1. Introduction
Despite tremendous progress being made in its management, sepsis constitutes a significant healthcare problem. In 2017, there were approximately 48 million cases of sepsis and 11 million sepsis-related deaths globally [
1]. According to a wide consensus, sepsis has been defined as “life-threatening organ dysfunction caused by dysregulated host response to infection” [
2]. Sepsis is considered a medical emergency because its prognosis very much depends on the speed of diagnosis and adequate early management [
3]. Despite enormous progress being made in the management of sepsis, it is associated with high mortality, with the mortality rate in a septic shock reaching more than 50%, especially in elderly patients [
4,
5]. By comparison, the mortality rate in myocardial infarction with ST-segment elevation is currently at about 8%, ranging from 4% to 15% in recent years [
6,
7,
8]. Predictive scores have been developed for both the diagnosis and prediction of sepsis. Numerous scoring systems for the severity of illness include abnormalities in the complete blood count, although white blood cell and erythrocyte parameters are mostly under-represented. The most frequently used score for both the diagnosis and prediction of sepsis is the Sepsis-related Organ Failure Assessment (SOFA) [
9]. Its abbreviated form is called “quick SOFA score” (qSOFA) and is used to improve the diagnosis of sepsis in hospital departments outside intensive care units (ICUs) [
10]. The quick SOFA score is also used to identify patients at increased mortality risk and requiring ICU hospitalization for more than 72 h. The predictive value of an original SOFA score and its abbreviated form for diagnosing sepsis is not excellent. It has been shown that the area under the receiver operating characteristic (ROC) curve (AUROC) for the SOFA in ICUs and for qSOFA outside ICUs for the prediction of sepsis is approximately 0.80 [
11].
Complete blood count with differential (CBC w/diff) is a standard, inexpensive laboratory test that aids in determining a patient’s immune competency and response to infection [
12]. Nucleated red blood cells (NRBCs) are precursors of erythrocytes. They rarely appear in healthy adults’ blood smears but, if present, are considered a credible extended inflammatory parameter for outcome prediction [
13].
This study aimed to search for hematological parameters that could predict short-term sepsis-related mortality.
2. Materials and Methods
This retrospective cohort study was carried out in a mixed medical–surgical ICU in a large academic hospital with 663 hospital beds, and the analyzed period was from September 2021 to March 2025. All patients diagnosed with either sepsis or septic shock were analyzed. Sepsis and septic shock were diagnosed using the most recent international definitions [
2]. The modern definitions of sepsis or septic shock do not involve laboratory determinations [
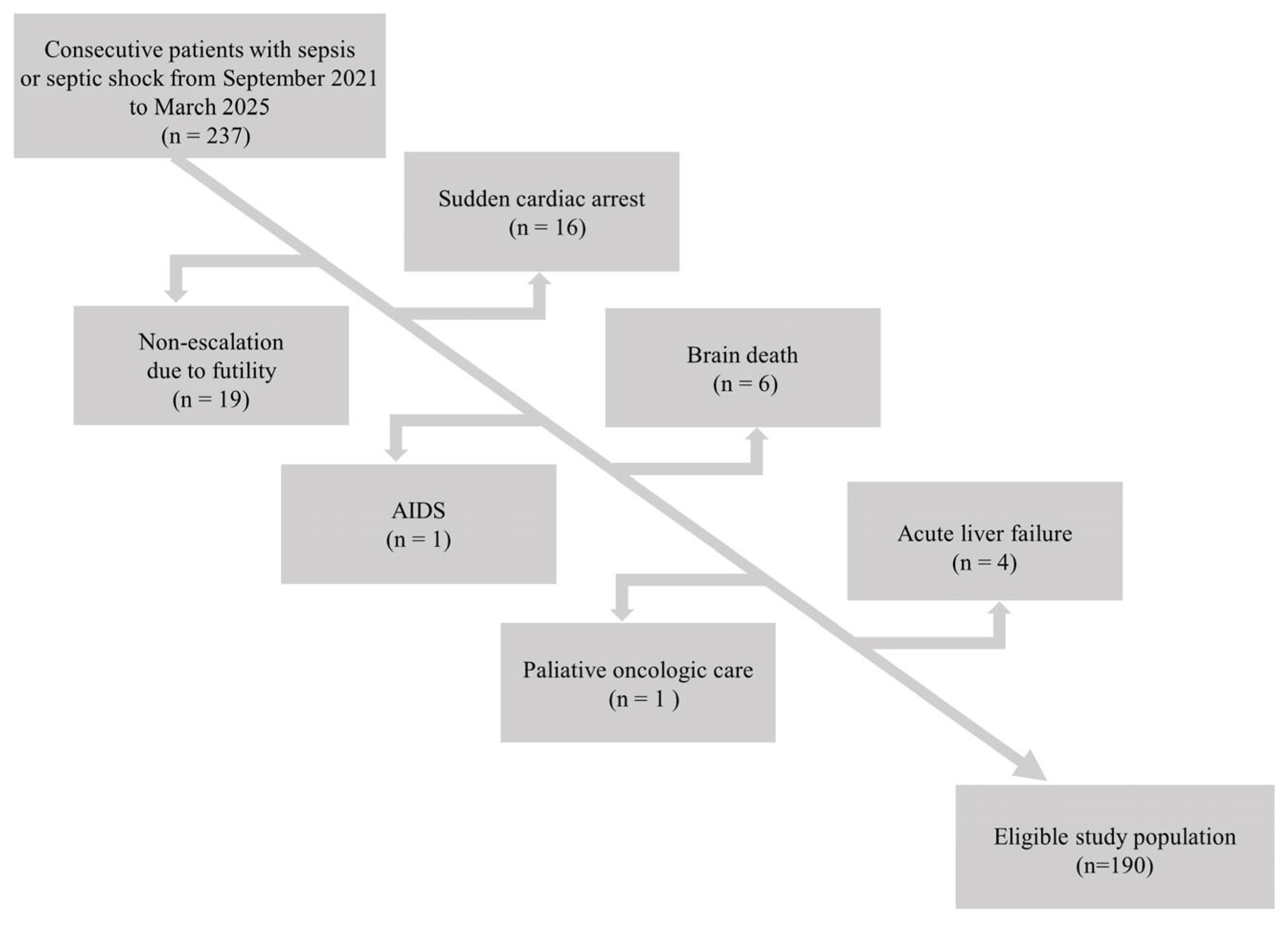
14]. We analyzed patients with sepsis and septic shock as a single group, as the primary factor contributing to mortality in both conditions is acute organ dysfunction caused by the host’s response to infection. Patients with additional diagnoses that could have an impact on mortality were excluded: non-escalation of therapy due to futility, sudden cardiac arrest, brain death, acute liver failure, etc. Only sepsis-related short-term (ICU) mortality was of interest to us. Complete blood counts with differential and reticulocyte parameters were compared between survivors and non-survivors. All data regarding demographics, clinical features, and laboratory parameters were taken from electronic medical records (AMMS, Asseco Medical Management Solutions, Rzeszów, Poland).
Demographics and clinical features included sex, age, primary infection site, time interval between the moment of ICU admission and collection of blood for hematological parameters, length of stay (LOS) in the ICU, mortality in the ICU, and SOFA score.
In the local ICU, the standard laboratory panel for sepsis patients includes the following: complete blood count with differential (CBC w/diff), reticulocyte panel (reticulocyte populations, reticulocyte maturity index, reticulocyte hemoglobin equivalent RET-He), PCT, C-reactive protein (CRP), creatinine, blood urea nitrogen (BUN), estimated glomerular filtration rate (eGFR), and total bilirubin. The hematological parameters were determined using a hematology analyzer (XN-1000, Sysmex, Kobe, Japan). A complete diagnostic panel in patients with sepsis requires the collection of blood for the following test tubes: an ethylenediaminetetraacetic acid (EDTA) tube, sodium citrate tube, and clot activator tube. Blood was collected from an artery through a cannula (BD Arterial Cannula, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) using a vacuum system (BD Vacutainer®, Franklin Lakes, NJ, USA). Care was taken to not pollute blood samples with unfractionated heparin (UFH) from the flashing solution of the arterial line system. Blood samples were collected directly following admission to the ICU.
Statistical analyses were carried out with the use of licensed statistical software (18.0 Basic Edition, Stata, StataCorp LLC, College Station, TX, USA). Continuous variables were expressed as medians and interquartile ranges (IQRs, i.e., 25pc–75pc). Categorical variables were expressed as frequencies and percentages. The Shapiro–Wilk test and histograms were used to verify the type of distribution of continuous variables. Differences for continuous variables were assessed by independent samples Student’s t-test or the Mann–Whitney U test, depending on the type of variable distribution. Pearson’s χ2 test was utilized for categorical variables, with Fisher’s exact test used when it was necessary. The ROC curve was drawn for hematological parameters, and the AUROC was calculated to determine their value to predict short-term mortality. Multiple logistic regression was performed to exclude the impact of confounders (SOFA, age, sex). An ROC analysis was also carried out to assess the best cut-off values using the Liu method. The statistical tests were two-sided. Statistical significance was considered at p < 0.05.
The local bioethics committee approved this study. Because this study was retrospective in nature, the need for informed consent was waived. This study was performed according to the regulations of the Declaration of Helsinki. The results of this study were reported according to research reporting guidelines for observational studies (STROBE).
3. Results
Data for 237 patients with sepsis admitted to the local ICU were taken from electronic health records. Patients with diagnoses other than sepsis that could have an impact on mortality were excluded. The flow chart for this study is presented in
Figure 1.
Following exclusions, 190 patients were finally included in this study. The median age in the study group was 65.0 (IQR 51.0–71.0) years. There were 94 (49.5%) women and 96 (50.5%) men.
The majority of patients’ primary anatomical site of infection was intra-abdominal infection (n = 58, 30.8%), pneumonia (n = 57, 30.3%), or urinary tract infection (n = 43, 22.9%). The median values for biochemical parameters were as follows: PCT 3.68 (IQR 1.21–18.60) ng mL−1, CRP 177.5 (IQR 108.0–265.0) mg L−1, creatinine 1.19 (IQR 0.71–1.95) mg dL−1, blood urea nitrogen 30.0 (IQR 20.7–45.9) mg dL−1, and bilirubin 0.58 (IQR 0.30–1.00) mg dL−1. The median SOFA score in the cohort was 8.0 (IQR 6.0–11.0) points.
There were 130 (70.0%) survivors and 57 (30.0%) non-survivors. There was no association between patients’ sex and mortality (24.5 vs. 35.4% in females and males, respectively,
p = 0.1). Non-survivors were older compared to survivors (68.0 (IQR 59.0–73.0) vs. 64.0 (IQR 46.0–71.0) years,
p = 0.02). There were differences in biochemical parameters in survivors and non-survivors (
Table 1).
The median SOFA score among survivors and non-survivors was 7.0 (IQR 5.0–10.0) and 11.0 (9.0–14.0) points, respectively (
p < 0.001). There were significant differences between survivors and non-survivors in leukocyte parameters (monocyte percentage, monocyte number), platelet parameters (platelet number, platelet distribution width (PDW), mean platelet volume (MPV), platelet larger cell ratio (P-LCR)), and erythrocyte parameters (red blood cell distribution width (RDW), RDW standard deviation (RDW-SD), percentage of nucleated red blood cells (NRBCs), number of NRBCs). All aforementioned parameters were higher in non-survivors compared to survivors except platelet number and monocyte percentage and number (
Table 2 and
Table 3).
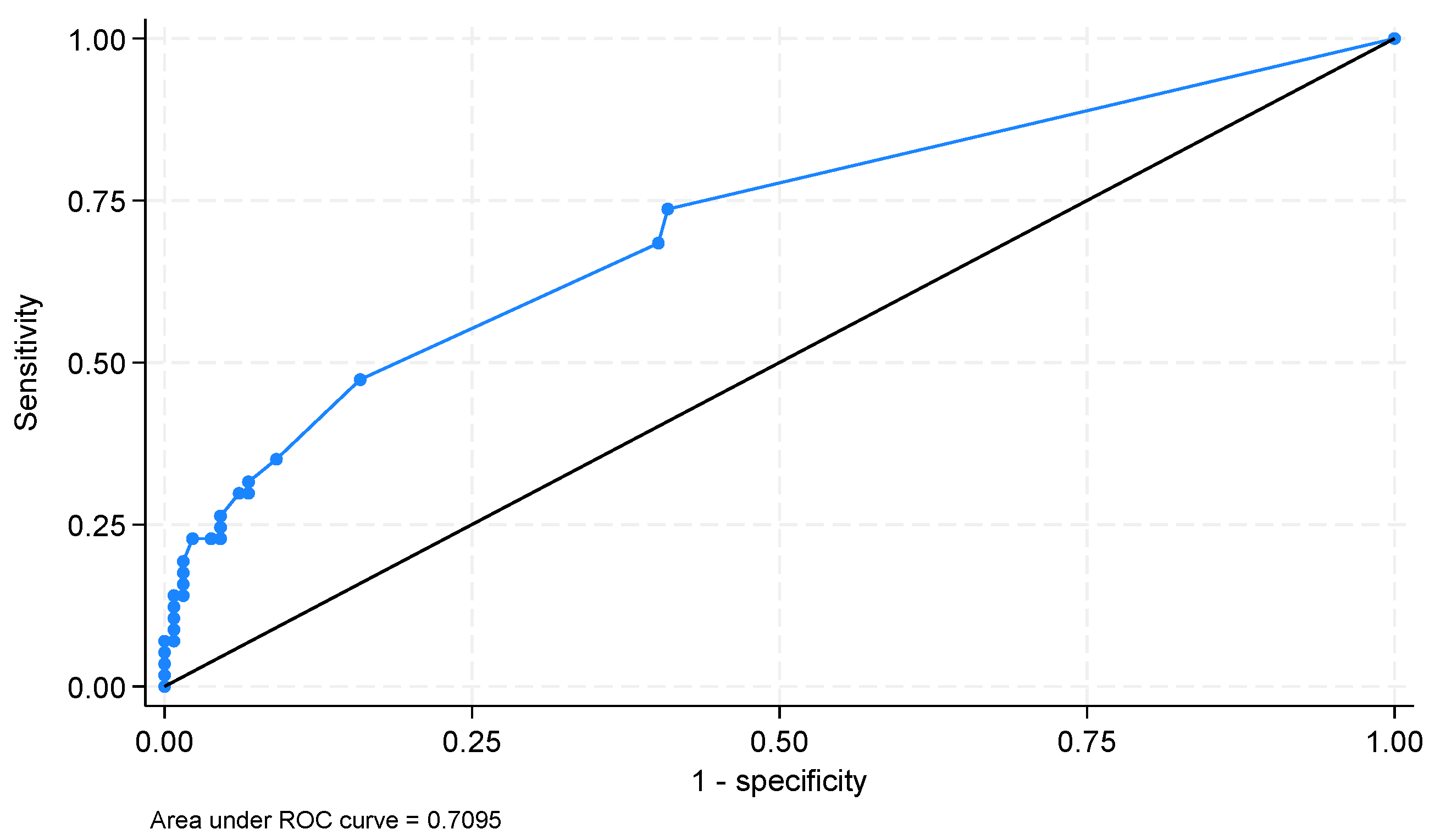
The AUROCs for potential hematological parameters that could be used for ICU mortality prediction were as follows (only parameters with an AUROC > 0.500): PLT—0.607 (95%CI 0.516–0.697,
p = 0.039), PDW—0.646 (95%CI 0.557–0.735;
p = 0.002), RDW-SD—0.649 (95%CI 0.568–0.732;
p = 0.002), MPV—0.656 (95%CI 0.568–0.743;
p = 0.001), P-LCR—0.667 (95%CI 0.579–0.755;
p < 0.001), number of NRBCs—0.708 (95%CI 0.625–0.791;
p = 0.01), percentage of NRBCs—0.709 (95%CI 0.629–0.789;
p = 0.006) (
Figure 2).
The optimal cut-off for NRBCs was >0.02 × 103 µL−1 (sensitivity 54.4%; specificity 84.1%); for the percentage of NRBCs, the cut-off was 0% (sensitivity 73.7%; specificity 59.1%).
We performed multiple logistic regression to exclude potential confounding factors that could impact the validity of NRBCs as a predictor of ICU mortality. NRBCs continued to be a statistically significant factor in the prediction of ICU mortality in the multiple logistic model. Other statistically significant variables were SOFA scoring and the age of the patients (
Table 4).
4. Discussion
This study aimed to search for hematological parameters to predict sepsis-related ICU mortality. Relatively well-known and seemingly simple, routinely applied laboratory tests, such as blood count, have some untapped potential for patient diagnostics, with parameters that may be primarily overlooked in daily clinical practice. Complete blood count is the most commonly ordered hematological laboratory test. However, its value is limited, as it only gives information on the quantity of major essential blood components provided by automatic, algorithm-based analyzers. To better determine the types and subtypes of blood cells, their size and volume distribution, maturity, and cell type ratios, the analysis needs to be expanded by adding differential smears [
15].
The WBC count with differential determines the total number of leukocytes, alongside the enumeration and percentage of each subtype. Leukocytes are primary components of systemic defensive reactions towards pathogens and injury, resulting in infectious, inflammatory, and immune responses [
12]. The WBC count with differential traditionally should be examined in patients presenting signs, symptoms, or conditions associated with infections, inflammation, bone marrow alterations, and altered immune competency, particularly in an attempt to discern between infection with inflammation and inflammatory state without concomitant infection [
12].
Routine hematological parameters have been analyzed to state their potential as extended inflammatory parameters to determine timely sepsis diagnosis and prognostication [
16]. In a recent study by Herawati et al., sepsis patients presented with varied activation stages of neutrophils and lymphocytes, with higher total white blood cell count, neutrophil count, and percentage relative to total WBC, and lower monocyte percentage relative to total WBC, compared to non-sepsis and healthy controls, which is partially in line with our findings [
17]. Through logistic regression model analysis, the authors concluded that standard hematological results in combination with inflammatory parameters could aid in timely clinical decision-making in suspected sepsis cases without imposing additional burdens on patients. In our study, we showed that confounding factors, like SOFA scoring, age, or sex, did not have impact on the conclusions of our study.
Amundsen et al. discussed the role of NRBCs in evaluating the prognosis of patients with suspected sepsis and their role as a marker that would potentially be useful in the emergency department (ED) setting [
18]. There is a constant need for reliable, time-saving, broadly available parameters during initial diagnostics in the ED environment. Timely recognition of patients’ probable deterioration should lead to timely admission to the ICU or any other proper hospital ward for treatment. NRBCs can be measured accurately by the most accessible analyzers, even at deficient concentrations, which makes them possible prognostic markers [
19]. The authors evaluated patients with suspected sepsis, noticing increased 30-day mortality in patients with elevated NRBCs, with a higher number of NRBC-positive patients in a subgroup of patients in whom sepsis was confirmed. The study concluded that NRBCs add prognostic value to clinical scoring systems and standard laboratory tests in the ED, with the prognostic value of NRBCs being more significant than some components of the SOFA score [
18]. In our study, we showed that the odds ratio (OR) for NRBCs in the prediction of ICU mortality was higher than the OR for the SOFA.
Purtle et al. analyzed the occurrence of the circulating NRBCs in the peripheral blood as an indicator of an extreme increase in erythropoietic activity, detected in stress states like inflammation, hematological malignancy, pathological hematopoiesis, massive hemorrhage, or severe hypoxia [
20]. The researchers emphasized the need to craft and test new models that could predict post-discharge outcomes in ICU patients and ICU survivors, aimed at tailoring interventions and improving outcomes [
21]. They attempted to determine if critically ill patients who manifested NRBCs have higher 90-day mortality following hospital discharge. Sepsis patients were isolated as a study subgroup, and sepsis events were included in the adjustment for the accuracy of statistical models. Overall, the elevated NRBCs were connected to a raised risk of hospital readmission and delayed mortality. Additionally, RDW was correlated with NRBC count [
21].
Studies indicate that hematological parameters, including RDW, PDW, MPV, and NRBC counts, serve as key indices for bloodstream infections and sepsis-related acute organ failure. These indices offer diagnostic performance that is on par with procalcitonin and surpass CRP, similar to laboratory markers of renal and hepatic function [
21,
22,
23].
Our study is not devoid of limitations. The obvious limitation of this study is its retrospective mature, and previously recorded data may lack completeness or accuracy. However, we have taken every effort to make sure that the data were as complete and accurate as possible. Another limitation of a retrospective study is the lack of a control group Yet another limitation is the fact that this was a single-center study, which may limit the generalizability of our findings to other settings. However, to increase the reliability of our results, we performed additional a posteriori power calculations regarding our study’s sample size for the study group. We discovered that we would require a minimum of 168 participants to confirm a significant between-group difference with an alpha of <0.05 and a beta of 0.20. We also performed a sample size calculation to verify the significance of the ROC analysis for the ICU mortality prediction. We discovered that we would require at least 147 participants to establish the significance of an AUROC value of at least 0.7, with a ratio of sample sizes between negative and positive groups of 2 to 1, an alpha of <0.001, and a beta of 0.20. Therefore, while we acknowledge that our sample size is small, it is sufficient to draw conclusions, and our study was in no way underpowered. We included more than the minimal number of subjects calculated for both statistical purposes.
5. Conclusions
Among different hematological parameters in sepsis patients, the only acceptable parameter for predicting ICU mortality is the percentage of NRBCs. The presence of NRBCs in a blood smear is associated with a worse prognosis in patients with sepsis.
Author Contributions
Conceptualization, P.F.C.; methodology, P.F.C. and A.W.; investigation, P.C and A.W.; data curation, P.F.C.; writing—original draft preparation, A.W.; writing—review and editing, P.F.C.; visualization, A.W.; funding acquisition, P.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Medical University of Silesia, Katowice, Poland.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the retrospective nature of the study.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The data presented in this study are only available on request from the corresponding author due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 2050312119835043. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Nasa, P.; Juneja, D.; Singh, O. Severe sepsis and septic shock in the elderly: An overview. World J. Crit. Care Med. 2012, 1, 23–30. [Google Scholar] [CrossRef]
- Choudhury, T.; West, N.E.; El-Omar, M. ST elevation myocardial infarction. Clin. Med. 2016, 16, 277–282. [Google Scholar] [CrossRef]
- Pascual, I.; Hernandez-Vaquero, D.; Almendarez, M.; Lorca, R.; Escalera, A.; Díaz, R.; Alperi, A.; Carnero, M.; Silva, J.; Morís, C.; et al. Observed and Expected Survival in Men and Women After Suffering a STEMI. J. Clin. Med. 2020, 9, 1174. [Google Scholar] [CrossRef]
- Oraii, A.; Shafeghat, M.; Ashraf, H.; Soleimani, A.; Kazemian, S.; Sadatnaseri, A.; Saadat, N.; Danandeh, K.; Akrami, A.; Balali, P.; et al. Risk assessment for mortality in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: A retrospective cohort study. Health Sci. Rep. 2024, 7, e1867. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Fernandes, S.; Wyawahare, M. Utility of quick sepsis-related organ failure assessment (qSOFA) score to predict outcomes in out-of-ICU patients with suspected infections. J. Fam. Med. Prim. Care 2020, 9, 3251–3255. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis: For the third international consensus definition for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- George-Gay, B.; Parker, K. Understanding the complete blood count with differential. J. Perianesth. Nurs. 2003, 18, 96–114. [Google Scholar] [CrossRef] [PubMed]
- Pikora, K.; Krętowska-Grunwald, A.; Krawczuk-Rybak, M.; Sawicka-Żukowska, M. Diagnostic Value and Prognostic Significance of Nucleated Red Blood Cells (NRBCs) in Selected Medical Conditions. Cells 2023, 12, 1817. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Rahmnitarini, A.; Hernaningsih, Y.; Indrasari, Y.N. The stability of sample storage for complete blood count (CBC) toward the blood cell morphology. Bali Med. J. 2019, 8, 391–395. [Google Scholar] [CrossRef]
- Ekici-Günay, N.; Çakır, I.; Çelik, T. Is there clinical value in counting nucleated red blood cells and platelet indices in primary immunodeficiency disease? Turk. J. Pediatr. 2017, 59, 657–663. [Google Scholar] [CrossRef]
- Herawati, S.; Somia, I.K.A.; Kosasih, S.; Wande, I.N.; Felim, J.; Payana, I.M.D. Integrating Routine Hematological and Extended Inflammatory Parameters as a Novel Approach for Timely Diagnosis and Prognosis in Sepsis Management. Diagnostics 2024, 14, 956. [Google Scholar] [CrossRef]
- Amundsen, E.K.; Binde, C.; Christensen, E.E.; Klingenberg, O.; Kvale, D.; Holten, A.R. Prognostic Value of Nucleated RBCs for Patients With Suspected Sepsis in the Emergency Department: A Single-Center Prospective Cohort Study. Crit. Care Explor. 2021, 3, e0490. [Google Scholar] [CrossRef]
- Da Rin, G.; Vidali, M.; Balboni, F.; Benegiamo, A.; Borin, M.; Ciardelli, M.L.; Dima, F.; Di Fabio, A.; Fanelli, A.; Fiorini, F.; et al. Performance evaluation of the automated nucleated red blood cell count of five commercial hematological analyzers. Int. J. Lab. Hematol. 2017, 39, 663–670. [Google Scholar] [CrossRef]
- Danise, P.; Maconi, M.; Barrella, F.; Di Palma, A.; Avino, D.; Rovetti, A.; Gioia, M.; Amendola, G. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin. Chem. Lab. Med. 2011, 50, 357–360. [Google Scholar] [CrossRef]
- Purtle, S.W.; Horkan, C.M.; Moromizato, T.; Gibbons, F.K.; Christopher, K.B. Nucleated red blood cells, critical illness survivors and postdischarge outcomes: A cohort study. Crit. Care 2017, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, W.; Li, X.; Cheng, J.; Liu, Z.; Zhou, Q.; Guan, S. Hematological parameters in patients with bloodstream infection: A retrospective observational study. J. Infect. Dev. Ctries. 2020, 14, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Chen, J.; Lan, Q.F.; Ma, X.J.; Zhang, S.Y. Diagnostic values of red cell distribution width, platelet distribution width and neutrophil-lymphocyte count ratio for sepsis. Exp. Ther. Med. 2016, 12, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).