Comparing Auto-Machine Learning and Expert-Designed Models in Diagnosing Vitreomacular Interface Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Building the Dataset
2.2. Designing Expert Model
2.2.1. Dataset and Image Preprocessing
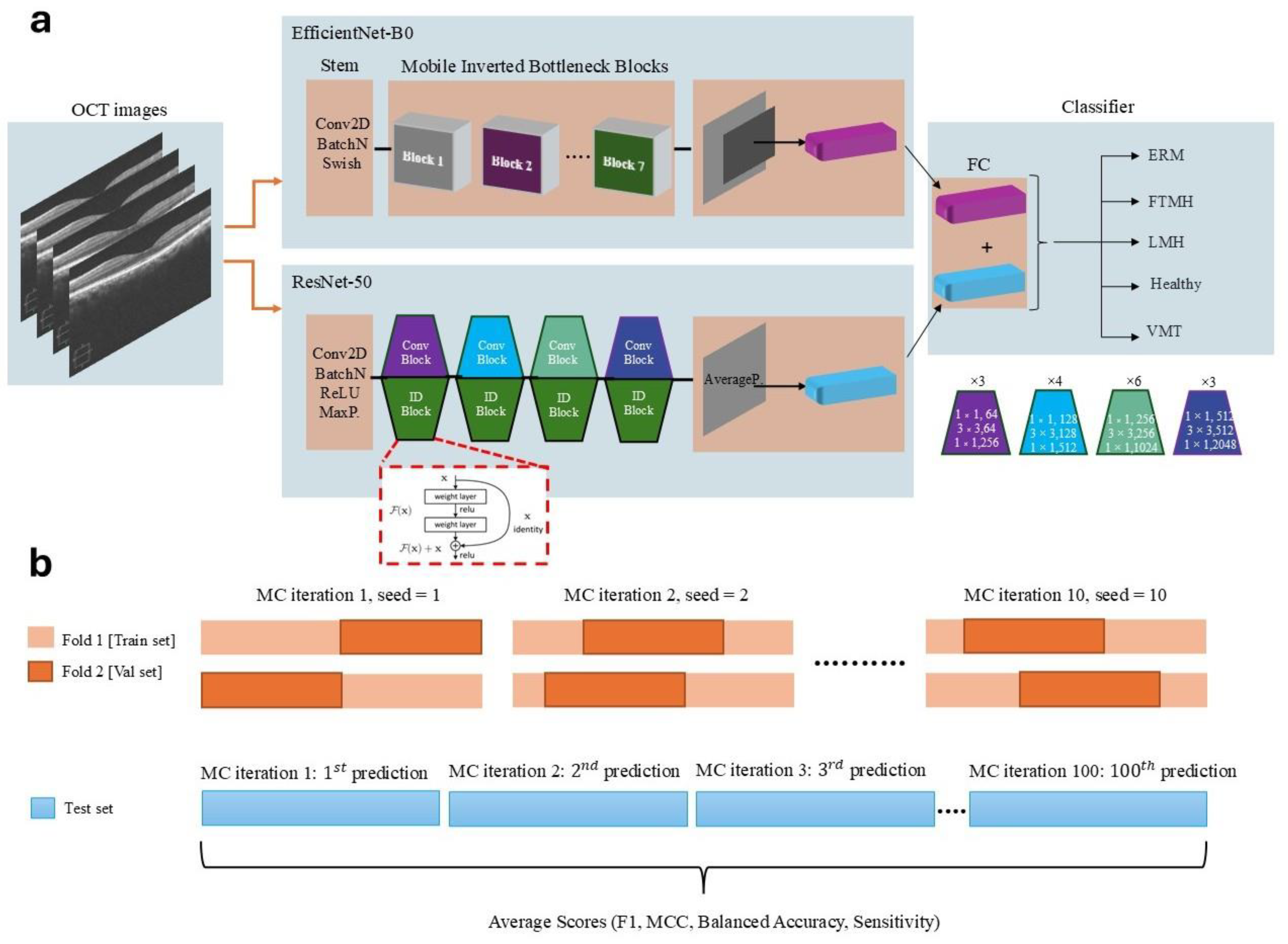
2.2.2. Model Architecture and Feature Extraction
2.2.3. Cross-Validation and Monte Carlo Sampling
2.2.4. Training Procedure
2.3. Designing AutoML Model
2.3.1. Dataset Preparation
2.3.2. Model Development
2.3.3. Training and Optimization
2.4. Evaluation Metrics
3. Results
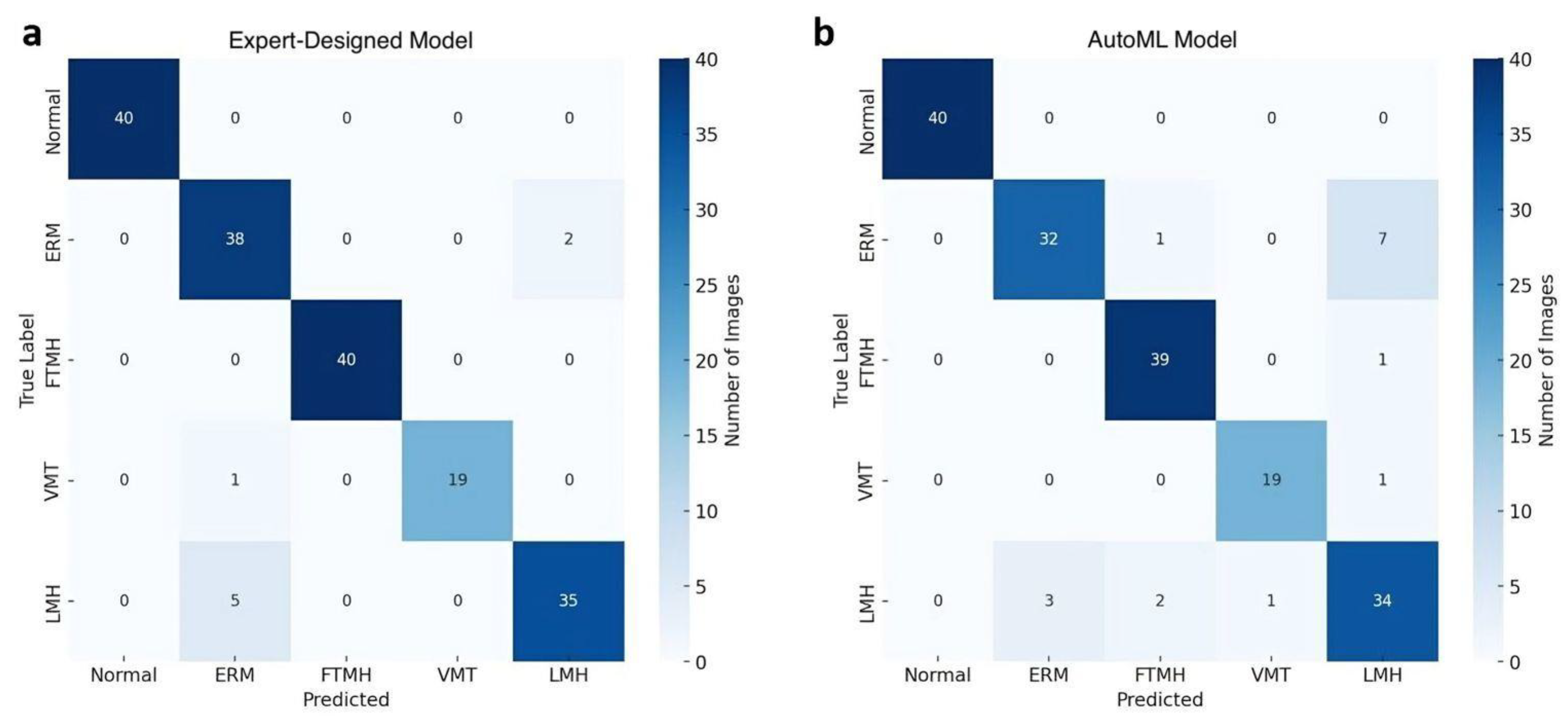
3.1. Expert-Designed Model
3.2. AutoML Model
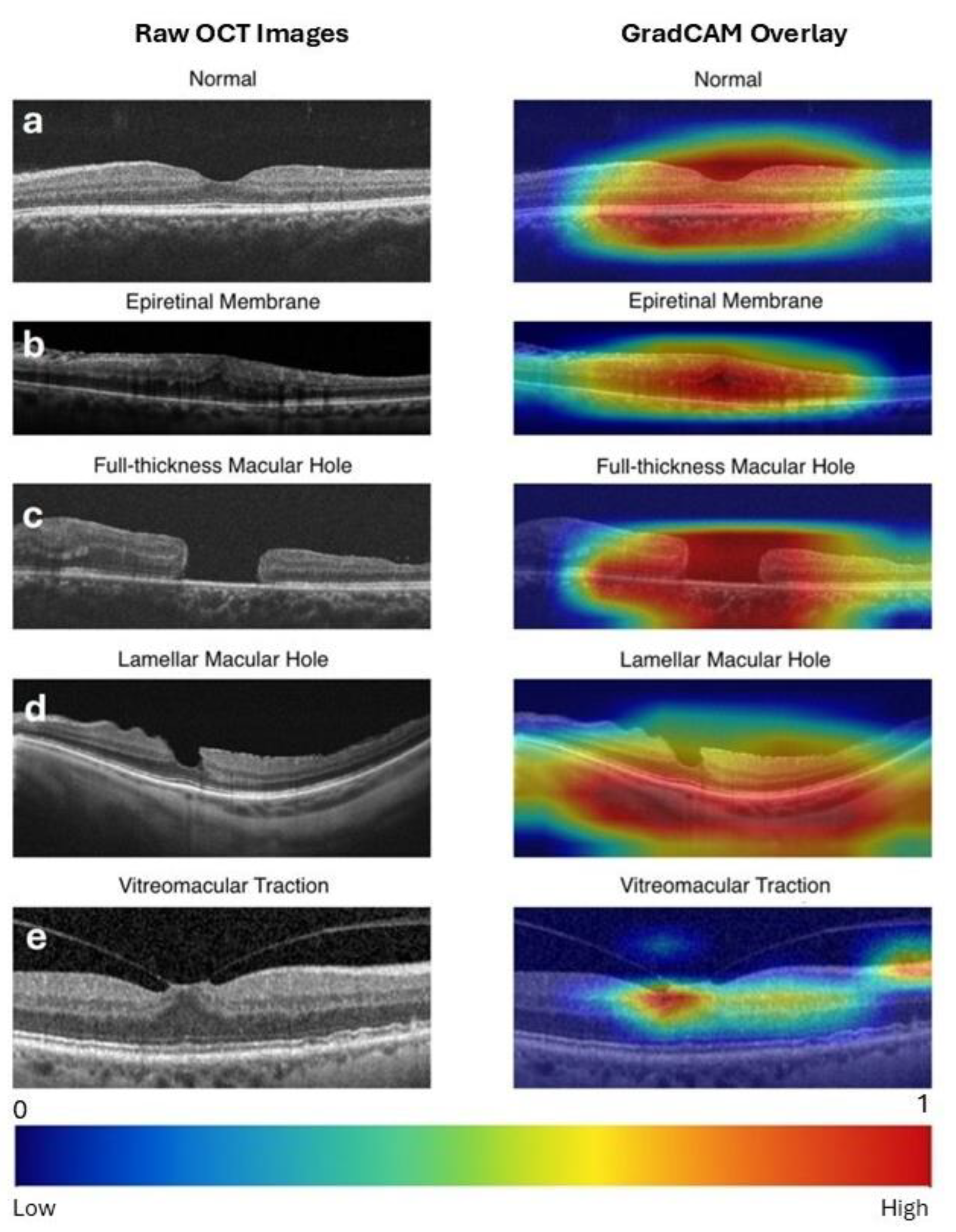
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AUC | Area Under the Receiver Operating Characteristic Curve |
| CNN | Convolutional Neural Network |
| DL | Deep Learning |
| ERM | Epiretinal Membrane |
| FTMH | Full-Thickness Macular Hole |
| Grad-CAM | Gradient-weighted Class Activation Mapping |
| LMH | Lamellar Macular Hole |
| MC | Monte Carlo |
| MCC | Matthews Correlation Coefficient |
| ML | Machine Learning |
| OCT | Optical Coherence Tomography |
| VMI | Vitreomacular Interface |
| VMT | Vitreomacular Traction |
References
- Valentim, C.C.S.; Wu, A.K.; Yu, S.; Manivannan, N.; Zhang, Q.; Cao, J.; Song, W.; Wang, V.; Kang, H.; Kalur, A.; et al. Deep learning-based algorithm for the detection of idiopathic full thickness macular holes in spectral domain optical coherence tomography. Int. J. Retin. Vitr. 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hu, Y.; Quan, W.; Yang, Y.; Lai, W.; Wang, X.; Zhang, X.; Zhang, B.; Wu, Y.; Wu, Q.; et al. Development and validation of a deep learning system to classify aetiology and predict anatomical outcomes of macular hole. Br. J. Ophthalmol. 2023, 107, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Touma, S.; Antaki, F.; Duval, R. Development of a code-free machine learning model for the classification of cataract surgery phases. Sci. Rep. 2022, 12, 2398. [Google Scholar] [CrossRef]
- Jacoba, C.M.P.; Doan, D.; Salongcay, R.P.; Aquino, L.A.C.; Silva, J.P.Y.; Salva, C.M.G.; Zhang, D.; Alog, G.P.; Zhang, K.; Locaylocay, K.L.R.B.; et al. Performance of automated machine learning for diabetic retinopathy image classification from multi-field handheld retinal images. Ophthalmol. Retin. 2023, 7, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Antaki, F.; Coussa, R.G.; Kahwati, G.; Hammamji, K.; Sebag, M.; Duval, R. Accuracy of automated machine learning in classifying retinal pathologies from ultra-widefield pseudocolour fundus images. Br. J. Ophthalmol. 2023, 107, 90–95. [Google Scholar] [CrossRef]
- Kirik, F.; Ozbas, C.; Aslanoglu, C.E.; Koytak, I.A.; Ozdemir, H. Efficiency of web-based code-free artificial intelligence platform in classification of vitreomacular interface diseases. Eur. Eye Res. 2024, 4, 57–62. [Google Scholar] [CrossRef]
- Wong, C.Y.T.; O’Byrne, C.; Taribagil, P.; Liu, T.; Antaki, F.; Keane, P.A. Comparing code-free and bespoke deep learning approaches in ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 262, 2785–2798. [Google Scholar] [CrossRef]
- Levison, A.L.; Kaiser, P.K. Vitreomacular interface diseases: Diagnosis and management. Taiwan J. Ophthalmol. 2014, 4, 63–68. [Google Scholar] [CrossRef]
- Crincoli, E.; Savastano, M.C.; Savastano, A.; Caporossi, T.; Bacherini, D.; Miere, A.; Gambini, G.; De Vico, U.; Baldascino, A.; Minnella, A.M.; et al. New artificial intelligence analysis for prediction of long-term visual improvement after epiretinal membrane surgery. Retina 2023, 43, 173–181. [Google Scholar] [CrossRef]
- Parra-Mora, E.; Cazañas-Gordon, A.; Proença, R.; da Silva Cruz, L.A. Epiretinal membrane detection in optical coherence tomography retinal images using deep learning. IEEE Access 2021, 9, 99201–99219. [Google Scholar] [CrossRef]
- Hsia, Y.; Lin, Y.-Y.; Wang, B.-S.; Su, C.-Y.; Lai, Y.-H.; Hsieh, Y.-T. Prediction of Visual Impairment in Epiretinal Membrane and Feature Analysis: A Deep Learning Approach Using Optical Coherence Tomography. Asia-Pac. J. Ophthalmol. 2023, 12, 21–28. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, H.; Yang, S.; Kim, S.S.; Lee, J.H. Deep learning-based prediction of outcomes following noncomplicated epiretinal membrane surgery. Retina 2022, 42, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Monte Carlo methods for medical imaging research. Biomed. Eng. Lett. 2024, 14, 1195–1205. [Google Scholar] [CrossRef]
- Touma, S.; Hammou, B.A.; Antaki, F.; Boucher, M.C.; Duval, R. Comparing code-free deep learning models to expert-designed models for detecting retinal diseases from optical coherence tomography. Int. J. Retin. Vitr. 2024, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Mutasa, S.; Sun, S.; Ha, R. Understanding artificial intelligence based radiology studies: What is overfitting? Clin. Imaging 2020, 65, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.H.; Kumar, S.; Gandomi, A.H. Breaking the data barrier: A review of deep learning techniques for democratizing AI with small datasets. Artif. Intell. Rev. 2024, 57, 226. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar] [CrossRef]
- Tan, M.; Le, Q. Efficientnet: Rethinking model scaling for convolutional neural networks. In Proceedings of the International Conference on Machine Learning, PMLR, Long Beach, CA, USA, 9–15 June 2019; pp. 6105–6114. [Google Scholar] [CrossRef]
- Usmani, E.; Bacchi, S.; Zhang, H.; Guymer, C.; Kraczkowska, A.; Qinfeng Shi, J.; Gilhotra, J.; Chan, W.O. Prediction of vitreomacular traction syndrome outcomes with deep learning: A pilot study. Eur. J. Ophthalmol. 2025, 35, 335–339. [Google Scholar] [CrossRef]
- Crincoli, E.; Parolini, B.; Catania, F.; Savastano, A.; Savastano, M.C.; Rizzo, C.; Kilian, R.; Matello, V.; Allegrini, D.; Romano, M.R.; et al. Prediction of functional and anatomical progression in lamellar macular holes. Ophthalmol. Sci. 2024, 4, 100529. [Google Scholar] [CrossRef]
- Ganaie, M.A.; Hu, M.; Malik, A.K.; Tanveer, M.; Suganthan, P.N. Ensemble deep learning: A review. Eng. Appl. Artif. Intell. 2022, 115, 105151. [Google Scholar] [CrossRef]
- Rosly, R.; Makhtar, M.; Awang, M.K.; Awang, M.I.; Rahman, M.N.A.; Mahdin, H. Comprehensive study on ensemble classification for medical applications. Int. J. Eng. Technol. 2018, 7, 186–190. [Google Scholar] [CrossRef]
- Pandey, P.U.; Ballios, B.G.; Christakis, P.G.; Kaplan, A.J.; Mathew, D.J.; Tone, S.O.; Wan, M.J.; Micieli, J.A.; Wong, J.C.Y. Ensemble of deep convolutional neural networks is more accurate and reliable than board-certified ophthalmologists at detecting multiple diseases in retinal fundus photographs. Br. J. Ophthalmol. 2024, 108, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gupta, S.; Bharany, S.; Almogren, A.; Altameem, A.; Rehman, A.U. Ensemble Deep Learning Models for Enhanced Brain Tumor Classification by Leveraging ResNet50 and EfficientNet-B7 on High-Resolution MRI Images. IEEE Access 2024, 12, 178623–178641. [Google Scholar] [CrossRef]
| Average Precision | Precision (%) | Recall (%) | ||||
|---|---|---|---|---|---|---|
| Expert | AutoML | Expert | AutoML | Expert | AutoML | |
| Normal | 1.000 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| FTMH | 100.0 | 97.8 | 100.0 | 92.7 | 100.0 | 97.4 |
| LMH | 90.3 | 86.0 | 95.0 | 72.3 | 88.0 | 87.2 |
| ERM | 95.3 | 83.0 | 86.0 | 93.9 | 95.0 | 79.5 |
| VMT | 99.5 | 96.0 | 100 | 100 | 95.0 | 94.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durmaz Engin, C.; Gokkan, M.O.; Koksaldi, S.; Kayabasi, M.; Besenk, U.; Selver, M.A.; Grzybowski, A. Comparing Auto-Machine Learning and Expert-Designed Models in Diagnosing Vitreomacular Interface Disorders. J. Clin. Med. 2025, 14, 2774. https://doi.org/10.3390/jcm14082774
Durmaz Engin C, Gokkan MO, Koksaldi S, Kayabasi M, Besenk U, Selver MA, Grzybowski A. Comparing Auto-Machine Learning and Expert-Designed Models in Diagnosing Vitreomacular Interface Disorders. Journal of Clinical Medicine. 2025; 14(8):2774. https://doi.org/10.3390/jcm14082774
Chicago/Turabian StyleDurmaz Engin, Ceren, Mahmut Ozan Gokkan, Seher Koksaldi, Mustafa Kayabasi, Ufuk Besenk, Mustafa Alper Selver, and Andrzej Grzybowski. 2025. "Comparing Auto-Machine Learning and Expert-Designed Models in Diagnosing Vitreomacular Interface Disorders" Journal of Clinical Medicine 14, no. 8: 2774. https://doi.org/10.3390/jcm14082774
APA StyleDurmaz Engin, C., Gokkan, M. O., Koksaldi, S., Kayabasi, M., Besenk, U., Selver, M. A., & Grzybowski, A. (2025). Comparing Auto-Machine Learning and Expert-Designed Models in Diagnosing Vitreomacular Interface Disorders. Journal of Clinical Medicine, 14(8), 2774. https://doi.org/10.3390/jcm14082774






