Inter-Relationships Between the Deep Learning-Based Pachychoroid Index and Clinical Features Associated with Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Ophthalmic Examinations
2.3. The Neural Network for Our Deep Learning-Based Pachychoroid Index
2.4. Endpoints and Statistical Analyses
3. Results
3.1. Clinical Background
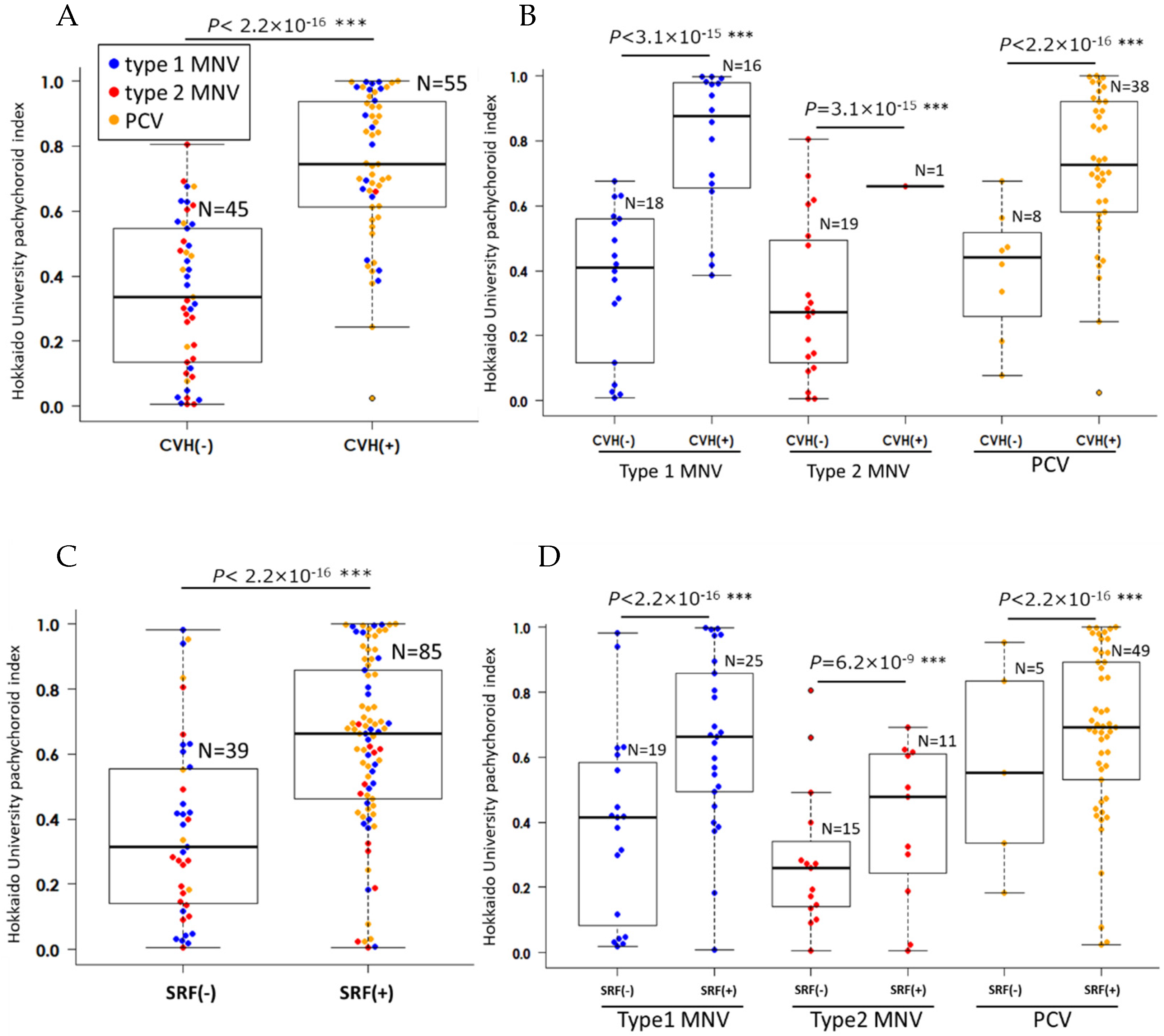
3.2. Comparison of HUPI Between Types of nAMD
3.3. Analysis of Factors Contributing to HUPI
3.4. The Interactions Between Clinical nAMD Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Warrow, D.J.; Hoang, Q.V.; Freund, K.B. Pachychoroid pigment epitheliopathy. Retina 2013, 33, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef]
- Borooah, S.; Sim, P.Y.; Phatak, S.; Moraes, G.; Wu, C.Y.; Cheung, C.M.G.; Pal, B.; Bujarborua, D. Pachychoroid spectrum disease. Acta Ophthalmol. 2021, 99, e806–e822. [Google Scholar] [CrossRef]
- Kido, A.; Miyake, M.; Tamura, H.; Hiragi, S.; Kimura, T.; Yoshida, S.; Takeuchi, M.; Ohtera, S.; Takahashi, A.; Ooto, S.; et al. Incidence and clinical practice of exudative age-related macular degeneration: A nationwide population-based cohort study. Ophthalmol. Sci. 2022, 2, 100215. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, Y.; Miyake, M.; Yamashiro, K.; Ooto, S.; Takahashi, A.; Oishi, A.; Miyata, M.; Uji, A.; Muraoka, Y.; Tsujikawa, A. Deep phenotype unsupervised machine learning revealed the significance of pachychoroid features in etiology and visual prognosis of age-related macular degeneration. Sci. Rep. 2020, 10, 18423. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Kishi, S.; Akiyama, H. Clinical characteristics and pachychoroid incidence in Japanese patients with neovascular age-related macular degeneration. Sci. Rep. 2022, 12, 4492. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Yamashiro, K.; Yanagi, Y.; Koizumi, H.; Matsumoto, H.; Cheung, C.M.G.; Gomi, F.; Iida, T.; Tsujikawa, A. Relationship between Pachychoroid and Polypoidal Choroidal Vasculopathy. J. Clin. Med. 2022, 11, 4614. [Google Scholar] [CrossRef]
- Fukutsu, K.; Saito, M.; Noda, K.; Murata, M.; Kase, S.; Shiba, R.; Isogai, N.; Asano, Y.; Hanawa, N.; Dohke, M.; et al. A Deep Learning Architecture for Vascular Area Measurement in Fundus Images. Ophthalmol. Sci. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Fukutsu, K.; Saito, M.; Noda, K.; Murata, M.; Kase, S.; Shiba, R.; Isogai, N.; Asano, Y.; Hanawa, N.; Dohke, M.; et al. Relationship between Brachial-Ankle Pulse Wave Velocity and Fundus Arteriolar Area Calculated Using a Deep-Learning Algorithm. Curr. Eye Res. 2022, 47, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Mitamura, M.; Fukutsu, K.; Dong, Z.; Ando, R.; Kase, S.; Noda, K.; Katsuta, S.; Ishida, S. Retinal arteriovenous information improves the prediction accuracy of deep learning-based pulse wave velocity from color fundus photographs. Investig. Ophthalmol. Vis. Sci. 2025, 66, 63. [Google Scholar] [CrossRef]
- Mitamura, M.; Saito, M.; Fukutsu, K.; Dong, Z.; Ando, R.; Kase, S.; Noda, K.; Shiba, R.; Isogai, N.; Dohke, M.; et al. Sex differences in age-related changes in retinal arteriovenous area based on deep learning segmentation model. Ophthalmol. Sci. 2025, 5, 100719. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Mitamura, M.; Kimura, M.; Ito, Y.; Endo, H.; Katsuta, S.; Kase, M.; Ishida, S. Grad-CAM-Based Investigation into Acute-Stage Fluorescein Angiography Images to Predict Long-Term Visual Prognosis of Branch Retinal Vein Occlusion. J. Clin. Med. 2024, 13, 5271. [Google Scholar] [CrossRef]
- Mitamura, M.; Saito, M.; Hirooka, K.; Dong, Z.; Ando, R.; Kase, S.; Ishida, S. Differences in artificial intelligence-based macular fluid parameters between clinical stages of diabetic macular edema and their relationship with visual acuity. J. Clin. Med. 2025, 14, 1007. [Google Scholar] [CrossRef]
- Saito, M.; Mitamura, M.; Ito, Y.; Endo, H.; Katsuta, S.; Ishida, S. A novel deep learning-based pachychoroid index based on choroidal image patterns of enhanced-depth-imaging optical coherence tomography. Hokkaido University, Sapporo, Japan. 2025; to be submitted. [Google Scholar]
- Invernizzi, A.; Benatti, E.; Cozzi, M.; Erba, S.; Vaishnavi, S.; Vupparaboina, K.K.; Staurenghi, G.; Chhablani, J.; Gillies, M.; Viola, F. Choroidal Structural Changes Correlate With Neovascular Activity in Neovascular Age Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3836–3841. [Google Scholar] [CrossRef]
- Kumar, J.B.; Wai, K.M.; Ehlers, J.P.; Singh, R.P.; Rachitskaya, A.V. Subfoveal choroidal thickness as a prognostic factor in exudative age-related macular degeneration. Br. J. Ophthalmol. 2019, 103, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Azar, G.; Wolff, B.; De Bats, F.; Halfon, J.; Streho, M.; Tick, S.; Castelnovo, L.; Michel, G.; Masse, H.; Vasseur, V.; et al. Morphological Predictive Features on Spectral-Domain Optical Coherence Tomography for Visual Outcomes in Neovascular Age-Related Macular Degeneration Treated with Ranibizumab. BioMed Res. Int. 2018, 2018, 7438083. [Google Scholar] [CrossRef]
- Takahashi, Y.; Koizumi, H.; Hasegawa, T.; Izumi, T.; Maruko, I.; Sonoda, S.; Sakamoto, T.; Iida, T. Comparison of subfoveal choroidal structures in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Jpn. J. Ophthalmol. 2018, 62, 576–583. [Google Scholar] [CrossRef]
- Wei, X.; Ting, D.S.W.; Ng, W.Y.; Khandelwal, N.; Agrawal, R.; Cheung, C.M.G. Choroidal Vascularity Index: A Novel Optical Coherence Tomography Based Parameter in Patients With Exudative Age-Related Macular Degeneration. Retina 2017, 37, 1120–1125. [Google Scholar] [CrossRef]
- Bakthavatsalam, M.; Ng, D.S.; Lai, F.H.; Tang, F.Y.; Brelén, M.E.; Tsang, C.W.; Lai, T.Y.-Y.; Cheung, C.Y.-L. Choroidal structures in polypoidal choroidal vasculopathy, neovascular age-related maculopathy, and healthy eyes determined by binarization of swept source optical coherence tomographic images. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 935–943. [Google Scholar] [CrossRef]
- Daizumoto, E.; Mitamura, Y.; Sano, H.; Akaiwa, K.; Niki, M.; Yamanaka, C.; Kinoshita, T.; Egawa, M.; Sonoda, S.; Sakamoto, T. Changes of choroidal structure after intravitreal aflibercept therapy for polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2017, 101, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y. Pachychoroid disease: A new perspective on exudative maculopathy. Jpn. J. Ophthalmol. 2020, 64, 323–337. [Google Scholar] [CrossRef]
- Tong, J.P.; Chan, W.M.; Liu, D.T.; Lai, T.Y.; Choy, K.-W.; Pang, C.-P.; Lam, D.S. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am. J. Ophthalmol. 2006, 141, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, Y.; Yoshikawa, M.; Miyake, M.; Tabara, Y.; Ahn, J.; Woo, S.J.; Honda, S.; Sakurada, Y.; Shiragami, C.; Nakanishi, H.; et al. CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proc. Natl. Acad. Sci. USA 2018, 115, 6261–6266. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, N.K.; Ahn, S.J.; Park, K.H.; Ahn, J.; Seo, J.; Han, J.W.; Kim, K.W.; Woo, S.J. Thickness of retina and choroid in the elderly population and its association with Complement Factor H polymorphism: KLoSHA Eye study. PLoS ONE 2018, 13, e0209276. [Google Scholar] [CrossRef]
- Tittl, M.; Polska, E.; Kircher, K.; Kruger, A.; Maar, N.; Stur, M.; Schmetterer, L. Topical fundus pulsation measurement in patients with active central serous chorioretinopathy. Arch. Ophthalmol. 2003, 121, 975–978. [Google Scholar] [CrossRef]
- Saito, M.; Saito, W.; Hashimoto, Y.; Yoshizawa, C.; Fujiya, A.; Noda, K.; Ishida, S. Macular choroidal blood flow velocity decreases with regression of acute central serous chorioretinopathy. Br. J. Ophthalmol. 2013, 97, 775–780. [Google Scholar] [CrossRef]
- Saito, M.; Noda, K.; Saito, W.; Ishida, S. Relationship between choroidal blood flow velocity and choroidal thickness in patients with regression of acute central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 227–229. [Google Scholar] [CrossRef]
- Saito, M.; Saito, W.; Hirooka, K.; Hashimoto, Y.; Mori, S.; Noda, K.; Ishida, S. Pulse Waveform Changes in Macular Choroidal Hemodynamics With Regression of Acute Central Serous Chorioretinopathy. Invest. Ophthalmol. Vis. Sci. 2015, 56, 6515–6522. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Imanaga, N.; Terao, N. Central serous chorioretinopathy and the sclera: What we have learned so far. Jpn. J. Ophthalmol. 2024, 68, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Saito, M.; Yamashita, Y.; Hashimoto, Y.; Terao, N.; Koizumi, H.; Noda, K.; Ishida, S. Imbalanced choroidal circulation in eyes with asymmetric dilated vortex vein. Jpn. J. Ophthalmol. 2022, 66, 14–18. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | |
|---|---|
| Age (years) | 75.9 ± 8.6 |
| Sex (male: female) | 71:40 |
| Type of MNV | n (%) |
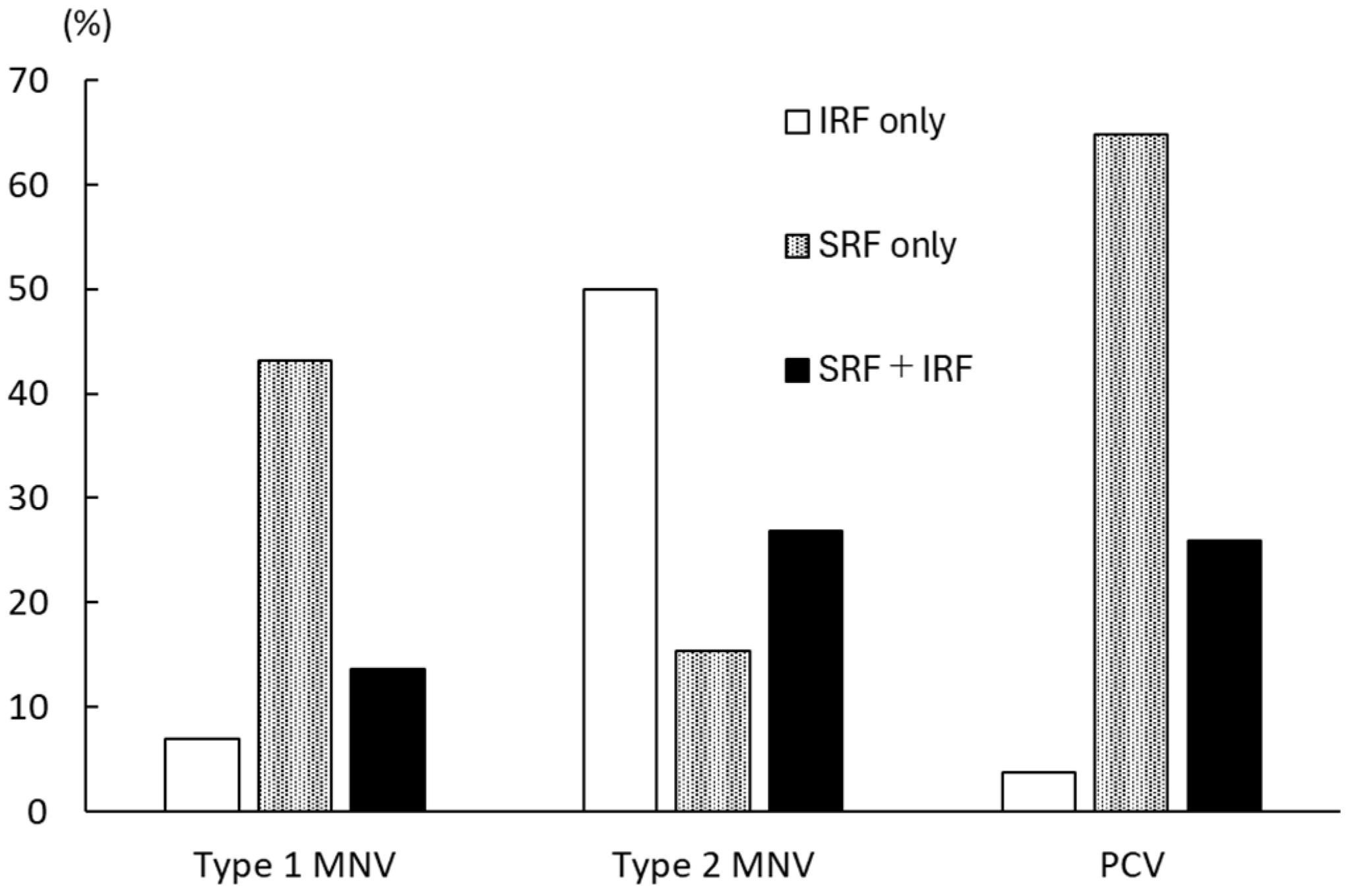
| Type 1 MNV | 44 (35.5) |
| Type 2 MNV | 26 (21.0) |
| PCV | 54 (43.5) |
| Subtype of retinal fluid | n (%) |
| IRF only | 58 (46.8) |
| SRF only | 24 (19.4) |
| IRF + SRF | 18 (14.5) |
| HUPI | ||||
|---|---|---|---|---|
| Simple Linear Regression Analysis | Multiple Linear Regression Analysis | |||
| r | p Value | β | p Value | |
| Age (years) | −0.31 | 3.5 × 10−4 | - | - |
| Sex (male/female: 1/0) | 0.045 | 0.62 | - | - |
| LogMAR BCVA | −0.06 | 0.51 | - | - |
| SRF | 0.42 | 1.1 × 10−6 | 0.16 | 0.017 |
| IRF | −0.11 | 0.21 | - | - |
| CVH | 0.64 | 4.5 × 10−13 | 0.49 | 2.6 × 10−6 |
| Polypoidal lesions | 0.34 | 1.0 × 10−4 | - | - |
| Type 1 MNV | 0.12 | 0.19 | - | - |
| Type 2 MNV | −0.11 | 0.23 | - | - |
| Objective Variables | r | p Value | β | p Value |
|---|---|---|---|---|
| Explanatory Variables | ||||
| LogMAR BCVA | ||||
| IRF | 0.46 | 4.6 × 10−8 | 0.84 | 1.5 × 10−5 |
| SRF | ||||
| HUPI | 0.42 | 1.1 × 10−6 | 0.51 | 0.031 |
| Polypoidal lesions | 0.41 | 1.1 × 10−6 | 0.61 | 0.028 |
| IRF | ||||
| Type 1 MNV | 0.11 | 0.22 | 0.2 | 0.047 |
| Type 2 MNV | 0.69 | 2.2 × 10−16 | 0.6 | 0.0015 |
| CVH | ||||
| Polypoidal lesions | 0.52 | 1.9 × 10−8 | 0.44 | 0.0021 |
| HUPI | 0.64 | 4.5 × 10−13 | 0.99 | 4.6 × 10−7 |
| Polypoidal lesions | ||||
| CVH | 0.52 | 1.9 × 10−8 | 0.86 | 0.06 |
| Type 1 MNV | ||||
| Not detected | - | - | - | - |
| Type 2 MNV | ||||
| IRF | 0.69 | 2.2 × 10−6 | 0.67 | 3.5 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, M.; Mitamura, M.; Ito, Y.; Endo, H.; Katsuta, S.; Ishida, S. Inter-Relationships Between the Deep Learning-Based Pachychoroid Index and Clinical Features Associated with Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2025, 14, 3245. https://doi.org/10.3390/jcm14093245
Saito M, Mitamura M, Ito Y, Endo H, Katsuta S, Ishida S. Inter-Relationships Between the Deep Learning-Based Pachychoroid Index and Clinical Features Associated with Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine. 2025; 14(9):3245. https://doi.org/10.3390/jcm14093245
Chicago/Turabian StyleSaito, Michiyuki, Mizuho Mitamura, Yuki Ito, Hiroaki Endo, Satoshi Katsuta, and Susumu Ishida. 2025. "Inter-Relationships Between the Deep Learning-Based Pachychoroid Index and Clinical Features Associated with Neovascular Age-Related Macular Degeneration" Journal of Clinical Medicine 14, no. 9: 3245. https://doi.org/10.3390/jcm14093245
APA StyleSaito, M., Mitamura, M., Ito, Y., Endo, H., Katsuta, S., & Ishida, S. (2025). Inter-Relationships Between the Deep Learning-Based Pachychoroid Index and Clinical Features Associated with Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine, 14(9), 3245. https://doi.org/10.3390/jcm14093245






