Schlüter, M.; Elvan, A.; Braegelmann, K.M.; Kueffer, F.J.; et al. Impact of Female Sex on Clinical Outcomes in the FIRE AND ICE Trial of Catheter Ablation for Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006204. [Google Scholar] [CrossRef]
- Guta, A.C.; Badano, L.P.; Tomaselli, M.; Mihalcea, D.; Bartos, D.; Parati, G.; Muraru, D. The Pathophysiological Link between Right Atrial Remodeling and Functional Tricuspid Regurgitation in Patients with Atrial Fibrillation: A Three-Dimensional Echocardiography Study. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021, 34, 585–594.e1. [Google Scholar] [CrossRef] [PubMed]
- Suffee, N.; Baptista, E.; Piquereau, J.; Ponnaiah, M.; Doisne, N.; Ichou, F.; Lhomme, M.; Pichard, C.; Galand, V.; Mougenot, N.; et al. Impacts of a High-Fat Diet on the Metabolic Profile and the Phenotype of Atrial Myocardium in Mice. Cardiovasc. Res. 2022, 118, 3126–3139. [Google Scholar] [CrossRef] [PubMed]
- Hopman, L.H.G.A.; Bhagirath, P.; Mulder, M.J.; Eggink, I.N.; van Rossum, A.C.; Allaart, C.P.; Götte, M.J.W. Quantification of Left Atrial Fibrosis by 3D Late Gadolinium-Enhanced Cardiac Magnetic Resonance Imaging in Patients with Atrial Fibrillation: Impact of Different Analysis Methods. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1182–1190. [Google Scholar] [CrossRef]
- Kawasaki, M.; Meulendijks, E.R.; van den Berg, N.W.E.; Nariswari, F.A.; Neefs, J.; Wesselink, R.; Baalman, S.W.E.; Jongejan, A.; Schelfhorst, T.; Piersma, S.R.; et al. Neutrophil Degranulation Interconnects Over-Represented Biological Processes in Atrial Fibrillation. Sci. Rep. 2021, 11, 2972. [Google Scholar] [CrossRef]
- Meulendijks, E.R.; Al-Shama, R.F.M.; Kawasaki, M.; Fabrizi, B.; Neefs, J.; Wesselink, R.; Ernault, A.C.; Piersma, S.; Pham, T.V.; Jimenez, C.R.; et al. Atrial Epicardial Adipose Tissue Abundantly Secretes Myeloperoxidase and Activates Atrial Fibroblasts in Patients with Atrial Fibrillation. J. Transl. Med. 2023, 21, 366. [Google Scholar] [CrossRef]
- van den Berg, N.W.E.; Neefs, J.; Kawasaki, M.; Nariswari, F.A.; Wesselink, R.; Fabrizi, B.; Jongejan, A.; Klaver, M.N.; Havenaar, H.; Hulsman, E.L.; et al. Extracellular Matrix Remodeling Precedes Atrial Fibrillation: Results of the PREDICT-AF Trial. Heart Rhythm 2021, 18, 2115–2125. [Google Scholar] [CrossRef]
- Heijman, J.; Muna, A.P.; Veleva, T.; Molina, C.E.; Sutanto, H.; Tekook, M.; Wang, Q.; Abu-Taha, I.H.; Gorka, M.; Künzel, S.; et al. Atrial Myocyte NLRP3/CaMKII Nexus Forms a Substrate for Postoperative Atrial Fibrillation. Circ. Res. 2020, 127, 1036–1055. [Google Scholar] [CrossRef]
- Tong, D.; Schiattarella, G.G.; Jiang, N.; Daou, D.; Luo, Y.; Link, M.S.; Lavandero, S.; Gillette, T.G.; Hill, J.A. Impaired AMP-Activated Protein Kinase Signaling in Heart Failure with Preserved Ejection Fraction-Associated Atrial Fibrillation. Circulation 2022, 146, 73–76. [Google Scholar] [CrossRef]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory Signalling in Atrial Cardiomyocytes: A Novel Unifying Principle in Atrial Fibrillation Pathophysiology. Nat. Rev. Cardiol. 2023, 20, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Li, N.; Dobrev, D. Role of Inflammatory Signaling in Atrial Fibrillation. Int. J. Cardiol. 2019, 287, 195–200. [Google Scholar] [CrossRef]
- Bapat, A.; Schloss, M.J.; Yamazoe, M.; Grune, J.; Hulsmans, M.; Milan, D.J.; Nahrendorf, M.; Ellinor, P.T. A Mouse Model of Atrial Fibrillation in Sepsis. Circulation 2023, 147, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Fender, A.C.; Saljic, A.; Li, L.; Chen, X.; Wang, X.; Linz, D.; Lang, J.; Hohl, M.; Twomey, D.; et al. NLRP3 Inflammasome Is a Key Driver of Obesity-Induced Atrial Arrhythmias. Cardiovasc. Res. 2021, 117, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K. Novel Macrophage Targets for the Treatment of Atrial Fibrillation. Nat. Rev. Cardiol. 2023, 20, 648. [Google Scholar] [CrossRef]
- Ninni, S.; Dombrowicz, D.; de Winther, M.; Staels, B.; Montaigne, D.; Nattel, S. Genetic Factors Altering Immune Responses in Atrial Fibrillation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2024, 83, 1163–1176. [Google Scholar] [CrossRef]
- Tao, S.-M.; Yang, M.; Zorzi, A. Immune Regulation in Atrial Cardiomyopathy. Rev. Cardiovasc. Med. 2025, 26, 26897. [Google Scholar] [CrossRef]
- Harada, M.; Van Wagoner, D.R.; Nattel, S. Role of Inflammation in Atrial Fibrillation Pathophysiology and Management. Circ. J. 2015, 79, 495–502. [Google Scholar] [CrossRef]
- McCutcheon, K.; Nqebelele, U.; Murray, L.; Thomas, T.S.; Mpanya, D.; Tsabedze, N. Cardiac and Renal Comorbidities in Aging People Living With HIV. Circ. Res. 2024, 134, 1636–1660. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and Inflammatory Cardiomyopathy: Current Evidence and Future Directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- La Fazia, V.M.; Pierucci, N.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Compagnucci, P.; MacDonald, B.; Mayedo, A.; Torlapati, P.G.; Bassiouny, M.; et al. Catheter Ablation Approach and Outcome in HIV+ Patients with Recurrent Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2023, 34, 2527–2534. [Google Scholar] [CrossRef]
- Peterson, T.E.; Hahn, V.S.; Moaddel, R.; Zhu, M.; Haberlen, S.A.; Palella, F.J.; Plankey, M.; Bader, J.S.; Lima, J.A.C.; Gerszten, R.E.; et al. Proteomic Signature of HIV-Associated Subclinical Left Atrial Remodeling and Incident Heart Failure. Nat. Commun. 2025, 16, 610. [Google Scholar] [CrossRef]
- Hiram, R.; Xiong, F.; Naud, P.; Xiao, J.; Sosnowski, D.K.; Le Quilliec, E.; Saljic, A.; Abu-Taha, I.H.; Kamler, M.; LeBlanc, C.-A.; et al. An Inflammation Resolution-Promoting Intervention Prevents Atrial Fibrillation Caused by Left Ventricular Dysfunction. Cardiovasc. Res. 2024, 120, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Hiram, R.; Xiong, F.; Naud, P.; Xiao, J.; Sirois, M.; Tanguay, J.-F.; Tardif, J.-C.; Nattel, S. The Inflammation-Resolution Promoting Molecule Resolvin-D1 Prevents Atrial Proarrhythmic Remodelling in Experimental Right Heart Disease. Cardiovasc. Res. 2021, 117, 1776–1789. [Google Scholar] [CrossRef]
- Liu, M.; He, H.; Chen, L. Protective Potential of Maresins in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 923413. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Yang, Y.-H.; Wang, Y.-C.; Chang, W.-M.; Wang, C.-W. Maresin: Macrophage Mediator for Resolving Inflammation and Bridging Tissue Regeneration-A System-Based Preclinical Systematic Review. Int. J. Mol. Sci. 2023, 24, 11012. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef]
- Vidar Hansen, T.; Serhan, C.N. Protectins: Their Biosynthesis, Metabolism and Structure-Functions. Biochem. Pharmacol. 2022, 206, 115330. [Google Scholar] [CrossRef]
- Kohli, P.; Levy, B.D. Resolvins and Protectins: Mediating Solutions to Inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef]
- Gawałko, M.; Agbaedeng, T.A.; Saljic, A.; Müller, D.N.; Wilck, N.; Schnabel, R.; Penders, J.; Rienstra, M.; van Gelder, I.; Jespersen, T.; et al. Gut Microbiota, Dysbiosis and Atrial Fibrillation. Arrhythmogenic Mechanisms and Potential Clinical Implications. Cardiovasc. Res. 2022, 118, 2415–2427. [Google Scholar] [CrossRef]
- Boutaud, O.; Montine, T.J.; Chang, L.; Klein, W.L.; Oates, J.A. PGH2-Derived Levuglandin Adducts Increase the Neurotoxicity of Amyloid Beta1-42. J. Neurochem. 2006, 96, 917–923. [Google Scholar] [CrossRef]
- Davies, S.S.; May-Zhang, L.S.; Boutaud, O.; Amarnath, V.; Kirabo, A.; Harrison, D.G. Isolevuglandins as Mediators of Disease and the Development of Dicarbonyl Scavengers as Pharmaceutical Interventions. Pharmacol. Ther. 2020, 205, 107418. [Google Scholar] [CrossRef] [PubMed]
- May-Zhang, L.S.; Kirabo, A.; Huang, J.; Linton, M.F.; Davies, S.S.; Murray, K.T. Scavenging Reactive Lipids to Prevent Oxidative Injury. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, L.M.; Driver, P.M.; Fuller, J.C.J.; Akers, W.S.; Abumrad, N.N.; Amarnath, V.; Milne, G.L.; Chen, S.-C.; Ye, F.; Roberts, L.J., 2nd; et al. Safety, Tolerability, and Pharmacokinetics of Repeated Oral Doses of 2-Hydroxybenzylamine Acetate in Healthy Volunteers: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. BMC Pharmacol. Toxicol. 2020, 21, 3. [Google Scholar] [CrossRef]
- Pitchford, L.M.; Rathmacher, J.A.; Fuller, J.C.J.; Daniels, J.S.; Morrison, R.D.; Akers, W.S.; Abumrad, N.N.; Amarnath, V.; Currey, P.M.; Roberts, L.J.; et al. First-in-Human Study Assessing Safety, Tolerability, and Pharmacokinetics of 2-Hydroxybenzylamine Acetate, a Selective Dicarbonyl Electrophile Scavenger, in Healthy Volunteers. BMC Pharmacol. Toxicol. 2019, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.-M.; Hu, W.-C.; Wu, M.-H.; Tai, C.-T.; Lin, Y.-J.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Wu, T.-J.; et al. Quantitative Analysis of Quantity and Distribution of Epicardial Adipose Tissue Surrounding the Left Atrium in Patients with Atrial Fibrillation and Effect of Recurrence after Ablation. Am. J. Cardiol. 2011, 107, 1498–1503. [Google Scholar] [CrossRef]
- Al Chekakie, M.O.; Welles, C.C.; Metoyer, R.; Ibrahim, A.; Shapira, A.R.; Cytron, J.; Santucci, P.; Wilber, D.J.; Akar, J.G. Pericardial Fat Is Independently Associated with Human Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 56, 784–788. [Google Scholar] [CrossRef]
- Wong, C.X.; Abed, H.S.; Molaee, P.; Nelson, A.J.; Brooks, A.G.; Sharma, G.; Leong, D.P.; Lau, D.H.; Middeldorp, M.E.; Roberts-Thomson, K.C.; et al. Pericardial Fat Is Associated with Atrial Fibrillation Severity and Ablation Outcome. J. Am. Coll. Cardiol. 2011, 57, 1745–1751. [Google Scholar] [CrossRef]
- Gan, L.; Xie, D.; Liu, J.; Bond Lau, W.; Christopher, T.A.; Lopez, B.; Zhang, L.; Gao, E.; Koch, W.; Ma, X.-L.; et al. Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice. Circulation 2020, 141, 968–983. [Google Scholar] [CrossRef]
- Rommel, C.; Rösner, S.; Lother, A.; Barg, M.; Schwaderer, M.; Gilsbach, R.; Bömicke, T.; Schnick, T.; Mayer, S.; Doll, S.; et al. The Transcription Factor ETV1 Induces Atrial Remodeling and Arrhythmia. Circ. Res. 2018, 123, 550–563. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial Adipose Tissue in Contemporary Cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.E.; Ross, J.L.; Xie, C.; Su, K.N.; Zaha, V.G.; Wu, X.; Palmeri, M.; Ashraf, M.; Akar, J.G.; Russell, K.S.; et al. LKB1 Deletion Causes Early Changes in Atrial Channel Expression and Electrophysiology Prior to Atrial Fibrillation. Cardiovasc. Res. 2015, 108, 197–208. [Google Scholar] [CrossRef][Green Version]
- Hulsurkar, M.M.; Lahiri, S.K.; Moore, O.; Moreira, L.M.; Abu-Taha, I.; Kamler, M.; Dobrev, D.; Nattel, S.; Reilly, S.; Wehrens, X.H.T. Atrial-Specific LKB1 Knockdown Represents a Novel Mouse Model of Atrial Cardiomyopathy with Spontaneous Atrial Fibrillation. Circulation 2021, 144, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Ernault, A.C.; Meijborg, V.M.F.; Coronel, R. Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1730–1745. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Zeng, Z.; Li, Q.; Zhou, S.; Zhou, M.; Xue, Y.; Cheng, X.; Xia, Y.; Wang, Q.; et al. Regulation of SCN3B/Scn3b by Interleukin 2 (IL-2): IL-2 Modulates SCN3B/Scn3b Transcript Expression and Increases Sodium Current in Myocardial Cells. BMC Cardiovasc. Disord. 2016, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hurtado, N.; Domínguez-Rodríguez, A.; Mateo, P.; Fernández-Velasco, M.; Val-Blasco, A.; Aizpún, R.; Sabourin, J.; Gómez, A.M.; Benitah, J.-P.; Delgado, C. Beneficial Effects of Leptin Treatment in a Setting of Cardiac Dysfunction Induced by Transverse Aortic Constriction in Mouse. J. Physiol. 2017, 595, 4227–4243. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Zhang, P.; Chen, Y.; Li, C.; Chen, J.; Wang, Y.; Li, Y. The Crucial Role of Activin A/ALK4 Pathway in the Pathogenesis of Ang-II-Induced Atrial Fibrosis and Vulnerability to Atrial Fibrillation. Basic Res. Cardiol. 2017, 112, 47. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human Epicardial Adipose Tissue Induces Fibrosis of the Atrial Myocardium through the Secretion of Adipo-Fibrokines. Eur. Heart J. 2015, 36, 795–805a. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Chaumont, C.; Suffee, N.; Gandjbakhch, E.; Balse, E.; Anselme, F.; Hatem, S.N. Epicardial Origin of Cardiac Arrhythmias: Clinical Evidences and Pathophysiology. Cardiovasc. Res. 2022, 118, 1693–1702. [Google Scholar] [CrossRef]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial Fibrillation Is Associated with the Fibrotic Remodelling of Adipose Tissue in the Subepicardium of Human and Sheep Atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Teshima, Y.; Kondo, H.; Kaku, H.; Kira, S.; Ikebe, Y.; Saito, S.; Fukui, A.; Shinohara, T.; Yufu, K.; et al. Association of Fibrotic Remodeling and Cytokines/Chemokines Content in Epicardial Adipose Tissue with Atrial Myocardial Fibrosis in Patients with Atrial Fibrillation. Heart Rhythm 2018, 15, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, M.G.; Pandozzi, C.; Muscogiuri, G.; Sironi, S.; Pujia, A.; Lenzi, A.; Giannetta, E. Epicardial Adipose Tissue: A Novel Potential Imaging Marker of Comorbidities Caused by Chronic Inflammation. Nutrients 2022, 14, 2926. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Bell, J.R.; Raaijmakers, A.J.A.; Waddell, H.M.; Wells, S.P.; Bernasochi, G.B.; Montgomery, M.K.; Binny, S.; Watts, T.; Joshi, S.B.; et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. J. Am. Coll. Cardiol. 2020, 76, 1197–1211. [Google Scholar] [CrossRef]
- Gharaviri, A.; Bidar, E.; Potse, M.; Zeemering, S.; Verheule, S.; Pezzuto, S.; Krause, R.; Maessen, J.G.; Auricchio, A.; Schotten, U. Epicardial Fibrosis Explains Increased Endo-Epicardial Dissociation and Epicardial Breakthroughs in Human Atrial Fibrillation. Front. Physiol. 2020, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, F.; Masè, M.; Cristoforetti, A.; Avogaro, L.; D’Amato, E.; Tessarolo, F.; Piccoli, F.; Graffigna, A. Quantitative Assessment of Transmural Fibrosis Profile in the Human Atrium: Evidence for a Three-Dimensional Arrhythmic Substrate by Slice-to-Slice Histology. Europace 2023, 25, 739–747. [Google Scholar] [CrossRef]
- De Coster, T.; Claus, P.; Kazbanov, I.V.; Haemers, P.; Willems, R.; Sipido, K.R.; Panfilov, A. V Arrhythmogenicity of Fibro-Fatty Infiltrations. Sci. Rep. 2018, 8, 2050. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Cavallero, S.; Patterson, M.; Shen, H.; Xu, J.; Kumar, S.R.; Sucov, H.M. Adipogenesis and Epicardial Adipose Tissue: A Novel Fate of the Epicardium Induced by Mesenchymal Transformation and PPARγ Activation. Proc. Natl. Acad. Sci. USA 2015, 112, 2070–2075. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial Progenitors Contribute to the Cardiomyocyte Lineage in the Developing Heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef]
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Tregouet, D.A.; Pucéat, M.; et al. Reactivation of the Epicardium at the Origin of Myocardial Fibro-Fatty Infiltration During the Atrial Cardiomyopathy. Circ. Res. 2020, 126, 1330–1342. [Google Scholar] [CrossRef]
- Suffee, N.; Moore-Morris, T.; Farahmand, P.; Rücker-Martin, C.; Dilanian, G.; Fradet, M.; Sawaki, D.; Derumeaux, G.; LePrince, P.; Clément, K.; et al. Atrial Natriuretic Peptide Regulates Adipose Tissue Accumulation in Adult Atria. Proc. Natl. Acad. Sci. USA 2017, 114, E771–E780. [Google Scholar] [CrossRef]
- Arndt, M.; Lendeckel, U.; Röcken, C.; Nepple, K.; Wolke, C.; Spiess, A.; Huth, C.; Ansorge, S.; Klein, H.U.; Goette, A. Altered Expression of ADAMs (A Disintegrin And Metalloproteinase) in Fibrillating Human Atria. Circulation 2002, 105, 720–725. [Google Scholar] [CrossRef]
- Kwak, S.; Lim, J.; Yang, S.; Rhee, T.-M.; Choi, Y.-J.; Lee, H.-J.; Hwang, I.-C.; Lee, H.; Yoon, Y.E.; Park, H.E.; et al. Atrial Functional Tricuspid Regurgitation: Importance of Atrial Fibrillation and Right Atrial Remodeling and Prognostic Significance. JACC. Cardiovasc. Imaging 2023, 16, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leon, X.A.; Posada-Martinez, E.L.; Trejo-Paredes, M.C.; Ivey-Miranda, J.B.; Pereira, J.; Crandall, I.; DaSilva, P.; Bouman, E.; Brooks, A.; Gerardi, C.; et al. Understanding Tricuspid Valve Remodelling in Atrial Fibrillation Using Three-Dimensional Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Schwammenthal, E. Ischemic Mitral Regurgitation on the Threshold of a Solution: From Paradoxes to Unifying Concepts. Circulation 2005, 112, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Levine, R.A.; Flachskampf, F.; Grayburn, P.; Gillam, L.; Leipsic, J.; Thomas, J.D.; Kwong, R.Y.; Vandervoort, P.; Chandrashekhar, Y. Atrial Functional Mitral Regurgitation: A JACC: Cardiovascular Imaging Expert Panel Viewpoint. JACC. Cardiovasc. Imaging 2022, 15, 1870–1882. [Google Scholar] [CrossRef]
- Gertz, Z.M.; Raina, A.; Saghy, L.; Zado, E.S.; Callans, D.J.; Marchlinski, F.E.; Keane, M.G.; Silvestry, F.E. Evidence of Atrial Functional Mitral Regurgitation Due to Atrial Fibrillation: Reversal with Arrhythmia Control. J. Am. Coll. Cardiol. 2011, 58, 1474–1481. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Verbrugge, F.H.; Verhaert, D.; Rega, F.; Thomas, J.D.; Vandervoort, P.M. Atrial Functional Mitral Regurgitation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2465–2476. [Google Scholar] [CrossRef]
- Dziadzko, V.; Dziadzko, M.; Medina-Inojosa, J.R.; Benfari, G.; Michelena, H.I.; Crestanello, J.A.; Maalouf, J.; Thapa, P.; Enriquez-Sarano, M. Causes and Mechanisms of Isolated Mitral Regurgitation in the Community: Clinical Context and Outcome. Eur. Heart J. 2019, 40, 2194–2202. [Google Scholar] [CrossRef]
- Tamargo, M.; Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Lin, G.; Egbe, A.C.; Nishimura, R.A.; Borlaug, B.A. Functional Mitral Regurgitation and Left Atrial Myopathy in Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2020, 22, 489–498. [Google Scholar] [CrossRef]
- Patel, R.B.; Shah, S.J. Therapeutic Targeting of Left Atrial Myopathy in Atrial Fibrillation and Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2020, 5, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Obokata, M.; Gersh, B.J.; Borlaug, B.A. High Prevalence of Occult Heart Failure with Preserved Ejection Fraction Among Patients with Atrial Fibrillation and Dyspnea. Circulation 2018, 137, 534–535. [Google Scholar] [CrossRef]
- Packer, M.; Lam, C.S.P.; Lund, L.H.; Redfield, M.M. Interdependence of Atrial Fibrillation and Heart Failure with a Preserved Ejection Fraction Reflects a Common Underlying Atrial and Ventricular Myopathy. Circulation 2020, 141, 4–6. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Verbrugge, F.H.; Lin, G.; Borlaug, B.A. Atrial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.; Shantsila, E.; Lip, G.Y.H. Mechanisms of Thrombogenesis in Atrial Fibrillation: Virchow’s Triad Revisited. Lancet 2009, 373, 155–166. [Google Scholar] [CrossRef]
- Unni, P.A.; Pillai, G.G.; Sajithalulu, S. Biological Processes and Key Druggable Targets Involved in Age-Associated Memory Loss: A Systematic Review. Life Sci. 2021, 270, 119079. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial Dysfunction and Oxidative Stress in Aging and Cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Opstad, T.B.; Tveit, A.; Solheim, S.; Arnesen, H.; Seljeflot, I. Biomarkers of Ageing and Cardiac Remodeling Are Associated with Atrial Fibrillation. Scand. Cardiovasc. J. 2021, 55, 213–219. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular Inflammation: Underpinnings of Aging and Age-Related Diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Aguilar, M.; Thorin, E.; Ferbeyre, G.; Nattel, S. The Role of Cellular Senescence in Cardiac Disease: Basic Biology and Clinical Relevance. Nat. Rev. Cardiol. 2022, 19, 250–264. [Google Scholar] [CrossRef]
- Ramos, K.S.; Brundel, B.J.J.M. DNA Damage, an Innocent Bystander in Atrial Fibrillation and Other Cardiovascular Diseases? Front. Cardiovasc. Med. 2020, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Kwan, G.F.; Benjamin, E.J. Global Epidemiology of Atrial Fibrillation. Nat. Rev. Cardiol. 2014, 11, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Westaby, J.D.; Zullo, E.; Bicalho, L.M.; Anderson, R.H.; Sheppard, M.N. Effect of Sex, Age and Body Measurements on Heart Weight, Atrial, Ventricular, Valvular and Sub-Epicardial Fat Measurements of the Normal Heart. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2023, 63, 107508. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, C.; Liao, P.; Cui, X.; Tian, W.; Wang, Q.; Sun, J.; Yang, M.; Luo, L.; Wu, H.; et al. Epidemiology, Management, and Outcomes of Atrial Fibrillation among 30 Million Citizens in Shanghai, China from 2015 to 2020: A Medical Insurance Database Study. Lancet Reg. Health West. Pacific 2022, 23, 100470. [Google Scholar] [CrossRef]
- Fenger-Grøn, M.; Overvad, K.; Tjønneland, A.; Frost, L. Lean Body Mass Is the Predominant Anthropometric Risk Factor for Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 69, 2488–2497. [Google Scholar] [CrossRef]
- Emdin, C.A.; Wong, C.X.; Hsiao, A.J.; Altman, D.G.; Peters, S.A.; Woodward, M.; Odutayo, A.A. Atrial Fibrillation as Risk Factor for Cardiovascular Disease and Death in Women Compared with Men: Systematic Review and Meta-Analysis of Cohort Studies. BMJ 2016, 532, h7013. [Google Scholar] [CrossRef]
- Volgman, A.S.; Benjamin, E.J.; Curtis, A.B.; Fang, M.C.; Lindley, K.J.; Naccarelli, G.V.; Pepine, C.J.; Quesada, O.; Vaseghi, M.; Waldo, A.L.; et al. Women and Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2021, 32, 2793–2807. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, Q.; Gao, L.; Liu, J.; Qin, S.; Zhang, D. Sex-Related Differences in Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Europace 2019, 21, 1509–1518. [Google Scholar] [CrossRef]
- Wang, L.; Selzman, K.A.; Shah, R.U. Peri-Procedural Complications in Women: An Alarming and Consistent Trend. Eur. Heart J. 2019, 40, 3044–3045. [Google Scholar] [CrossRef]
- Cheung, J.W.; Cheng, E.P.; Wu, X.; Yeo, I.; Christos, P.J.; Kamel, H.; Markowitz, S.M.; Liu, C.F.; Thomas, G.; Ip, J.E.; et al. Sex-Based Differences in Outcomes, 30-Day Readmissions, and Costs Following Catheter Ablation of Atrial Fibrillation: The United States Nationwide Readmissions Database 2010-14. Eur. Heart J. 2019, 40, 3035–3043. [Google Scholar] [CrossRef]
- Wong, G.R.; Nalliah, C.J.; Lee, G.; Voskoboinik, A.; Chieng, D.; Prabhu, S.; Parameswaran, R.; Sugumar, H.; Al-Kaisey, A.; McLellan, A.; et al. Sex-Related Differences in Atrial Remodeling in Patients with Atrial Fibrillation: Relationship to Ablation Outcomes. Circ. Arrhythm. Electrophysiol. 2022, 15, e009925. [Google Scholar] [CrossRef]
- Tadros, R.; Ton, A.-T.; Fiset, C.; Nattel, S. Sex Differences in Cardiac Electrophysiology and Clinical Arrhythmias: Epidemiology, Therapeutics, and Mechanisms. Can. J. Cardiol. 2014, 30, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.; Leonardo, F.; Dicandia, C.; Sheiban, I.; Pagnotta, P.; Pappone, C.; Chierchia, S.L. Acute Electrophysiologic Effect of Estradiol 17beta in Menopausal Women. Am. J. Cardiol. 2000, 86, 1385–1387. [Google Scholar] [CrossRef]
- Müller-Edenborn, B.; Moreno-Weidmann, Z.; Venier, S.; Defaye, P.; Park, C.-I.; Guerra, J.; Alonso-Martín, C.; Bazan, V.; Vinolas, X.; Rodriguez-Font, E.; et al. Determinants of Fibrotic Atrial Cardiomyopathy in Atrial Fibrillation. A Multicenter Observational Study of the RETAC (Reseau Européen de Traîtement d’arrhythmies Cardiaques)-Group. Clin. Res. Cardiol. 2022, 111, 1018–1027. [Google Scholar] [CrossRef]
- Cochet, H.; Mouries, A.; Nivet, H.; Sacher, F.; Derval, N.; Denis, A.; Merle, M.; Relan, J.; Hocini, M.; Haïssaguerre, M.; et al. Age, Atrial Fibrillation, and Structural Heart Disease Are the Main Determinants of Left Atrial Fibrosis Detected by Delayed-Enhanced Magnetic Resonance Imaging in a General Cardiology Population. J. Cardiovasc. Electrophysiol. 2015, 26, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Z.; Yin, Z.; Zhang, Y.; Xue, X.; Han, J.; Zhu, Y.; Zhang, J.; Emmert, M.Y.; Wang, H. Gender Differences in Fibrosis Remodeling in Patients with Long-Standing Persistent Atrial Fibrillation. Oncotarget 2017, 8, 53714–53729. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Abed, H.S.; Wittert, G.A.; Leong, D.P.; Shirazi, M.G.; Bahrami, B.; Middeldorp, M.E.; Lorimer, M.F.; Lau, D.H.; Antic, N.A.; Brooks, A.G.; et al. Effect of Weight Reduction and Cardiometabolic Risk Factor Management on Symptom Burden and Severity in Patients with Atrial Fibrillation: A Randomized Clinical Trial. JAMA 2013, 310, 2050–2060. [Google Scholar] [CrossRef]
- Dublin, S.; Glazer, N.L.; Smith, N.L.; Psaty, B.M.; Lumley, T.; Wiggins, K.L.; Page, R.L.; Heckbert, S.R. Diabetes Mellitus, Glycemic Control, and Risk of Atrial Fibrillation. J. Gen. Intern. Med. 2010, 25, 853–858. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort. The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef]
- Fenger-Grøn, M.; Vinter, N.; Frost, L. Body Mass and Atrial Fibrillation Risk: Status of the Epidemiology Concerning the Influence of Fat versus Lean Body Mass. Trends Cardiovasc. Med. 2020, 30, 205–211. [Google Scholar] [CrossRef]
- Gong, M.; Yuan, M.; Meng, L.; Zhang, Z.; Tse, G.; Zhao, Y.; Zhang, Y.; Yuan, M.; Liang, X.; Fan, G.; et al. Wenxin Keli Regulates Mitochondrial Oxidative Stress and Homeostasis and Improves Atrial Remodeling in Diabetic Rats. Oxid. Med. Cell. Longev. 2020, 2020, 2468031. [Google Scholar] [CrossRef]
- Habibi, M.; Zareian, M.; Ambale Venkatesh, B.; Samiei, S.; Imai, M.; Wu, C.; Launer, L.J.; Shea, S.; Gottesman, R.F.; Heckbert, S.R.; et al. Left Atrial Mechanical Function and Incident Ischemic Cerebrovascular Events Independent of AF: Insights From the MESA Study. JACC. Cardiovasc. Imaging 2019, 12, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Aromolaran, A.S.; Colecraft, H.M.; Boutjdir, M. High-Fat Diet-Dependent Modulation of the Delayed Rectifier K(+) Current in Adult Guinea Pig Atrial Myocytes. Biochem. Biophys. Res. Commun. 2016, 474, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J.; Trent, C.M.; Schulze, P.C. Lipid Metabolism and Toxicity in the Heart. Cell Metab. 2012, 15, 805–812. [Google Scholar] [CrossRef]
- Vyas, V.; Blythe, H.; Wood, E.G.; Sandhar, B.; Sarker, S.-J.; Balmforth, D.; Ambekar, S.G.; Yap, J.; Edmondson, S.J.; Di Salvo, C.; et al. Obesity and Diabetes Are Major Risk Factors for Epicardial Adipose Tissue Inflammation. JCI Insight 2021, 6, e145495. [Google Scholar] [CrossRef] [PubMed]
- Smorodinova, N.; Bláha, M.; Melenovský, V.; Rozsívalová, K.; Přidal, J.; Ďurišová, M.; Pirk, J.; Kautzner, J.; Kučera, T. Analysis of Immune Cell Populations in Atrial Myocardium of Patients with Atrial Fibrillation or Sinus Rhythm. PLoS ONE 2017, 12, e0172691. [Google Scholar] [CrossRef]
- Guo, Y.; Lip, G.Y.H.; Apostolakis, S. Inflammation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 60, 2263–2270. [Google Scholar] [CrossRef]
- Corradi, D. Atrial Fibrillation from the Pathologist’s Perspective. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2014, 23, 71–84. [Google Scholar] [CrossRef]
- Nattel, S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [CrossRef]
- Li, D.; Fareh, S.; Leung, T.K.; Nattel, S. Promotion of Atrial Fibrillation by Heart Failure in Dogs: Atrial Remodeling of a Different Sort. Circulation 1999, 100, 87–95. [Google Scholar] [CrossRef]
- Teh, A.W.; Kistler, P.M.; Lee, G.; Medi, C.; Heck, P.M.; Spence, S.J.; Sparks, P.B.; Morton, J.B.; Kalman, J.M. Electroanatomic Remodeling of the Left Atrium in Paroxysmal and Persistent Atrial Fibrillation Patients without Structural Heart Disease. J. Cardiovasc. Electrophysiol. 2012, 23, 232–238. [Google Scholar] [CrossRef]
- Corradi, D.; Callegari, S.; Maestri, R.; Benussi, S.; Alfieri, O. Structural Remodeling in Atrial Fibrillation. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Caixal, G.; Althoff, T.; Garre, P.; Alarcón, F.; NuñezGarcia, M.; Benito, E.M.; Borras, R.; Perea, R.J.; Prat-González, S.; Gunturiz, C.; et al. Proximity to the Descending Aorta Predicts Regional Fibrosis in the Adjacent Left Atrial Wall: Aetiopathogenic and Prognostic Implications. Europace 2021, 23, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Callegari, S.; Macchi, E.; Monaco, R.; Magnani, L.; Tafuni, A.; Croci, S.; Nicastro, M.; Garrapa, V.; Banchini, A.; Becchi, G.; et al. Clinicopathological Bird’s-Eye View of Left Atrial Myocardial Fibrosis in 121 Patients With Persistent Atrial Fibrillation: Developing Architecture and Main Cellular Players. Circ. Arrhythm. Electrophysiol. 2020, 13, e007588. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, X.-H.; Yin, X.; Liu, Y.-J.; Han, C.; Yang, J.; Huang, X.; Yu, X.; Gao, L.; Yang, Y.-Z.; et al. Elevated Circulating Fibrocytes Is a Marker of Left Atrial Fibrosis and Recurrence of Persistent Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7, e008083. [Google Scholar] [CrossRef]
- Christensen, G.; Herum, K.M.; Lunde, I.G. Sweet, yet Underappreciated: Proteoglycans and Extracellular Matrix Remodeling in Heart Disease. Matrix Biol. 2019, 75–76, 286–299. [Google Scholar] [CrossRef]
- Yue, L.; Xie, J.; Nattel, S. Molecular Determinants of Cardiac Fibroblast Electrical Function and Therapeutic Implications for Atrial Fibrillation. Cardiovasc. Res. 2011, 89, 744–753. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Barallobre-Barreiro, J.; Gupta, S.K.; Zoccarato, A.; Kitazume-Taneike, R.; Fava, M.; Yin, X.; Werner, T.; Hirt, M.N.; Zampetaki, A.; Viviano, A.; et al. Glycoproteomics Reveals Decorin Peptides with Anti-Myostatin Activity in Human Atrial Fibrillation. Circulation 2016, 134, 817–832. [Google Scholar] [CrossRef]
- Ausma, J.; Wijffels, M.; Thoné, F.; Wouters, L.; Allessie, M.; Borgers, M. Structural Changes of Atrial Myocardium Due to Sustained Atrial Fibrillation in the Goat. Circulation 1997, 96, 3157–3163. [Google Scholar] [CrossRef]
- Harada, M.; Tadevosyan, A.; Qi, X.; Xiao, J.; Liu, T.; Voigt, N.; Karck, M.; Kamler, M.; Kodama, I.; Murohara, T.; et al. Atrial Fibrillation Activates AMP-Dependent Protein Kinase and Its Regulation of Cellular Calcium Handling: Potential Role in Metabolic Adaptation and Prevention of Progression. J. Am. Coll. Cardiol. 2015, 66, 47–58. [Google Scholar] [CrossRef]
- Gunturiz-Beltrán, C.; Borràs, R.; Alarcón, F.; Garre, P.; Figueras, I.; Ventura, R.M.; Benito, E.M.; Caixal, G.; Althoff, T.F.; Tolosana, J.M.; et al. Quantification of Right Atrial Fibrosis by Cardiac Magnetic Resonance: Verification of the Method to Standardize Thresholds. Rev. Esp. Cardiol. (Engl. Ed.) 2023, 76, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.G.; King, J.B.; Kholmovski, E.G.; Ma, J.; Gal, P.; Marashly, Q.; AlJuaid, M.A.; Kaur, G.; Silver, M.A.; Johnson, K.A.; et al. Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging and Catheter Ablation of Atrial Fibrillation: 5-Year Follow-Up Data. J. Am. Heart Assoc. 2018, 7, e006313. [Google Scholar] [CrossRef] [PubMed]
- Siebermair, J.; Kholmovski, E.G.; Marrouche, N. Assessment of Left Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging: Methodology and Clinical Implications. JACC Clin. Electrophysiol. 2017, 3, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.S.; Maltês, S.; Marques, H.; Nunes, R.G.; Ferreira, A. Myocardial T1 Mapping with Magnetic Resonance Imaging—A Useful Tool to Understand the Diseased Heart. Rev. Port. De Cardiol. 2022, 41, 61–69. [Google Scholar] [CrossRef]
- Mills, M.T.; Calvert, P.; Phenton, C.; Worthington, N.; Todd, D.; Modi, S.; Ashrafi, R.; Snowdon, R.; Gupta, D.; Luther, V. An Approach to Electroanatomical Mapping with a Pentaspline Pulsed Field Catheter to Guide Atrial Fibrillation Ablation. J. Interv. Card. Electrophysiol. 2025. [Google Scholar] [CrossRef]
- Chen, Y.; Zhuang, J.; Li, X.; Zhang, C.; Cao, X.; Xu, Z.; Feng, X. The Relationship between the 3D Electroanatomical Mapping Parameters of the Left Atrial Posterior Wall and the Recurrence of Paroxysmal Atrial Fibrillation. Front. Cardiovasc. Med. 2025, 12, 1522807. [Google Scholar] [CrossRef]
- Rodríguez-Mañero, M.; Valderrábano, M.; Baluja, A.; Kreidieh, O.; Martínez-Sande, J.L.; García-Seara, J.; Saenen, J.; Iglesias-Álvarez, D.; Bories, W.; Villamayor-Blanco, L.M.; et al. Validating Left Atrial Low Voltage Areas During Atrial Fibrillation and Atrial Flutter Using Multielectrode Automated Electroanatomic Mapping. JACC Clin. Electrophysiol. 2018, 4, 1541–1552. [Google Scholar] [CrossRef]
- Black, N.; Mohammad, F.; Saraf, K.; Morris, G. Endothelial Function and Atrial Fibrillation: A Missing Piece of the Puzzle? J. Cardiovasc. Electrophysiol. 2022, 33, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Grego, A.; Fernandes, C.; Fonseca, I.; Dias-Neto, M.; Costa, R.; Leite-Moreira, A.; Oliveira, S.M.; Trindade, F.; Nogueira-Ferreira, R. Endothelial Dysfunction in Cardiovascular Diseases: Mechanisms and in Vitro Models. Mol. Cell. Biochem. 2025. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Tokuyama, T.; Takahashi, S.; Hiyama, T.; Okubo, Y.; Okamura, S.; Miyamoto, S.; Oguri, N.; Takasaki, T.; Katayama, K.; et al. Relationship Between Fibrosis, Endocardial Endothelial Damage, and Thrombosis of Left Atrial Appendage in Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 1158–1168. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE Expert Consensus on Atrial Cardiomyopathies: Definition, Characterisation, and Clinical Implication. J. Arrhythmia 2016, 32, 247–278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lin, Y.; Ding, H.; Lian, L.; Chen, J.; Wu, M.; Chen, X.; Zhang, J. Vascular Cell Adhesion Molecule 1 in Atrial Fibrillation. BMC Cardiovasc. Disord. 2024, 24, 725. [Google Scholar] [CrossRef]
- Yamaguchi, T. Atrial Structural Remodeling and Atrial Fibrillation Substrate: A Histopathological Perspective. J. Cardiol. 2025, 85, 47–55. [Google Scholar] [CrossRef]
- Rahimi, M.; Faridi, L.; Nikniaz, L.; Daneshvar, S.; Naseri, A.; Taban-Sadeghi, M.; Manaflouyan, H.; Shahabi, J.; Sarrafzadegan, N. Effect of Endothelial Adhesion Molecules on Atrial Fibrillation: A Systematic Review and Meta-Analysis. Heart Int. 2022, 16, 75–84. [Google Scholar] [CrossRef]
- van den Berg, N.W.E.; Kawasaki, M.; Fabrizi, B.; Nariswari, F.A.; Verduijn, A.C.; Neefs, J.; Wesselink, R.; Al-Shama, R.F.M.; van der Wal, A.C.; de Boer, O.J.; et al. Epicardial and Endothelial Cell Activation Concurs with Extracellular Matrix Remodeling in Atrial Fibrillation. Clin. Transl. Med. 2021, 11, e558. [Google Scholar] [CrossRef]
- Bukowska, A.; Hammwöhner, M.; Corradi, D.; Mahardhika, W.; Goette, A. Atrial Thrombogenesis in Atrial Fibrillation: Results from Atrial Fibrillation Models and AF-Patients. Herzschrittmacherther. Elektrophysiol. 2018, 29, 76–83. [Google Scholar] [CrossRef]
- Chalazan, B.; Freeth, E.; Mohajeri, A.; Ramanathan, K.; Bennett, M.; Walia, J.; Halperin, L.; Roston, T.; Lazarte, J.; Hegele, R.A.; et al. Genetic Testing in Monogenic Early-Onset Atrial Fibrillation. Eur. J. Hum. Genet. 2023, 31, 769–775. [Google Scholar] [CrossRef]
- McNally, E.M.; Khan, S.S. Genetic Testing for Early-Onset Atrial Fibrillation-Is It Time to Personalize Care? JAMA Cardiol. 2022, 7, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Jurgens, S.J.; Rämö, J.T.; Christophersen, I.E.; Rienstra, M.; Chung, M.K.; Olesen, M.S.; Ackerman, M.J.; McNally, E.M.; Semsarian, C.; et al. Genetic Testing in Early-Onset Atrial Fibrillation. Eur. Heart J. 2024, 45, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Abou Ziki, M.D.; Bhat, N.; Neogi, A.; Driscoll, T.P.; Ugwu, N.; Liu, Y.; Smith, E.; Abboud, J.M.; Chouairi, S.; Schwartz, M.A.; et al. Epistatic Interaction of PDE4DIP and DES Mutations in Familial Atrial Fibrillation with Slow Conduction. Hum. Mutat. 2021, 42, 1279–1293. [Google Scholar] [CrossRef]
- Yoneda, Z.T.; Anderson, K.C.; Quintana, J.A.; O’Neill, M.J.; Sims, R.A.; Glazer, A.M.; Shaffer, C.M.; Crawford, D.M.; Stricker, T.; Ye, F.; et al. Early-Onset Atrial Fibrillation and the Prevalence of Rare Variants in Cardiomyopathy and Arrhythmia Genes. JAMA Cardiol. 2021, 6, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Z.T.; Anderson, K.C.; Ye, F.; Quintana, J.A.; O’Neill, M.J.; Sims, R.A.; Sun, L.; Glazer, A.M.; Davogustto, G.; El-Harasis, M.; et al. Mortality Among Patients With Early-Onset Atrial Fibrillation and Rare Variants in Cardiomyopathy and Arrhythmia Genes. JAMA Cardiol. 2022, 7, 733–741. [Google Scholar] [CrossRef]
- Marston, N.A.; Garfinkel, A.C.; Kamanu, F.K.; Melloni, G.M.; Roselli, C.; Jarolim, P.; Berg, D.D.; Bhatt, D.L.; Bonaca, M.P.; Cannon, C.P.; et al. A Polygenic Risk Score Predicts Atrial Fibrillation in Cardiovascular Disease. Eur. Heart J. 2023, 44, 221–231. [Google Scholar] [CrossRef]
- Vad, O.B.; Paludan-Müller, C.; Diederichsen, S.Z.; McKaige, E.A.; Monfort, L.M.; Lundegaard, P.R.; Bertelsen, L.; Andreasen, L.; Svendsen, J.H.; Olesen, M.S. Genetic Determinants of Left Atrial Function Are Associated With Stroke. J. Am. Heart Assoc. 2024, 13, e037490. [Google Scholar] [CrossRef]
- Anderson, O.G.; Cappola, T.P.; Day, S.M. Genetic Testing in Early-Onset Atrial Fibrillation. JAMA Cardiol. 2025. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Kim, D.; Park, B.-E.; Kang, T.S.; Lim, S.-H.; Lee, S.Y.; Chung, Y.H.; Lee, M.-Y.; Yang, P.-S.; et al. Polygenic Risk and Cardiovascular Event Risk in Patients with Atrial Fibrillation with Low to Intermediate Stroke Risk. J. Am. Heart Assoc. 2025, 14, e037727. [Google Scholar] [CrossRef]
- Vad, O.B.; Monfort, L.M.; Paludan-Müller, C.; Kahnert, K.; Diederichsen, S.Z.; Andreasen, L.; Lotta, L.A.; Nielsen, J.B.; Lundby, A.; Svendsen, J.H.; et al. Rare and Common Genetic Variation Underlying Atrial Fibrillation Risk. JAMA Cardiol. 2024, 9, 732–740. [Google Scholar] [CrossRef]
- Miyazawa, K.; Ito, K.; Ito, M.; Zou, Z.; Kubota, M.; Nomura, S.; Matsunaga, H.; Koyama, S.; Ieki, H.; Akiyama, M.; et al. Cross-Ancestry Genome-Wide Analysis of Atrial Fibrillation Unveils Disease Biology and Enables Cardioembolic Risk Prediction. Nat. Genet. 2023, 55, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.T.; Rudd, J.H.F. Polygenic Risk Scores in Atrial Fibrillation: Associations and Clinical Utility in Disease Prediction. Heart Rhythm 2024, 21, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Chalazan, B.; Andrade, J.G.; Macle, L.; Nattel, S.; Tadros, R. Clinical Genetic Testing for Atrial Fibrillation: Are We There Yet? Can. J. Cardiol. 2024, 40, 540–553. [Google Scholar] [CrossRef]
- Marcoux, E.; Sosnowski, D.; Ninni, S.; Mackasey, M.; Cadrin-Tourigny, J.; Roberts, J.D.; Olesen, M.S.; Fatkin, D.; Nattel, S. Genetic Atrial Cardiomyopathies: Common Features, Specific Differences, and Broader Relevance to Understanding Atrial Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2023, 16, 675–698. [Google Scholar] [CrossRef]
- Savelieva, I.; Kakouros, N.; Kourliouros, A.; Camm, A.J. Upstream Therapies for Management of Atrial Fibrillation: Review of Clinical Evidence and Implications for European Society of Cardiology Guidelines. Part I: Primary Prevention. Europace 2011, 13, 308–328. [Google Scholar] [CrossRef]
- Pathak, R.K.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Wong, C.X.; Twomey, D.; Elliott, A.D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef]
- Pathak, R.K.; Elliott, A.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Hendriks, J.M.L.; Twomey, D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals with Atrial Fibrillation: The CARDIO-FIT Study. J. Am. Coll. Cardiol. 2015, 66, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Schön, N.; Kirchhof, P.; Breithardt, G.; Fetsch, T.; Häusler, K.G.; Klein, H.U.; Steinbeck, G.; Wegscheider, K.; Meinertz, T. Angiotensin II-Antagonist in Paroxysmal Atrial Fibrillation (ANTIPAF) Trial. Circ. Arrhythm. Electrophysiol. 2012, 5, 43–51. [Google Scholar] [CrossRef]
- Zhang, M.J.; Norby, F.L.; Lutsey, P.L.; Mosley, T.H.; Cogswell, R.J.; Konety, S.H.; Chao, T.-F.; Shah, A.M.; Solomon, S.D.; Alonso, A.; et al. Association of Left Atrial Enlargement and Atrial Fibrillation with Cognitive Function and Decline: The ARIC-NCS. J. Am. Heart Assoc. 2019, 8, e013197. [Google Scholar] [CrossRef]
- Rienstra, M.; Hobbelt, A.H.; Alings, M.; Tijssen, J.G.P.; Smit, M.D.; Brügemann, J.; Geelhoed, B.; Tieleman, R.G.; Hillege, H.L.; Tukkie, R.; et al. Targeted Therapy of Underlying Conditions Improves Sinus Rhythm Maintenance in Patients with Persistent Atrial Fibrillation: Results of the RACE 3 Trial. Eur. Heart J. 2018, 39, 2987–2996. [Google Scholar] [CrossRef]
- Neefs, J.; van den Berg, N.W.E.; Limpens, J.; Berger, W.R.; Boekholdt, S.M.; Sanders, P.; de Groot, J.R. Aldosterone Pathway Blockade to Prevent Atrial Fibrillation: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 231, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Popovic, D.S.; Pamporis, K.; Theofilis, P.; Nasoufidou, A.; Stachteas, P.; Samaras, A.; Tzikas, A.; Giannakoulas, G.; et al. Effects of Mineralocorticoid Receptor Antagonists on New-Onset or Recurrent Atrial Fibrillation: A Bayesian and Frequentist Network Meta-Analysis of Randomized Trials. Curr. Probl. Cardiol. 2024, 49, 102742. [Google Scholar] [CrossRef]
- Oraii, A.; Healey, J.S.; Kowalik, K.; Pandey, A.K.; Benz, A.P.; Wong, J.A.; Conen, D.; McIntyre, W.F. Mineralocorticoid Receptor Antagonists and Atrial Fibrillation: A Meta-Analysis of Clinical Trials. Eur. Heart J. 2024, 45, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Kapelios, C.J.; Murrow, J.R.; Nührenberg, T.G.; Montoro Lopez, M.N. Effect of Mineralocorticoid Receptor Antagonists on Cardiac Function in Patients with Heart Failure and Preserved Ejection Fraction: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Heart Fail. Rev. 2019, 24, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Sampaio Rodrigues, T.; Garcia Quarto, L.J.; Nogueira, S.C.; Koshy, A.N.; Mahajan, R.; Sanders, P.; Ekinci, E.I.; Burrell, L.M.; Farouque, O.; Lim, H.S. Incidence and Progression of Atrial Fibrillation in Patients with and without Heart Failure Using Mineralocorticoid Receptor Antagonists: A Meta-Analysis. Clin. Res. Cardiol. 2024, 113, 884–897. [Google Scholar] [CrossRef]
- Du, L.; Qin, M.; Yi, Y.; Chen, X.; Jiang, W.; Zhou, L.; Zhang, D.; Xu, K.; Yang, Y.; Li, C.; et al. Eplerenone Prevents Atrial Fibrosis via the TGF-β Signaling Pathway. Cardiology 2017, 138, 55–62. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Schuermans, A.; Vlachakis, P.K.; Klisic, A.; Rizzo, M.; Fragakis, N. Sodium-Glucose Cotransporter 2 Inhibitors and Outcomes in Transthyretin Amyloid Cardiomyopathy: Systematic Review and Meta-Analysis. Eur. J. Clin. Investig. 2025, e14392. [Google Scholar] [CrossRef] [PubMed]
- Dyck, J.R.B.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.C.; Light, P.E.; Zuurbier, C.J. Cardiac Mechanisms of the Beneficial Effects of SGLT2 Inhibitors in Heart Failure: Evidence for Potential off-Target Effects. J. Mol. Cell. Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef]
- Packer, M. SGLT2 Inhibitors: Role in Protective Reprogramming of Cardiac Nutrient Transport and Metabolism. Nat. Rev. Cardiol. 2023, 20, 443–462. [Google Scholar] [CrossRef]
- Søndergaard, E.; Lauritzen, E.S.; Lauritsen, K.M.; Åkerblom, A.; Nuutila, P.; Oldgren, J.; Gormsen, L.C. SGLT2 Inhibition Reduces Myocardial Oxygen Consumption. Metab. Open 2022, 15, 100207. [Google Scholar] [CrossRef]
- El-Saied, S.B.; El-Sherbeny, W.S.; El-Sharkawy, S.I. Impact of Sodium Glucose Co-Transporter-2 Inhibitors on Left Atrial Functions in Patients with Type-2 Diabetes and Heart Failure with Mildly Reduced Ejection Fraction. Int. J. Cardiol. Heart Vasc. 2024, 50, 101329. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.H. Anti-Inflammatory Role of Glucagon-like Peptide 1 Receptor Agonists and Its Clinical Implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222370. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.F.; Pusapati, S.; Anwar, M.S.; Lohana, D.; Kumar, P.; Nandula, S.A.; Nawaz, F.K.; Tracey, K.; Yang, H.; LeRoith, D.; et al. Glucagon-like Peptide-1: A Multi-Faceted Anti-Inflammatory Agent. Front. Immunol. 2023, 14, 1148209. [Google Scholar] [CrossRef]
- McNeish, D. On Using Bayesian Methods to Address Small Sample Problems. Struct. Equ. Model. A Multidiscip. J. 2016, 23, 750–773. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, G.Y.; Maeng, H.J.; Kim, H.; Bae, J.H.; Kim, K.M.; Lim, S. Effects of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination on the Atherosclerosis-Related Process in a Type 2 Diabetes Mouse Model. Endocrinol. Metab. 2021, 36, 157–170. [Google Scholar] [CrossRef]
- Yadav, P.; Khurana, A.; Bhatti, J.S.; Weiskirchen, R.; Navik, U. Glucagon-like Peptide 1 and Fibroblast Growth Factor-21 in Non-Alcoholic Steatohepatitis: An Experimental to Clinical Perspective. Pharmacol. Res. 2022, 184, 106426. [Google Scholar] [CrossRef]
- Li, Q.; Lin, Y.; Wang, S.; Zhang, L.; Guo, L. GLP-1 Inhibits High-Glucose-Induced Oxidative Injury of Vascular Endothelial Cells. Sci. Rep. 2017, 7, 8008. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.-S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-Analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef]
- Solomon, S.D.; Ostrominski, J.W.; Wang, X.; Shah, S.J.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Kitzman, D.W.; Verma, S.; Abildstrøm, S.Z.; et al. Effect of Semaglutide on Cardiac Structure and Function in Patients with Obesity-Related Heart Failure. J. Am. Coll. Cardiol. 2024, 84, 1587–1602. [Google Scholar] [CrossRef]
- Kramer, C.M.; Borlaug, B.A.; Zile, M.M.R.; Ruff, D.; DiMaria, J.M.; Menon, V.; Ou, Y.; Zarante, A.M.; Hurt, K.C.; Murakami, M.; et al. Tirzepatide Reduces LV Mass and Paracardiac Adipose Tissue in Obesity-Related Heart Failure: SUMMIT CMR Substudy. J. Am. Coll. Cardiol. 2024, 85, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Theofilis, P.; Pamporis, K.; Sagris, M.; Vlachakis, P.K.; Koufakis, T.; Antoniadis, A.P.; Fragakis, N. GLP-1 Receptor Agonists and Myocardial Perfusion: Bridging Mechanisms to Clinical Outcomes. Int. J. Mol. Sci. 2025, 26, 3050. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Sagris, M.; Koufakis, T.; Vlachakis, P.K.; Rangraze, I.R.; El Tanani, M.; Tsioufis, K.; et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines 2024, 12, 2112. [Google Scholar] [CrossRef]
- Jiménez, D.L.; Babkowski, M.C.; Miramontes González, J.P. GLP-1 and the Renin-Angiotensin-Aldosterone System. Lancet Diabetes Endocrinol. 2019, 7, 337. [Google Scholar] [CrossRef]
- Miyata, K.N.; Lo, C.-S.; Zhao, S.; Liao, M.-C.; Pang, Y.; Chang, S.-Y.; Peng, J.; Kretzler, M.; Filep, J.G.; Ingelfinger, J.R.; et al. Angiotensin II Up-Regulates Sodium-Glucose Co-Transporter 2 Expression and SGLT2 Inhibitor Attenuates Ang II-Induced Hypertensive Renal Injury in Mice. Clin. Sci. 2021, 135, 943–961. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Kassimis, G.; Koufakis, T.; Klisic, A.; Doumas, M.; Fragakis, N.; Rizzo, M. Therapeutic Potential of Sodium-Glucose Co-Transporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists for Patients with Acute Coronary Syndrome: A Review of Clinical Evidence. Curr. Pharm. Des. 2024, 30, 2109–2119. [Google Scholar] [CrossRef]
- Karakasis, P. Sodium-Glucose Cotransporter 2 Inhibitors in Transthyretin Amyloid Cardiomyopathy: Navigating Potential Benefits and Uncertainties. Curr. Med. Res. Opin. 2025, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Testani, J.M. SGLT2 Inhibitors and Diuretics in Heart Failure: Clicking Reset on the Renal Volume Setpoint? Eur. Heart J. 2023, 44, 2944–2946. [Google Scholar] [CrossRef]
- Blomström-Lundqvist, C.; Naccarelli, G.V.; McKindley, D.S.; Bigot, G.; Wieloch, M.; Hohnloser, S.H. Effect of Dronedarone vs. Placebo on Atrial Fibrillation Progression: A Post Hoc Analysis from ATHENA Trial. Europace 2023, 25, 845–854. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Crijns, H.J.G.M.; van Eickels, M.; Gaudin, C.; Page, R.L.; Torp-Pedersen, C.; Connolly, S.J. Effect of Dronedarone on Cardiovascular Events in Atrial Fibrillation. N. Engl. J. Med. 2009, 360, 668–678. [Google Scholar] [CrossRef]
- Rillig, A.; Borof, K.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Goette, A.; Kuck, K.-H.; Metzner, A.; Vardas, P.; Vettorazzi, E.; et al. Early Rhythm Control in Patients with Atrial Fibrillation and High Comorbidity Burden. Circulation 2022, 146, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Parkash, R.; Wells, G.A.; Rouleau, J.; Talajic, M.; Essebag, V.; Skanes, A.; Wilton, S.B.; Verma, A.; Healey, J.S.; Sterns, L.; et al. Randomized Ablation-Based Rhythm-Control Versus Rate-Control Trial in Patients with Heart Failure and Atrial Fibrillation: Results from the RAFT-AF Trial. Circulation 2022, 145, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-M.; Fang, F.; Zhang, Q.; Yip, G.W.K.; Li, C.M.; Chan, J.Y.-S.; Wu, L.; Fung, J.W.-H. Improvement of Atrial Function and Atrial Reverse Remodeling after Cardiac Resynchronization Therapy for Heart Failure. J. Am. Coll. Cardiol. 2007, 50, 778–785. [Google Scholar] [CrossRef]
- Su, L.; Cai, M.; Wu, S.; Wang, S.; Xu, T.; Vijayaraman, P.; Huang, W. Long-Term Performance and Risk Factors Analysis after Permanent His-Bundle Pacing and Atrioventricular Node Ablation in Patients with Atrial Fibrillation and Heart Failure. Europace 2020, 22, ii19–ii26. [Google Scholar] [CrossRef]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA Clinical Consensus Statement on Conduction System Pacing Implantation: Executive Summary. Endorsed by the Asia-Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS) and Latin-American Heart Rhythm Society (LAHRS). Europace 2023, 25, 1237–1248. [Google Scholar] [CrossRef]
- Marsan, N.A.; Maffessanti, F.; Tamborini, G.; Gripari, P.; Caiani, E.; Fusini, L.; Muratori, M.; Zanobini, M.; Alamanni, F.; Pepi, M. Left Atrial Reverse Remodeling and Functional Improvement after Mitral Valve Repair in Degenerative Mitral Regurgitation: A Real-Time 3-Dimensional Echocardiography Study. Am. Heart J. 2011, 161, 314–321. [Google Scholar] [CrossRef]
- John, B.; Stiles, M.K.; Kuklik, P.; Brooks, A.G.; Chandy, S.T.; Kalman, J.M.; Sanders, P. Reverse Remodeling of the Atria after Treatment of Chronic Stretch in Humans: Implications for the Atrial Fibrillation Substrate. J. Am. Coll. Cardiol. 2010, 55, 1217–1226. [Google Scholar] [CrossRef]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and Molecular Electrophysiology of Atrial Fibrillation Initiation, Maintenance, and Progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef]
- Walters, T.E.; Nisbet, A.; Morris, G.M.; Tan, G.; Mearns, M.; Teo, E.; Lewis, N.; Ng, A.; Gould, P.; Lee, G.; et al. Progression of Atrial Remodeling in Patients with High-Burden Atrial Fibrillation: Implications for Early Ablative Intervention. Heart Rhythm 2016, 13, 331–339. [Google Scholar] [CrossRef]
- Andrade, J.G.; Deyell, M.W.; Macle, L.; Wells, G.A.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; et al. Progression of Atrial Fibrillation after Cryoablation or Drug Therapy. N. Engl. J. Med. 2023, 388, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, H.; Prabhu, S.; Voskoboinik, A.; Young, S.; Gutman, S.J.; Wong, G.R.; Parameswaran, R.; Nalliah, C.J.; Lee, G.; McLellan, A.J.; et al. Atrial Remodeling Following Catheter Ablation for Atrial Fibrillation-Mediated Cardiomyopathy: Long-Term Follow-Up of CAMERA-MRI Study. JACC Clin. Electrophysiol. 2019, 5, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Sagris, M.; Pamporis, K.; Stachteas, P.; Sidiropoulos, G.; Vlachakis, P.K.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Artificial Intelligence in Atrial Fibrillation: From Early Detection to Precision Therapy. J. Clin. Med. 2025, 14, 2627. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Model/System | Key Findings | Mechanistic Insight | Implications for AF Pathogenesis |
|---|---|---|---|---|
| Yao et al., 2018 [16] | Human atrial samples, ATP dog model, CM-specific NLRP3 knock-in, and knockout mice | NLRP3 inflammasome activity is upregulated in atrial cardiomyocytes of patients with AF. Cardiomyocyte-specific activation of NLRP3 promotes atrial ectopy, shortened AERP, Ca2+ dysregulation, and structural remodeling. Genetic and pharmacological inhibition of NLRP3 reduces AF susceptibility. | NLRP3 activation in cardiomyocytes leads to increased RyR2-mediated Ca2+ leak, enhanced IKur via Kv1.5 upregulation, atrial fibrosis, and reentry substrate formation independent of immune cell infiltration. | Establishes a causative role for cardiomyocyte-intrinsic NLRP3 inflammasome signaling in AF initiation and maintenance. Identifies NLRP3 as a therapeutic target to mitigate electrical and structural remodeling in AF. |
| Song et al., 2024 [17] | Wildtype and Aim2−/− mice fed high-protein or normal chow diets | High-protein diet (HPD) enhances atrial arrhythmogenesis via AIM2 inflammasome activation. AIM2 deficiency attenuates AF susceptibility, diastolic Ca2+ leak, and mitochondrial ROS production. | HPD induces mitochondrial damage, increasing cytoplasmic dsDNA and activating AIM2 inflammasome. This leads to caspase-1 cleavage, IL-1β/IL-18 production, and aberrant Ca2+ handling without atrial fibrosis. | Establishes AIM2 as a novel inflammasome pathway promoting AF under dietary stress. Highlights the role of mitochondrial dsDNA and oxidative stress in atrial substrate formation. |
| Hulsmans et al., 2023 [18] | Human atrial tissue (scRNA-seq), HOMER mouse model, CCR2−/− and Spp1−/− mice | SPP1+ macrophages expand in human AF and HOMER mouse model. Recruited macrophages (via CCR2) and their secretion of SPP1 amplify atrial fibrosis and AF susceptibility. Deleting CCR2 or Spp1 reduces AF inducibility and burden in mice. | Monocyte-derived CCR2+ macrophages express SPP1, promoting fibroblast activation, inflammation, and fibrosis via integrin and CD44 signaling. SPP1 deletion limits macrophage proliferation and fibroblast collagen production. | Demonstrates macrophage-driven immunofibrotic remodeling in AF. SPP1 and CCR2 are highlighted as targets for immunomodulatory therapy in atrial cardiomyopathy. |
| Zhang et al., 2022 [19] | FMT rat model, primary atrial cells, clinical human data | Aged gut microbiota increases AF susceptibility by promoting LPS and glucose-induced NLRP3 inflammasome activation. Transplantation of aged microbiota induces atrial fibrosis, increased circulating LPS, and impaired glucose tolerance. MCC950 and LPS-RS reduce AF inducibility and fibrosis. Young microbiota transplantation reverses the aged phenotype. | Aged microbiota causes gut barrier dysfunction, elevating circulating LPS. LPS and glucose co-activate NLRP3 inflammasome in CFs via TLR4. This promotes atrial fibrosis through IL-1β/TGF-β1 signaling. CMs are not responsive to this pathway. | Establishes the gut–heart axis in ageing-related AF via microbiota-intestinal barrier–NLRP3 signaling. Highlights microbiota modulation and inflammasome inhibition as therapeutic strategies in elderly AF patients. |
| Ishii et al., 2021 [20] | Human left atrial appendage tissue and CT imaging in AF patients | Epicardial adipose tissue (EAT) adjacent to atrial myocardium shows greater fibrosis, inflammation, and smaller adipocytes than central EAT. The central-to-marginal adipocyte diameter ratio correlates with EAT and atrial myocardial fibrosis. CT-derived %change in EAT fat attenuation noninvasively reflects fibrotic remodeling. | Proinflammatory cytokines (IL-6, TNF-α, TGF-β1) impair adipogenesis and enhance fibrosis in marginal EAT, which in turn promotes atrial fibrosis. CT imaging detects fibrotic remodeling via increased fat attenuation linked to adipocyte size and fibrosis. | Establishes EAT fibrosis as a profibrotic substrate for AF. Highlights imaging-based biomarkers (fat attenuation change) to detect fibrotic remodeling and stratify AF progression risk. |
| Adili et al., 2022 [21] | Human LAA tissue, HL-1 atrial cardiomyocytes with tachypacing | Atrial fibrillation induces atrial cardiomyocyte senescence marked by increased SA-β-gal activity, p21/p16/p53 expression, and DNA damage (γH2AX). Senescence burden correlates with early AF recurrence after the maze procedure. Tachypacing induces senescence and SASP in HL-1 cells; siRNA p21 knockdown reduces senescence and restores SR Ca2+ protein expression. | Proinflammatory cytokines (IL-6, TNF-α, TGF-β1) impair adipogenesis and enhance fibrosis in marginal EAT, which in turn promotes atrial fibrosis. CT imaging detects fibrotic remodeling via increased fat attenuation linked to adipocyte size and fibrosis. | Identifies atrial cardiomyocyte senescence as a novel contributor to AF progression and recurrence. Suggests that anti-senescence strategies may ameliorate electrical and structural remodeling in AF. |
| Kuck et al., 2018 [22] | Clinical study (FIRE AND ICE trial, 750 patients with paroxysmal AF) | Female sex was independently associated with a 37% increased risk of AF recurrence and a 36% increased risk of cardiovascular rehospitalization after catheter ablation. Prior DCCV and hypertension were also independent predictors of poor outcomes. History of AF duration predicted repeat ablation. | Sex differences in clinical outcomes may reflect underlying differences in atrial substrate, referral patterns, or hormonal influences. Female patients often had more advanced disease at the time of ablation and were at higher risk for groin complications and procedural adverse events. | Highlights sex-specific vulnerability in AF outcomes, suggesting a potential role for sex-based atrial remodeling. Supports earlier intervention and tailored risk stratification in women with AF. |
| Guta et al., 2021 [23] | Clinical echocardiographic study (83 AF patients vs. 83 healthy controls) | RA dilation (RAVmin) is the primary determinant of tricuspid annulus (TA) dilation and FTR severity in patients with persistent AF. RV volume and function had limited contribution to FTR. TA area and RAVmin independently predicted FTR grade. TA dilation preceded leaflet tethering. | AF-induced RA remodeling leads to TA dilation, flattening of its saddle shape, and coaptation failure, promoting atriogenic FTR. Right atrial volume (especially RAVmin) is the most reliable indicator of TA geometry and FTR severity. | Supports a direct atriogenic mechanism linking RA dilation and FTR in AF. Emphasizes early rhythm control to prevent progressive RA/TA remodeling and right-sided valve dysfunction. |
| Perike et al., 2023 [13] | Human RAA tissue, HL-1 atrial cells, lentiviral PPP1R12C mouse model | PPP1R12C expression is upregulated in human AF, promoting PP1c targeting to MLC2a, reducing its phosphorylation, and leading to atrial hypocontractility. Overexpression in mice increases atrial dilation, reduces contractility, and enhances AF susceptibility. MRCK inhibition increases PPP1R12C activity and MLC2a dephosphorylation. | PPP1R12C regulates sarcomeric function by directing PP1c to dephosphorylate MLC2a. This reduces contractility independent of calcium handling. PPP1R12C is modulated by RhoA/MRCK signaling and represents a dynamic node in atrial myofilament remodeling. | Identifies PPP1R12C as a key mediator of atrial hypocontractility and a potential upstream regulator of stroke risk in AF. Therapeutic inhibition may restore contractility and reduce AF recurrence and thromboembolic risk. |
| Suffee et al., 2022 [24] | C57BL/6J mice subjected to 16-week high-fat diet (HFD) vs. normal diet | HFD mice developed AF vulnerability, atrial dilation, and a distinct atrial metabolic profile with lipid accumulation, enhanced β-oxidation, adipogenesis, and inflammation. Action potentials shortened due to activation of K-ATP channels. HADHA activity and palmitate oxidation increased. Adipogenic markers and immune infiltration (macrophages, T cells) were upregulated in the atria. | HFD induces a shift from glycolysis to fatty acid oxidation in atrial myocardium, activating K-ATP channels and shortening AP duration. FA accumulation triggers adipogenic transformation and low-grade inflammation, contributing to arrhythmogenic substrate. | Links diet-induced metabolic dysregulation to electrical, structural, and immunologic remodeling of atrial myocardium. Supports targeting atrial metabolic pathways as a strategy to prevent obesity-related AF. |
| Su et al., 2022 [12] | Atrial-specific AMPK α1/α2 knockout mice, in vitro AMPK knockdown in atrial myocytes | AMPK deletion in atrial cardiomyocytes leads to progressive conduction and repolarization abnormalities, atrial ectopy, and spontaneous AF. Electrical remodeling precedes fibrosis and chamber dilation. Pitx2c and Mef2c downregulation mediates transcriptional reprogramming of ion channels and connexins. | AMPK loss alters ion channel and gap junction protein expression (Nav1.5, Kir2.1, Gja1, Gja5) via suppression of Pitx2c and Mef2c. This causes early left atrial conduction delay and prolonged APD, promoting arrhythmogenesis. Right atrial dilation appears early, followed by biatrial fibrosis. | Reveals AMPK as a key homeostatic regulator of atrial electrophysiology and transcriptional identity. Suggests impaired AMPK signaling as a metabolic driver of AF, with potential for upstream preventive therapy. |
| Hopman et al., 2022 [25] | Clinical study (47 AF patients undergoing pre-ablation LGE-CMR) | Quantification of LA fibrosis using IIR 1.2 and 3SD methods shows significant variation in estimated fibrotic burden (29.80% vs. 8.43%, respectively). Despite good correlation (r = 0.85), agreement is poor (ICC = 0.19), and 34% of patients are reclassified into different fibrosis categories depending on the method. Agreement between CEMRG and ADAS 3D LA was high (ICC = 0.93) when using identical thresholds. | Different fibrosis quantification methods using the LA blood pool as reference produce inconsistent results due to dependence on blood pool signal-to-noise ratio (SNR). This affects both inter-method agreement and patient classification. | Highlights methodological variability in assessing LA fibrosis via LGE-CMR, with implications for risk stratification and treatment selection. Emphasizes the need for standardization and histological validation of fibrosis thresholds. |
| Kawasaki et al., 2021 [26] | Human left atrial tissue (AF vs. non-AF), proteomics and transcriptomics | Proteomics and GSEA reveal upregulation of neutrophil degranulation, oxidative phosphorylation, and ECM disassembly in AF. NETs and neutrophil granule genes (LCN2, S100A8/9) were elevated in AF tissue. MYH10, required for ciliogenesis, was downregulated in fibroblasts. MYH10 deficiency was associated with impaired primary cilia and fibrotic signaling. | Neutrophil degranulation proteins (MPO, ELANE, MMP9) interlink multiple biological processes (inflammation, oxidative stress, ECM remodeling). Cilium assembly is repressed in AF fibroblasts, potentially impairing antifibrotic signaling via TGF-β and angiotensin II. | Identifies neutrophil degranulation as a hub process coordinating inflammation, metabolism, and fibrosis in AF. Suggests primary cilium loss in fibroblasts contributes to profibrotic remodeling, providing novel mechanistic insight and therapeutic targets. |
| Meulendijks et al., 2023 [27] | Human EAT and LAA samples (AF, future-onset AF, and non-AF patients), atrial fibroblast culture | EAT secretome from AF patients induces COL1A1 and FN1 gene expression in atrial fibroblasts. Myeloperoxidase (MPO) is the most upregulated protein in EAT and EAT secretome in AF, especially in persistent and future-onset AF. NETs and MPO aggregates localize in fibrofatty infiltrates and subepicardial layers. | EAT-derived neutrophils secrete MPO and NETs, promoting fibroblast activation and ECM remodeling. MPO co-localizes with fibrofatty infiltrates, suggesting involvement in epithelial-to-mesenchymal transition and conduction abnormalities. | Reveals EAT neutrophil activity as a driver of atrial fibrosis and potential early biomarker of AF development. Targets like MPO and neutrophil pathways offer novel therapeutic avenues. |
| van den Berg et al., 2021 [28] | Clinical cohort (n = 150, LAA tissue, blood biomarkers), prospective 2-year follow-up | In patients without prior AF, increased atrial expression of COL1A1, COL3A1, COL8A2, TNC, THBS2, BGN, and EDN1 predicted incident AF. Plasma TNC and COL8A2 correlated with tissue levels and improved AF prediction. Histological remodeling was subtle but included increased epicardial fibrosis and mesenchymal cell content. | Subclinical ECM remodeling with upregulation of collagens, matricellular proteins, and fibroblast-activating factors precedes AF onset. Early transcriptional changes occur before visible interstitial fibrosis, suggesting epigenetic or cellular priming of the atrial substrate. | Establishes that atrial remodeling precedes AF onset in high-risk patients. Identifies early molecular biomarkers (COL8A2, TNC) and gene panels that may guide primary prevention strategies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Ktenopoulos, N.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts. J. Clin. Med. 2025, 14, 3250. https://doi.org/10.3390/jcm14093250
Karakasis P, Theofilis P, Vlachakis PK, Ktenopoulos N, Patoulias D, Antoniadis AP, Fragakis N. Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts. Journal of Clinical Medicine. 2025; 14(9):3250. https://doi.org/10.3390/jcm14093250
Chicago/Turabian StyleKarakasis, Paschalis, Panagiotis Theofilis, Panayotis K. Vlachakis, Nikolaos Ktenopoulos, Dimitrios Patoulias, Antonios P. Antoniadis, and Nikolaos Fragakis. 2025. "Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts" Journal of Clinical Medicine 14, no. 9: 3250. https://doi.org/10.3390/jcm14093250
APA StyleKarakasis, P., Theofilis, P., Vlachakis, P. K., Ktenopoulos, N., Patoulias, D., Antoniadis, A. P., & Fragakis, N. (2025). Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts. Journal of Clinical Medicine, 14(9), 3250. https://doi.org/10.3390/jcm14093250








