Oral Dydrogesterone Versus Vaginal Progesterone for Luteal Phase Support in Frozen–Thawed Embryo Transfer Cycles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources (Search Strategy)
2.2. Inclusion and Exclusion Criteria
2.3. Study Design
2.4. Data Extraction
2.5. Statistical Analysis
2.6. Risk of Bias (ROB) and Certainty of Evidence
| Author, Year | Location | Time Period | Sample Size (N, Total) | Type of FET | N, Oral P4 | N, Vaginal P4 | Age (Mean ± SD), Oral P4 | Age (Mean ± SD), Vaginal P4 | BMI (Mean ± SD), Oral P4 | BMI (Mean ± SD), Vaginal P4 | Oral P4 Daily Dosage | Vaginal P4 Daily Dosage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macedo et al. [19], 2023 | Brazil | 2019–2021 | 73 | artificial | 36 | 37 | 33.2 ± 4.4 | 34.1 ± 4.4 | 25.2 ± 5.0 | 26.5 ± 5.7 | 40 mg | 800 mg micronized |
| Pabuccu et al. [20], 2022 | Turkey | 2021–2022 | 109 | artificial | 54 | 55 | 32.8 ± 4.2 | 32.3 ± 4.4 | 22.0 ± 2.3 | 22.8 ± 2.2 | 40 mg | 180 mg vaginal gel |
| Ozer et al. [16], 2021 | Turkey | 2019 | 134 | mNC | 67 | 67 | 31.88 ± 5.20 | 32.4 ± 3.74 | 24.4 ± 4.85 | 23.23 ± 3.88 | 30 mg | 8% vaginal gel |
| Zarei et al. [21], 2016 | Iran | 2014–2015 | 440 | artificial | 100 | 100 | 32.90 ± 5.10 | 33.51 ± 5.20 | NA | NA | 20 mg | 800 mg vaginal suppository |
| Rashidi et al. [22], 2016 | Iran | 2015–2016 | 120 | artificial | 60 | 60 | 31.70 ± 6.48 | 33.27 ± 5.69 | 25.16 ± 2.89 | 24.56 ± 3.05 | 40 mg | 800 mg vaginal suppository |
3. Results
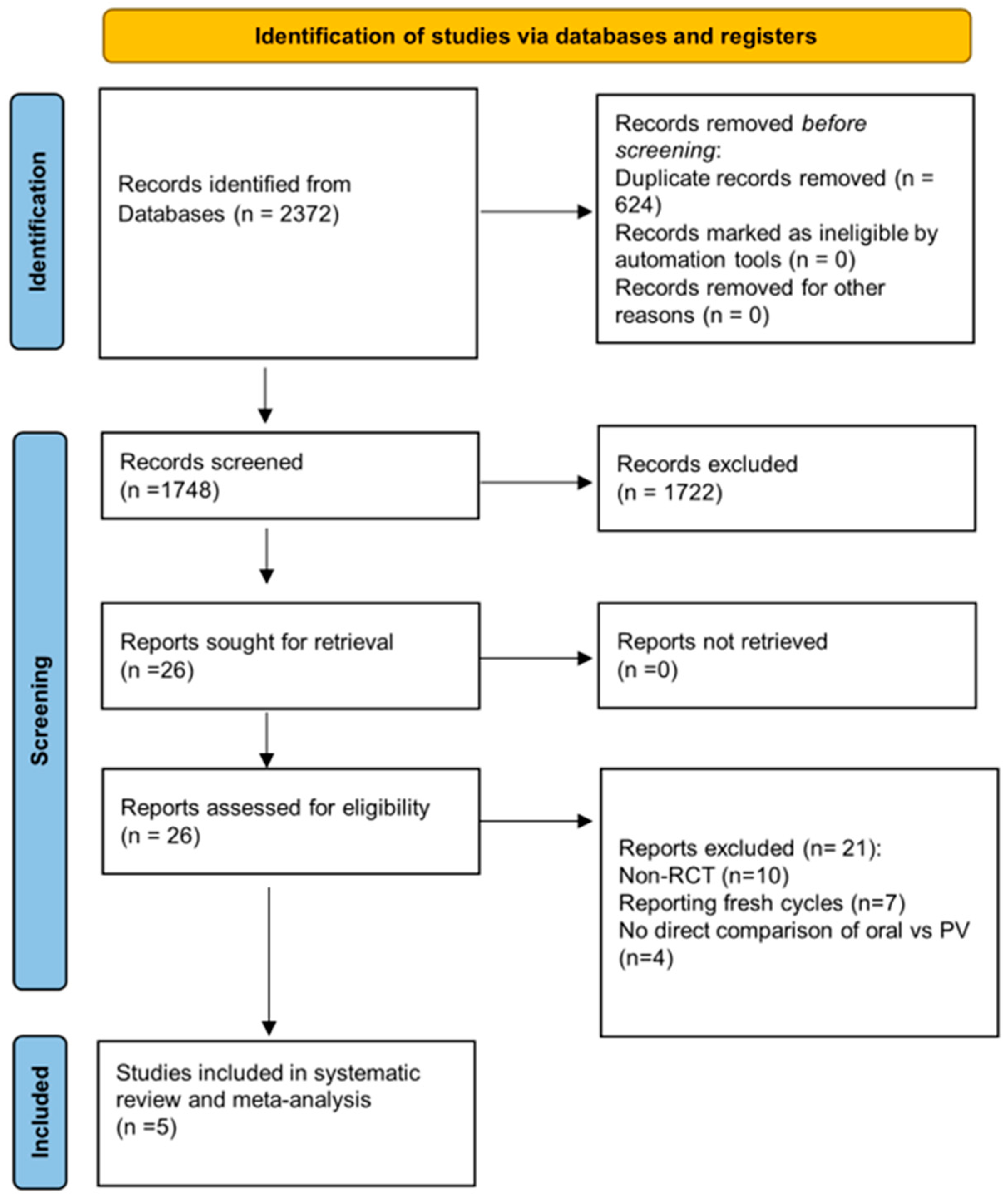
3.1. Study Selection
3.2. Study Characteristics
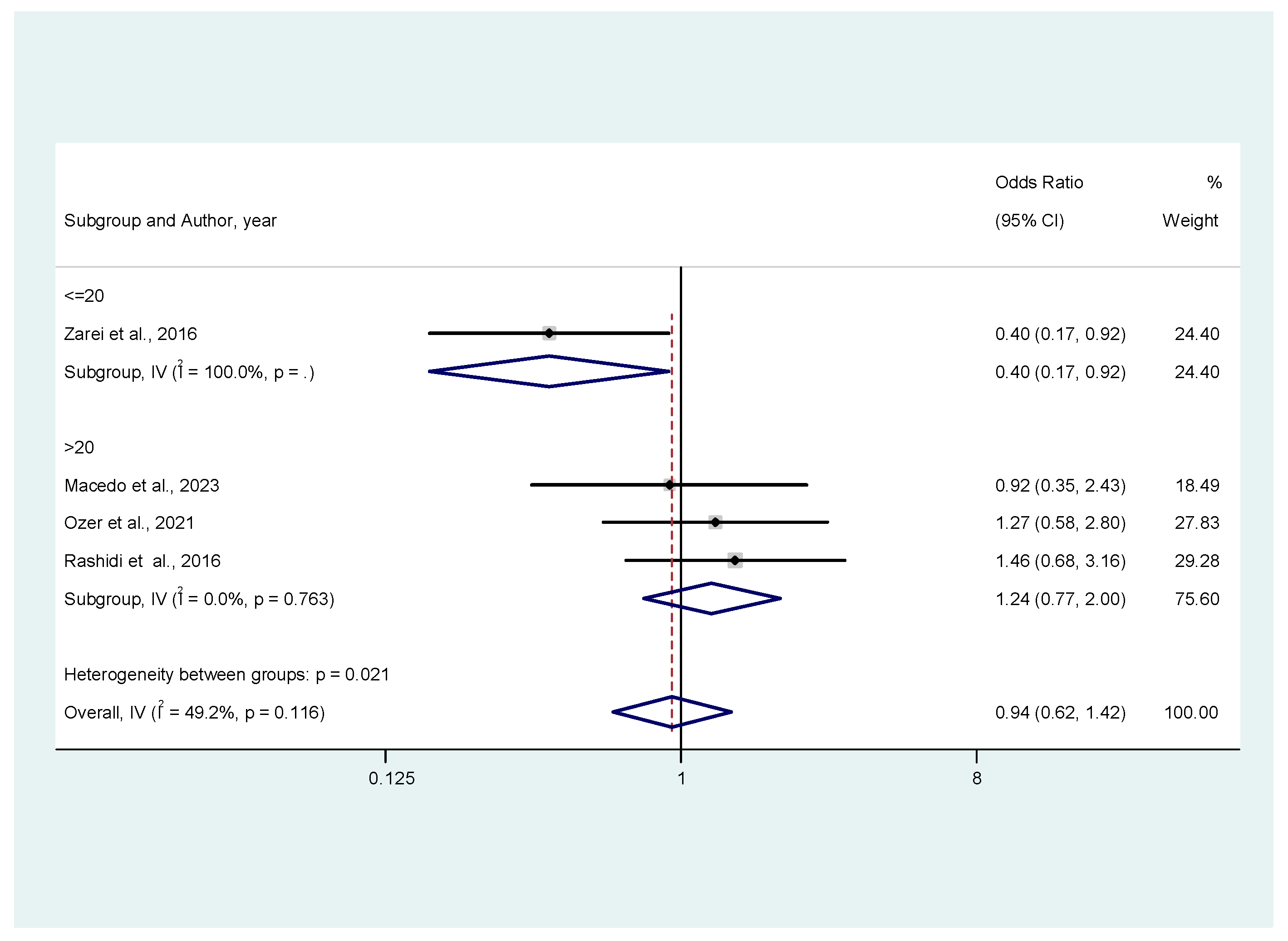
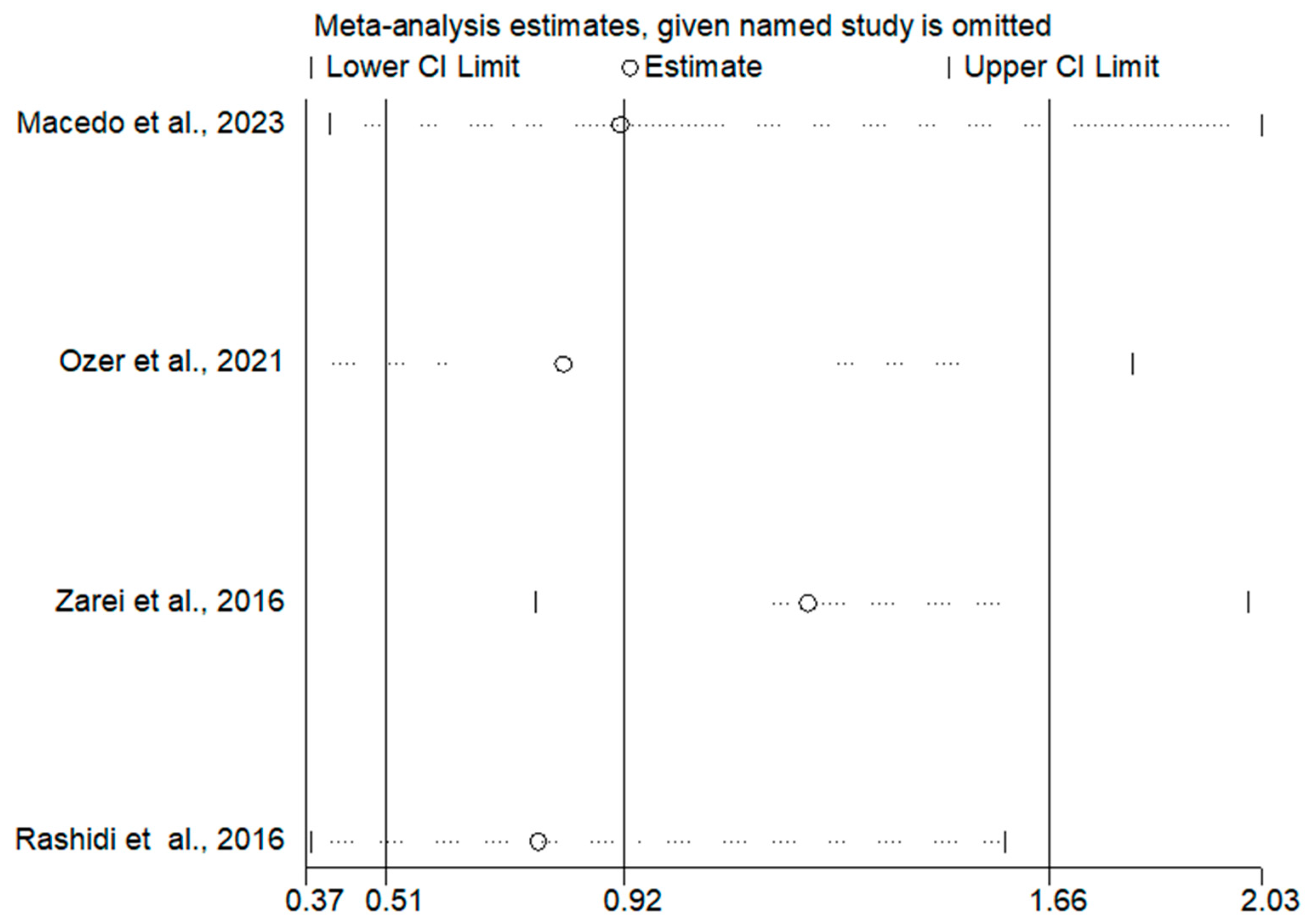
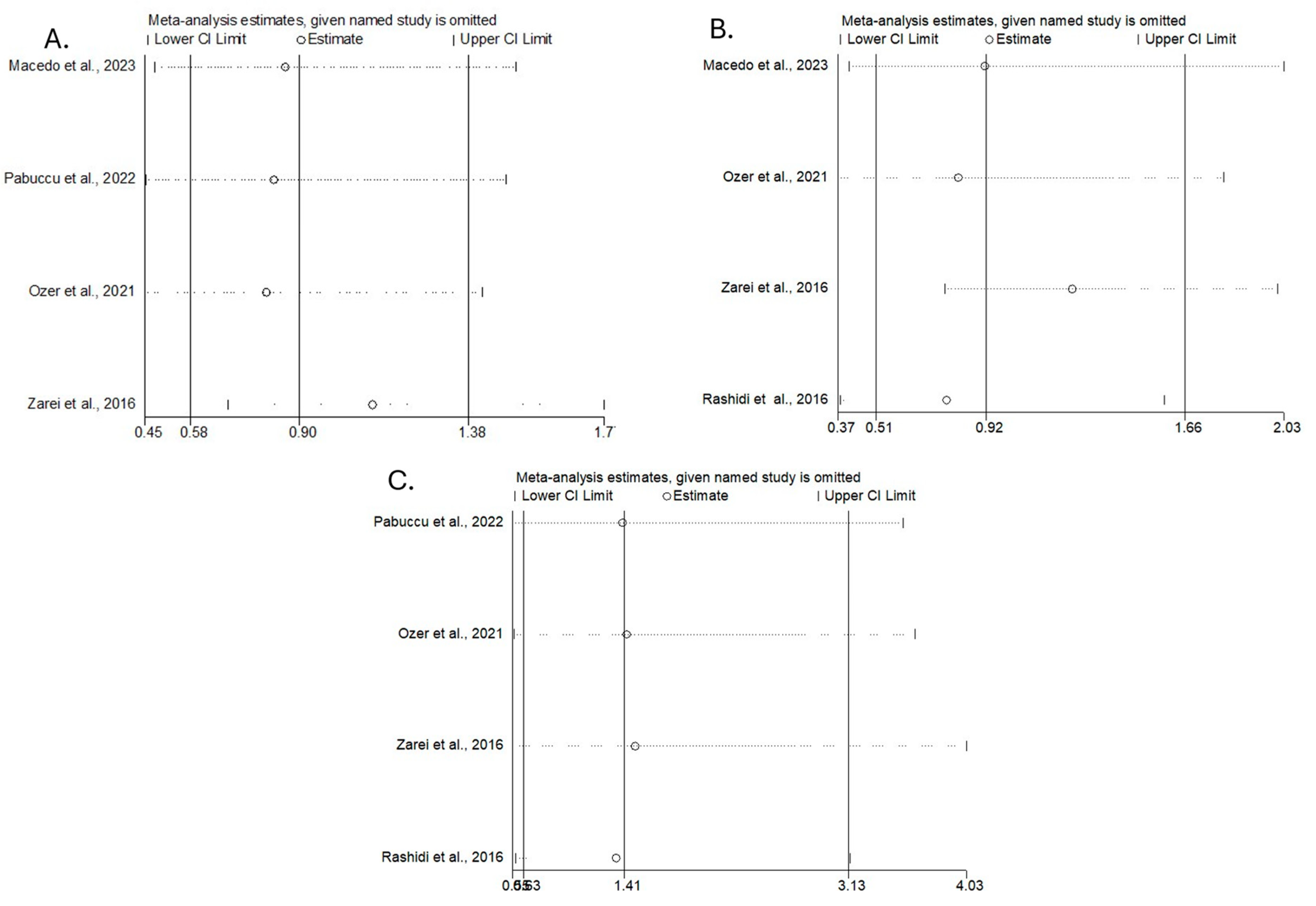
3.3. Meta-Analysis Results
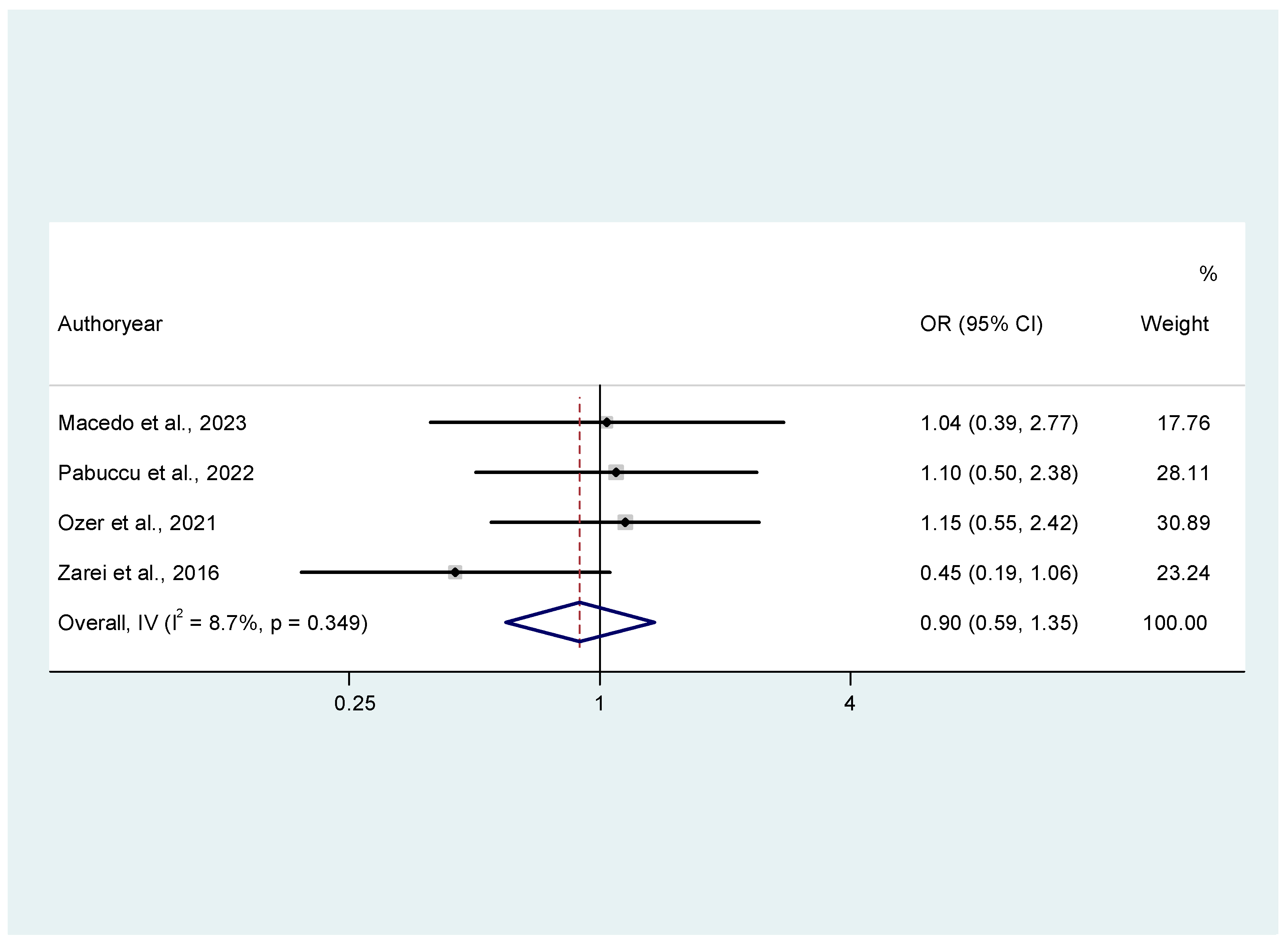
3.3.1. Ongoing Pregnancy Rates
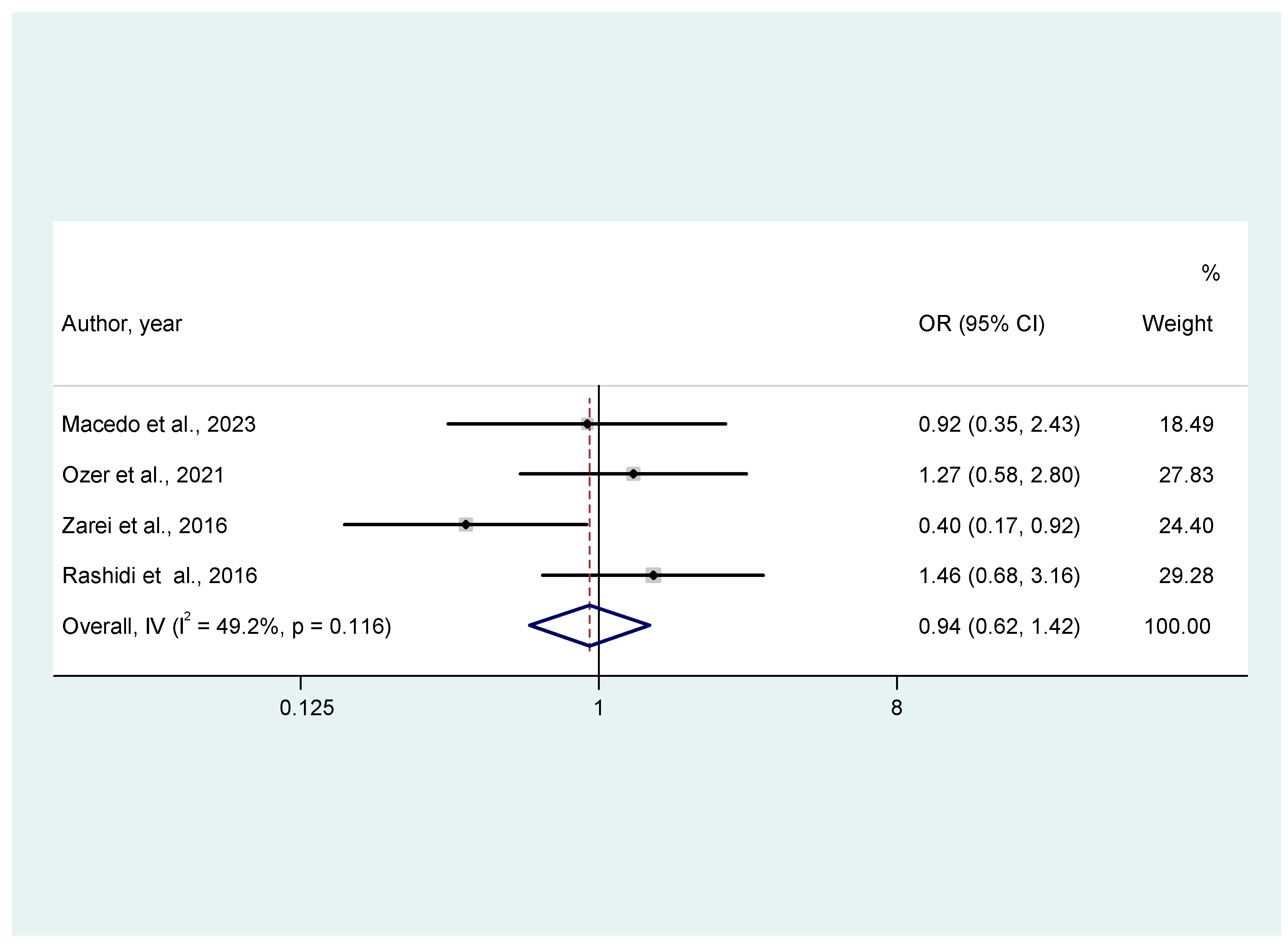
3.3.2. Secondary Outcomes (Miscarriage Rates, Clinical Pregnancy Rates, Live Birth Rates)
3.4. Risk of Bias
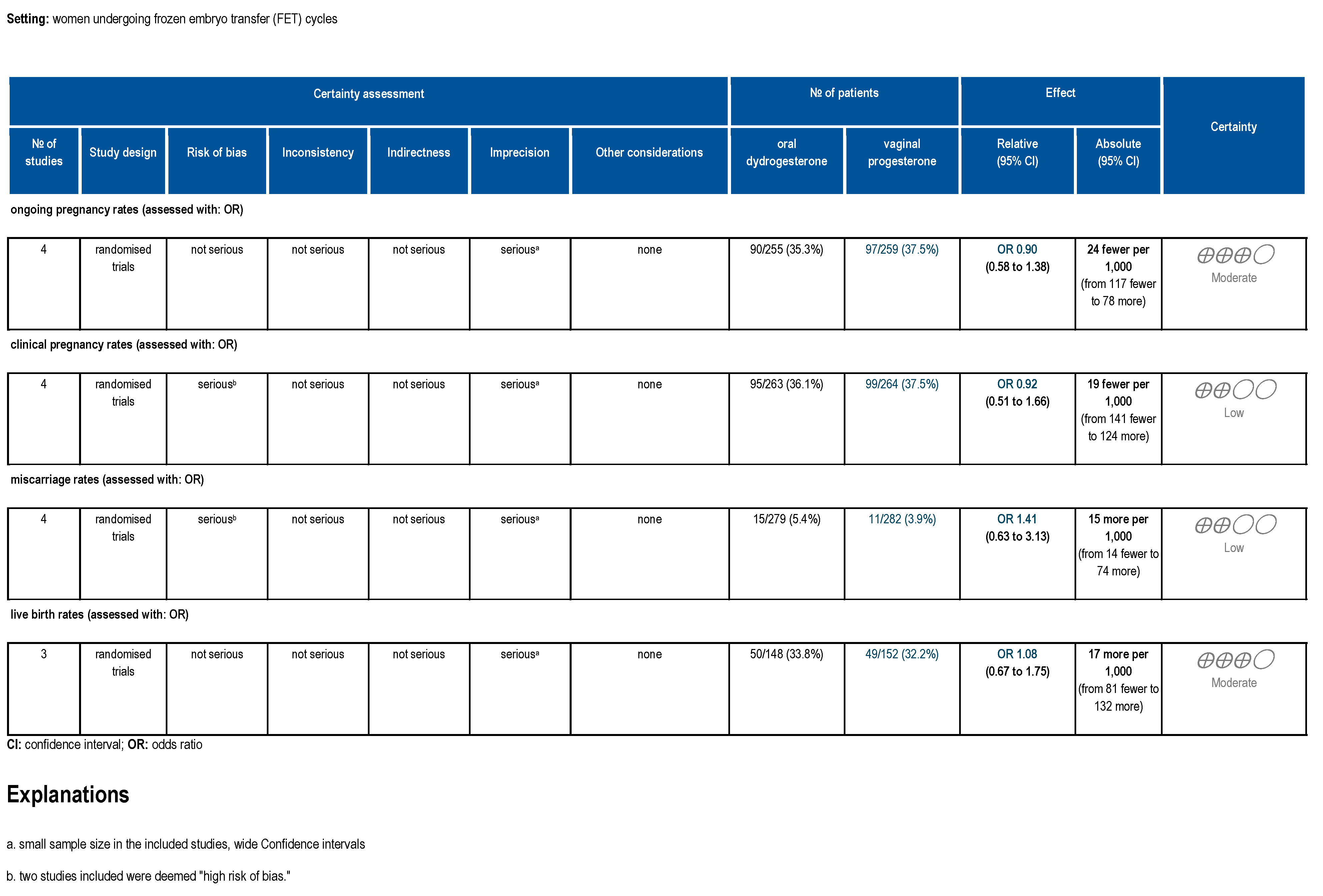
3.5. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FET | Frozen embryo transfer |
| OHSS | Ovarian hyperstimulation syndrome |
| COS | Controlled ovarian stimulation |
| IM | Intramuscular |
| LPS | Luteal phase support |
| PV | Vaginal |
Appendix A
| Author, Year | Title | Exclusion Reasons |
|---|---|---|
| Vinsonneau et al., 2022 | Impact of endometrial preparation on early pregnancy loss and live birth rate after frozen embryo transfer: A large multicenter cohort study (14 421 frozen cycles). | Retrospective cohort study comparing natural conception with artificial cycles, non-randomized design |
| Aygun et al., 2023 | The Effect of Different Luteal Phase Support Applications on Clinical Pregnancy Outcomes in Frozen-Thawed Embryo Transfer. | Retrospective, non-randomized design |
| Liu et al., 2023 | Association between duration of progesterone supplementation and clinical outcomes in artificial frozen-thawed embryo transfer cycles. | Prospective cohort study, only intramuscular progesterone is analyzed |
| Toriumi et al., 2023 | The addition of dydrogesterone improves the outcomes of pregnant women with low progesterone levels when receiving vaginal progesterone alone as luteal support in HRT-FET cycles. | Retrospective, non-randomized design |
| Vidal et al., 2023 | Supplementary dydrogesterone is beneficial as luteal phase support in artificial frozen-thawed embryo transfer cycles compared to micronized progesterone alone. | Retrospective, non-randomized design |
| Mackens et al., 2023 | Individualized luteal phase support using additional oral dydrogesterone in artificially prepared frozen embryo transfer cycles: is it beneficial? | Retrospective, non-randomized design |
| Ikechebelu et al., 2023 | A randomised control trial on oral dydrogesterone versus micronized vaginal progesterone pessary for luteal phase support in in vitro fertilization cycles. | Only fresh cycles were analyzed |
| Kao et al., 2022 | Clinical use of aqueous subcutaneous progesterone compared with vaginal progesterone as luteal support in in vitro fertilization: A randomized controlled study in Taiwan. | Only fresh cycles were analyzed |
| Stadelmann et al., 2022 | Vaginal progesterone as luteal phase support in natural cycle frozen-thawed embryo transfer (ProFET): protocol for a multicentre, open-label, randomised controlled trial. | Only vaginal progesterone is analyzed |
| Neumann et al., 2022 | Dydrogesterone and 20 alpha-dihydrodydrogesterone plasma levels on day of embryo transfer and clinical outcome in an anovulatory programmed frozen-thawed embryo transfer cycle: A prospective cohort study. | Dydrogesterone levels examined, no direct comparison with vaginal progesterone |
| Simon et al., 2022 | Comparison of two endometrial preparation methods for frozen-thawed embryo transfer in anovulatory PCOS patients: Impact on miscarriage rate. | Retrospective, non-randomized design |
| Ozgur et al., 2022 | Dydrogesterone versus medroxyprogesterone acetate co-treatment ovarian stimulation for IVF: a matched cohort study of 236 freeze-all-IVF cycles. | Only fresh cycles were analyzed |
| Vuong et al., 2021 | Micronized progesterone plus dydrogesterone versus micronized progesterone alone for luteal phase support in frozen-thawed cycles (MIDRONE): a prospective cohort study. | Prospective, non-randomized design |
| Deng et al., 2021 | Efficacy of vaginal administration of Crinone versus Utrogestan combined with oral dydrogesterone tablets for luteal support in PGT freeze-thaw embryo transfer cycles. [Chinese] | Prospective, non-randomized design |
| Noushin et al., 2021 | Dehydroepiandrosterone (DHEA) role in enhancement and maintenance of implantation (DREAM): Randomised double-blind placebo-controlled trial—Study protocol. | DHEA examined, not dydrogesterone |
| Devine et al., 2021 | Intramuscular progesterone optimizes live birth from programmed frozen embryo transfer: a randomized clinical trial. | Intramuscular versus vaginal progesterone were compared |
| Atzmon et al., 2021 | Comparable Outcomes Using Oral Dydrogesterone Vs. Micronized Vaginal Progesterone in Frozen Embryo Transfer: a Retrospective Cohort Study. | Retrospective, non-randomized design |
| Yang et al., 2020 | A Phase III randomized controlled trial of oral dydrogesterone versus intravaginal progesterone gel for luteal phase support in in vitro fertilization (Lotus II): results from the Chinese mainland subpopulation. | Only fresh cycles were analyzed |
| Cavagna et al., 2020 | Oral dydrogesterone compared to intravaginal micronized progesterone for endometrial preparation in frozen-thawed embryo transfer cycles: preliminary results of a randomized controlled trial. | Only fresh cycles were analyzed |
| Zargar et al., 2016 | Comparison the effectiveness of oral dydrogesterone, vaginal progesterone suppository and progesterone ampule for luteal phase support on pregnancy rate during ART cycles. | Only fresh cycles were analyzed |
| Tomic et al., 2015 | Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: Randomized controlled trial. | Only fresh cycles were analyzed |
| ROB 2 DOMAINS | Macedo et al. [19] | Pabuccu et al. [20] | Ozer et al. [16] | Zarei et al. [21] | Rashidi et al. [22] |
|---|---|---|---|---|---|
| 1: Bias arising from the randomization process | |||||
| Was the allocation sequence random? | Yes | Yes | Yes | Yes | Yes |
| Rationale/Notes (Quotes from the text to justify judgment) | The computer program used to randomize the patients into two groups was the R-Project | Therefore, 163 participants were randomly assigned based on a computer-generated list | In conclusion, a total of 134 women were assigned randomly in a ratio of 1:1 based on a computer-generated list to administer oral dydrogesterone (n = 67) or MVP (n = 67) for LPS | They were randomly assigned to four study groups using a computer-based random digit generator (each group including 100 patients) | Sequentially numbered sealed envelopes were prepared and provided by the study coordinator, according to random |
| Was the allocation sequence concealed until participants were enrolled and assigned to interventions? | Yes | NI | NI | NI | No |
| Rationale/Notes (Quotes from the text to justify judgment) | Participants received the study drugs through the institutional pharmacy, as required by the service’s protocol. | No information can be obtained from the manuscript | No information can be obtained from the manuscript | No information can be obtained from the manuscript | Envelopes were given before the intervention |
| Did baseline differences between intervention groups suggest a problem with the randomization process? | Probably No | No | No | No | No |
| Rationale/Notes (Quotes from the text to justify judgment) | Baseline differences do not suggest a problem based on Table 1 | Baseline differences do not suggest a problem based on Table 1 | Baseline differences do not suggest a problem based on Table 1 | Baseline differences do not suggest a problem based on Table 1 | Baseline differences do not suggest a problem based on Table 1 |
| Overall | Low risk | Low risk | Low risk | Low risk | High risk |
| 2. Bias due to deviations from intended interventions | |||||
| Were participants aware of their assigned intervention during the trial? | Yes | Yes | Yes | Yes | Yes |
| Rationale/Notes (Quotes from the text to justify judgment) | Open-label clinical trial. The different route of administration led to the participants knowing their group | Open-label clinical trial, technically not possible to make placebo arrangements | Open-label clinical trial | Participants were aware of their allocation due to the nature of the intervention (Oral versus vaginal) | Participants were aware of their allocation due to the nature of the intervention (Oral versus vaginal) |
| Were carers and people delivering the interventions aware of participants’ assigned intervention during the trial? | Yes | Yes | Yes | NI | No |
| Rationale/Notes (Quotes from the text to justify judgment) | Open-label clinical trial | Open-label clinical trial, technically not possible to make placebo arrangements | Open-label clinical trial | Not included throughout the text | Single-blind trial |
| Deviations that arose because of the trial context? (M) | No | No | No | No | No |
| Rationale/Notes (Quotes from the text to justify judgment) | No deviation | No deviation | No deviation | No deviation in the groups regarding the present study | No deviation |
| Deviations affect outcome? | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no |
| Rationale/Notes (Quotes from the text to justify judgment) | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no |
| Deviations balanced between groups? | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no |
| Rationale/Notes (Quotes from the text to justify judgment) | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no | Not answered because (M) is no |
| Was an appropriate analysis used to estimate the effect of assignment to intervention? (S) | Probably yes | No | Yes | No | Probably yes |
| Rationale/Notes (Quotes from the text to justify judgment) | Based on Figure 2 | All data were given as per the protocol analysis | Analyzed based on the groups to which participants were allocated | Analyzed based on the participants who adhered to the study | Based on diagram 1 |
| If N/PN/NI to (S): Was there potential for a substantial impact (on the result) of the failure to analyze participants in the group to which they were randomized? | Not answered because (S) is PY | Probably No | Not answered because (S) is Y | Probably No | Not answered because (S) is PY |
| Rationale/Notes (Quotes from the text to justify judgment) | Not answered because (S) is PY | Only a small number of people were excluded from the initial allocation | Not answered because (S) is Y | We also performed multivariate logistic regression to examine the effects of the four interventions on CPR, OPR, and MR by adjustment for infertility duration, endometrial thickness, and age | Not answered because (S) is PY |
| Overall | Low risk | Some concerns | Low risk | Some concerns | Low risk |
| 3. Bias due to missing outcome data | |||||
| Were data for this outcome available for all, or nearly all, participants randomized? (X) | Yes | Yes | Yes | No | Yes |
| Rationale/Notes (Quotes from the text to justify judgment) | Outcome data for nearly all participants and the lost to follow-up participants were lost independently of the outcome measure | No extreme losses to follow-up (93% analyzed from randomized participants) | No losses to follow-up | In the two groups concerning this meta-analysis, 10 patients were lost to follow-up, accounting for about 10% | Data were available for nearly all participants. Losses to follow-up were not important and were not associated with the outcome |
| If N/PN/NI to (X): Is there evidence that the result was not biased by missing outcome data? (Z) | Not answered because (X) is Yes | Not answered because (X) is Yes | Not answered because (X) is Yes | Probably no | Not answered because (X) is Yes |
| Rationale/Notes (Quotes from the text to justify judgment) | Not answered because (X) is Y | Not answered because (X) is Yes | Not answered because (X) is Yes | About 10% of participants in each group are lost, and no sensitivity analysis was carried out (e.g, LOCF) | Not answered because (X) is Yes |
| If N/PN to (Z): Could missingness in the outcome depend on its true value? (AB) | Not answered because (X) is Y | Not answered because (X) is Yes | Not answered because (X) is Yes | NI | Not answered because (X) is Yes |
| Rationale/Notes (Quotes from the text to justify judgment) | Not answered because (X) is Y | Not answered because (X) is Yes | Not answered because (X) is Yes | No information | Not answered because (X) is Yes |
| If Y/PY/NI to (AB): Is it likely that missingness in the outcome depended on its true value? | Not answered because (X) is Y | Not answered because (X) is Yes | Not answered because (X) is Yes | NI | Not answered because (X) is Yes |
| Rationale/Notes (Quotes from the text to justify judgment) | Not answered because (X) is Y | Not answered because (X) is Yes | Not answered because (X) is Yes | No information | Not answered because (X) is Yes |
| Overall | Low risk | Low risk | Low risk | Some concerns | Low risk |
| 4. Bias in measurement of the outcome | |||||
| Was the method of measuring the outcome inappropriate? (AG) | No | No | No | No | No |
| Rationale/Notes (Quotes from the text to justify judgment) | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured |
| Could measurement or ascertainment of the outcome have differed between intervention groups? (AI) | No | No | No | No | No |
| Rationale/Notes (Quotes from the text to justify judgment) | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured | Clinical pregnancy, ongoing pregnancy, and miscarriages are objectively measured through ultrasound. Live births are objectively measured |
| If N/PN/NI to (AG, AI): Were outcome assessors aware of the intervention received by study participants? (AK) | Yes | Yes | Yes | Yes | No |
| Rationale/Notes (Quotes from the text to justify judgment) | Open-label | Open-label | Open-label | Open-label | Trial blinded to assessors of the outcome |
| If Y/PY/NI to (AK): Could assessment of the outcome have been influenced by knowledge of intervention received? (AM) | No | No | No | No | Not answered because (AK) is No |
| Rationale/Notes (Quotes from the text to justify judgment) | Outcomes objectively measured | Outcomes objectively measured | Outcomes objectively measured | Outcomes objectively measured | Not answered because (AK) is No |
| If Y/PY/NI to (AM): Is it likely that assessment of the outcome was influenced by knowledge of intervention received? | Not answered because (AM) are No | Not answered because (AM) are No | Not answered because (AM) are No | Not answered because (AM) are No | Not answered because (AK) is No |
| Rationale/Notes (Quotes from the text to justify judgment) | Not answered because (AM) are No | Not answered because (AM) are No | Not answered because (AM) are No | Not answered because (AM) are No | Not answered because (AK) is No |
| Overall | Low risk | Low risk | Low risk | Low risk | Low risk |
| 5. Bias in selection of the reported result | |||||
| Were the data that produced this result analyzed in accordance with a pre-specified analysis plan that was finalized before unblinded outcome data were available for analysis? | Probably Yes | Probably Yes | 0 | NI | Probably Yes |
| Rationale/Notes (Quotes from the text to justify judgment) | Document Number: 3.453.065, Rapporteurship on 13 July 2019, and Brazilian Registry of Clinical Trials (ReBEC). UTN (Universal Trial Number): U1111-1247-1845, date: 31 March 2020 | The study was registered at clinicaltrials.gov (NCT03948022) | The study is also registered at ClinicalTrials.gov (NCT04124913) | No registration number | The registration number of the trial was IRCT201406255181N15. |
| Is the numerical result being assessed likely to have been selected? | Probably No | Probably No | Probably No | Probably No | Probably No |
| Rationale/Notes (Quotes from the text to justify judgment) | Reasonable outcomes are presented | Reasonable outcomes are presented | Reasonable outcomes are presented | Reasonable outcomes are presented | Reasonable outcomes are presented |
| Overall | Low risk | Low risk | Low risk | Some concerns | Low risk |
| OVERALL ASSESSMENT | Low risk | Some concerns | Low risk | High risk | High risk |
References
- Human Fertilisation and Embryology Authority. Fertility Treatment 2014–2016 Trends and Figures; HFEA: London, UK, 2018; Volume 479, p. 480. [Google Scholar]
- De Geyter, C.H.; Wyns, C.; Calhaz-Jorge, C.; de Mouzon, J.; Ferraretti, A.P.; Kupka, M.; Nyboe Andersen, A.; Nygren, K.G.; Goossens, V. 20 years of the European IVF-monitoring Consortium registry: What have we learned? A comparison with registries from two other regions. Hum. Reprod. 2020, 35, 2832–2849. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, C.; Lokshin, V.N. Assisted reproductive technology in Europe, 2011: Results generated from European registers by ESHRE. Hum. Reprod. 2016, 31, 233–248. [Google Scholar]
- Bourdon, M.; Maignien, C.; Pocate-Cheriet, K.; Bureau, G.P.; Marcellin, L.; Patrat, C.; Chapron, C.; Santulli, P. The freeze-all strategy after IVF: Which indications? Reprod. Biomed. Online 2021, 42, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, Y.; Hao, C.; Zhang, H.; Wei, D.; Zhang, Y.; Zhu, Y.; Deng, X.; Qi, X.; Li, H.; et al. Transfer of fresh versus frozen embryos in ovulatory women. N. Engl. J. Med. 2018, 378, 126–136. [Google Scholar] [CrossRef]
- Wong, K.M.; van Wely, M.; Mol, F.; Repping, S.; Mastenbroek, S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst. Rev. 2017, 3, CD011184. [Google Scholar] [CrossRef]
- Devine, K.; Richter, K.S.; Jahandideh, S.; Widra, E.A.; McKeeby, J.L. Intramuscular progesterone optimizes live birth from programmed frozen embryo transfer: A randomized clinical trial. Fertil. Steril. 2021, 116, 633–643. [Google Scholar] [CrossRef]
- Almohammadi, A.; Raveendran, A.; Black, M.; Maheshwari, A. The optimal route of progesterone administration for luteal phase support in a frozen embryo transfer: A systematic review. Arch. Gynecol. Obs. 2023, 308, 341–350. [Google Scholar] [CrossRef]
- Turgut, E.N.; Boynukalın, F.K.; Gültomruk, M.; Yarkıner, Z.; Bahçeci, M. Comparison of intramuscular versus subcutaneous aqueous progesterone for luteal phase support in artificially prepared frozen embryo transfer cycles. Turk. J. Obstet. Gynecol. 2020, 17, 240–246. [Google Scholar] [CrossRef]
- Arvidsson, C.; Hellborg, M.; Gemzell-Danielsson, K. Preference and acceptability of oral versus vaginal administration of misoprostol in medical abortion with mifepristone. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 87–91. [Google Scholar] [CrossRef]
- Griesinger, G.; Blockeel, C.; Sukhikh, G.T.; Patki, A.; Dhorepatil, B.; Yang, D.Z.; Chen, Z.J.; Kahler, E.; Pexman-Fieth, C.; Tournaye, H. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: A randomized clinical trial. Hum. Reprod. 2018, 33, 2212–2221. [Google Scholar] [CrossRef]
- Saharkhiz, N.; Zamaniyan, M.; Salehpour, S.; Zadehmodarres, S.; Hoseini, S.; Cheraghi, L.; Seif, S.; Baheiraei, N. A comparative study of dydrogesterone and micronized progesterone for luteal phase support during in vitro fertilization (IVF) cycles. Gynecol. Endocrino. 2016, 32, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H.; Sukhikh, G.T.; Kahler, E.; Griesinger, G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum. Reprod. 2017, 32, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- Ozer, G.; Yuksel, B.; Cicek, O.S.Y.; Kahraman, S. Oral dydrogesterone vs. micronized vaginal progesterone gel for luteal phase support in frozen-thawed single blastocyst transfer in good prognosis patients. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102030. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- de Macedo, L.C.G.M.; Neto, M.C.; Dzik, A.; do Rosário Rocha, A.; Lima, S.M.R.R. Randomized Clinical Trial Comparing Oral Dydrogesterone to Micronized Vaginal Progesterone for Endometrial Preparation in Frozen-Thawed Embryo Transfer Cycle. Clin. Exp. Obstet. Gynecol. 2023, 50, 8. [Google Scholar] [CrossRef]
- Pabuccu, E.; Kovanci, E.; Israfilova, G.; Tulek, F.; Demirel, C.; Pabuccu, R. Oral, vaginal or intramuscular progesterone in programmed frozen embryo transfer cycles: A pilot randomized controlled trial. Reprod. Biomed. Online 2022, 45, 1145–1151. [Google Scholar] [CrossRef]
- Zarei, A.; Sohail, P.; Parsanezhad, M.E.; Alborzi, S.; Samsami, A.; Azizi, M. Comparison of four protocols for luteal phase support in frozen-thawed Embryo transfer cycles: A randomized clinical trial. Arch. Gynecol. Obstet. 2017, 295, 239–246. [Google Scholar] [CrossRef]
- Rashidi, B.H.; Ghazizadeh, M.; Nejad, E.S.T.; Bagheri, M.; Gorginzadeh, M. Oral dydrogesterone for luteal support in frozen-thawed embryo transfer artificial cycles: A pilot randomized controlled trial. Asian Pac. J. Reprod. 2016, 5, 490–494. [Google Scholar] [CrossRef]
- Patki, A.; Pawar, V.C. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecol. Endocrinol. 2007, 23, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Huang, Y.; Zhou, Y. Dydrogesterone in the treatment of endometriosis: Evidence mapping and meta-analysis. Arch. Gynecol. Obs. 2021, 304, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, G.; Blockeel, C.; Tournaye, H. Oral dydrogesterone for luteal phase support in fresh in vitro fertilization cycles: A new standard? Fertil. Steril. 2018, 109, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, B.N.; Shirazee, H.H.; Dam, P.; Goswami, S.K.; Chatterjee, R.; Ghosh, S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: Results of a randomised study. J. Steroid Biochem. Mol. Biol. 2005, 97, 416–420. [Google Scholar] [CrossRef]
- Yang, D.Z.; Griesinger, G.; Wang, W.; Gong, F.; Liang, X.; Zhang, H.; Sun, Y.; Kahler, E.; Pexman-Fieth, C.; Olofsson, J.I.; et al. A Phase III randomized controlled trial of oral dydrogesterone versus intravaginal progesterone gel for luteal phase support in in vitro fertilization (Lotus II): Results from the Chinese mainland subpopulation. Gynecol. Endocrinol. 2020, 36, 175–183. [Google Scholar] [CrossRef]
- Barbosa, M.W.P.; Valadares, N.P.B.; Barbosa, A.C.P.; Amaral, A.S.; Iglesias, J.R.; Nastri, C.O.; de Paula Martins, W.; Nakagawa, H.M. Oral dydrogesterone vs. vaginal progesterone capsules for luteal-phase support in women undergoing embryo transfer: A systematic review and meta-analysis. JBRA Assist. Reprod. 2018, 22, 148–156. [Google Scholar] [CrossRef]
- Tomic, V.; Tomic, J.; Klaic, D.Z.; Kasum, M.; Kuna, K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: Randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 186, 49–53. [Google Scholar] [CrossRef]
- Queisser-Luft, A. Dydrogesterone use during pregnancy: Overview of birth defects reported since 1977. Early Hum. Dev. 2009, 85, 375–377. [Google Scholar] [CrossRef]
- Mackens, S.; Santos-Ribeiro, S.; Van De Vijver, A.; Racca, A.; Van Landuyt, L.; Tournaye, H.; Blockeel, C. Frozen embryo transfer: A review on the optimal endometrial preparation and timing. Hum. Reprod. 2017, 32, 2234–2242. [Google Scholar] [CrossRef]
- Blockeel, C.; Drakopoulos, P.; Santos-Ribeiro, S.; Polyzos, N.P.; Tournaye, H. A fresh look at the freeze—All protocol: A SWOT analysis. Hum. Reprod. 2016, 31, 491–497. [Google Scholar] [CrossRef]
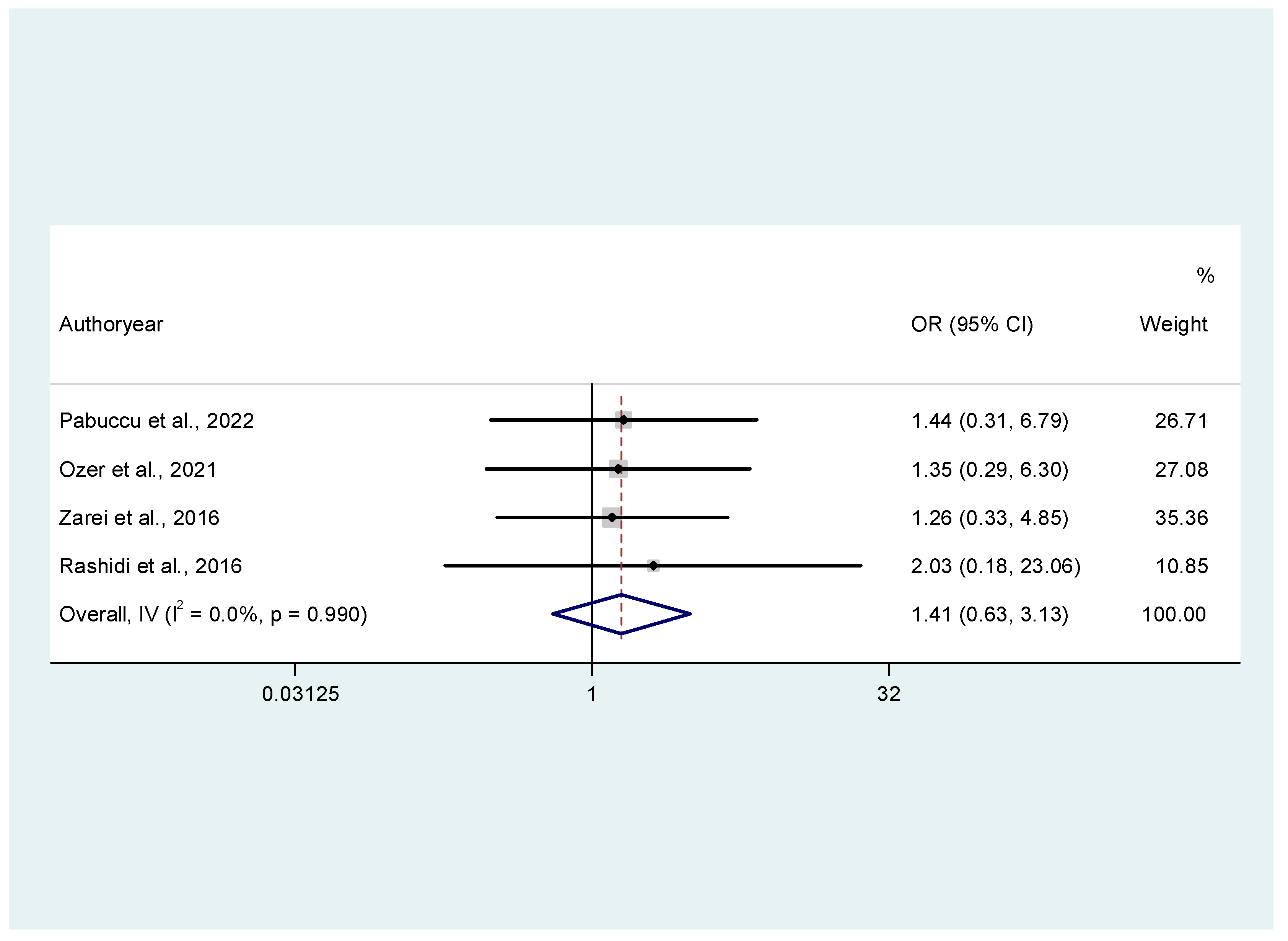


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavridis, K.; Balafoutas, D.; Kalampokas, T.; Benetou, V.; Samoli, E.; Vlahos, N.; Kasdagli, M.-I. Oral Dydrogesterone Versus Vaginal Progesterone for Luteal Phase Support in Frozen–Thawed Embryo Transfer Cycles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2025, 14, 3238. https://doi.org/10.3390/jcm14093238
Stavridis K, Balafoutas D, Kalampokas T, Benetou V, Samoli E, Vlahos N, Kasdagli M-I. Oral Dydrogesterone Versus Vaginal Progesterone for Luteal Phase Support in Frozen–Thawed Embryo Transfer Cycles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2025; 14(9):3238. https://doi.org/10.3390/jcm14093238
Chicago/Turabian StyleStavridis, Konstantinos, Dimitrios Balafoutas, Theodoros Kalampokas, Vassiliki Benetou, Evangelia Samoli, Nikolaos Vlahos, and Maria-Iosifina Kasdagli. 2025. "Oral Dydrogesterone Versus Vaginal Progesterone for Luteal Phase Support in Frozen–Thawed Embryo Transfer Cycles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 14, no. 9: 3238. https://doi.org/10.3390/jcm14093238
APA StyleStavridis, K., Balafoutas, D., Kalampokas, T., Benetou, V., Samoli, E., Vlahos, N., & Kasdagli, M.-I. (2025). Oral Dydrogesterone Versus Vaginal Progesterone for Luteal Phase Support in Frozen–Thawed Embryo Transfer Cycles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 14(9), 3238. https://doi.org/10.3390/jcm14093238







