Acid–Base Status in Critically Ill Patients: Physicochemical vs. Traditional Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Data Collection
2.4. Calculate Variables
2.5. Patient Stratification
2.6. Statistical Analysis
3. Results
3.1. On ICU Admission
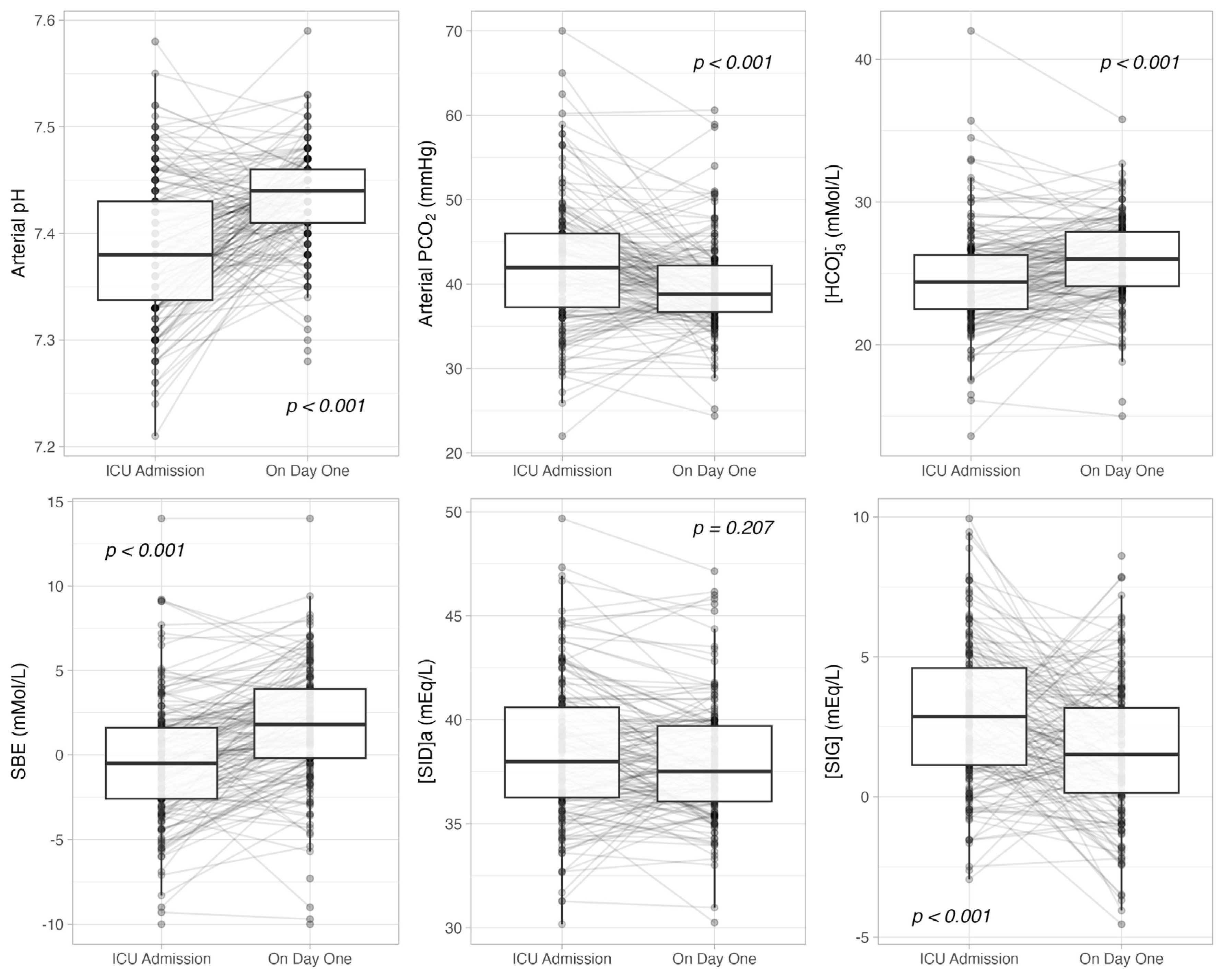
3.2. Admission vs. Day One
3.3. On Day One
3.4. Patients with and Without Renal Impairment
3.5. Diagnostic Accuracy of SBE and Apparent SID on Metabolic Acid–Base Derangements
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Jaghbeer, M.; Kellum, J.A. Acid–base disturbances in intensive care patients: Etiology, pathophysiology and treatment. Nephrol. Dial. Transplant. 2015, 30, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Gunnerson, K.J.; Saul, M.; He, S.; Kellum, J.A. Lactate versus non-lactate metabolic acidosis: A retrospective outcome evaluation of critically ill patients. Crit. Care 2006, 10, R22. [Google Scholar] [CrossRef]
- Lindner, G.; Schwarz, C.; Grüssing, H.; Kneidinger, N.; Fazekas, A.; Funk, G.-C. Rising serum sodium levels are associated with a concurrent development of metabolic alkalosis in critically ill patients. Intensive Care Med. 2013, 39, 399–405. [Google Scholar] [CrossRef]
- Achanti, A.; Szerlip, H.M. Acid-Base Disorders in the Critically Ill Patient. Clin. J. Am. Soc. Nephrol. 2023, 18, 102–112. [Google Scholar] [CrossRef]
- de Souza, S.P.; Caldas, J.R.; Lopes, M.B.; Silveira, M.A.D.; Coelho, F.O.; Queiroz, I.O.; Cury, P.D.; da Hora Passos, R. Physico-chemical characterization of acid base disorders in patients with COVID-19: A cohort study. World J Nephrol. 2024, 13, 92498. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Pozzi, T.; Fratti, I.; Modafferi, L.; Montante, M.; Papa, G.F.S.; Coppola, S. Acid-Base Disorders in COVID-19 Patients with Acute Respiratory Distress Syndrome. J. Clin. Med. 2022, 11, 2093. [Google Scholar] [CrossRef]
- Smith, I.; Kumar, P.; Molloy, S.; Rhodes, A.; Newman, P.J.; Grounds, R.M.; Bennett, E.D. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001, 27, 74–83. [Google Scholar] [CrossRef]
- Noritomi, D.T.; Soriano, F.G.; Kellum, J.A.; Cappi, S.B.; Biselli, P.J.C.; Libório, A.B.; Park, M. Metabolic acidosis in patients with severe sepsis and septic shock: A longitudinal quantitative study. Crit. Care Med. 2009, 37, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, I. Molecular mechanisms and regulation of urinary acidification. Compr. Physiol. 2014, 4, 1737–1774. [Google Scholar] [CrossRef]
- Berend, K.; de Vries, A.P.J.; Gans, R.O.B. Physiological Approach to Assessment of Acid–Base Disturbances. N. Engl. J. Med. 2014, 371, 1434–1445. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Gennari, F.J.; Galla, J.H.; Madias, N.E. Assessing acid–base disorders. Kidney Int. 2009, 76, 1239–1247. [Google Scholar] [CrossRef]
- Siggaard-Andersen, O. The Van Slyke Equation. Scand. J. Clin. Lab. Investig. 1977, 37, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Langer, T.; Brusatori, S.; Gattinoni, L. Understanding base excess (BE): Merits and pitfalls. Intensive Care Med. 2022, 48, 1080–1083. [Google Scholar] [CrossRef]
- Kellum, J.A. Determinants of blood pH in health and disease. Crit. Care 2000, 4, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stewart, P.A. Modern quantitative acid-base chemistry. Can. J. Physiol. Pharmacol. 1983, 61, 1444–1461. [Google Scholar] [CrossRef]
- Dubin, A.; Menises, M.M.; Masevicius, F.D.; Moseinco, M.C.; Kutscherauer, D.O.; Ventrice, E.; Laffaire, E.; Estenssoro, E. Comparison of three different methods of evaluation of metabolic acid-base disorders*. Crit. Care Med. 2007, 35, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Fencl, V.; Jabor, A.; Kazda, A.; Figge, J. Diagnosis of Metabolic Acid–Base Disturbances in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2000, 162, 2246–2251. [Google Scholar] [CrossRef]
- Figge, J.; Rossing, T.H.; Fencl, V. The role of serum proteins in acid-base equilibria. J. Lab. Clin. Med. 1991, 117, 453–467. [Google Scholar]
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Knaus, W.A.; Wagner, D.P.; Draper, E.A.; Zimmerman, J.E.; Bergner, M.; Bastos, P.G.; Sirio, C.A.; Murphy, D.J.; Lotring, T.; Damiano, A.; et al. The APACHE III Prognostic System. Chest 1991, 100, 1619–1636. [Google Scholar] [CrossRef]
- Kumar, V.; Karon, B.S. Comparison of Measured and Calculated Bicarbonate Values. Clin. Chem. 2008, 54, 1586–1587. [Google Scholar] [CrossRef]
- Gattinoni, L.; Busana, M. Venous and arterial base excess difference: Methodological error or physiological reality? Intensive Care Med. 2019, 45, 1686–1687. [Google Scholar] [CrossRef]
- Kellum, J.A.; Kramer, D.J.; Pinsky, M.R. Strong ion gap: A methodology for exploring unexplained anions. J. Crit. Care 1995, 10, 51–55. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.R.; Kulkarni, V. Metabolic Alkalosis in the Critically III. Crit. Rev. Clin. Lab. Sci. 1999, 36, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A. Determinants of Plasma Acid-Base Balance. Crit. Care Clin. 2005, 21, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Gunnerson, K.J. Clinical review: The meaning of acid-base abnormalities in the intensive care unit part I—Epidemiology. Crit. Care 2005, 9, 508. [Google Scholar] [CrossRef]
- Figge, J.; Jabor, A.; Kazda, A.; Fencl, V. Anion gap and hypoalbuminemia. Crit. Care Med. 1998, 26, 1807–1810. [Google Scholar] [CrossRef]
- Fidkowski, C.; Helstrom, J. Diagnosing metabolic acidosis in the critically ill: Bridging the anion gap, Stewart, and base excess methods. Can. J. Anesth./J. Can. D’anesthésie 2009, 56, 247–256. [Google Scholar] [CrossRef]
- Boniatti, M.M.; Cardoso, P.R.C.; Castilho, R.K.; Vieira, S.R.R. Acid–base disorders evaluation in critically ill patients: We can improve our diagnostic ability. Intensive Care Med. 2009, 35, 1377–1382. [Google Scholar] [CrossRef]
- Morris, C.G.; Low, J. Metabolic acidosis in the critically ill: Part 1. Classification and pathophysiology. Anaesthesia 2008, 63, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Maciel, A.T.; Park, M. Differences in acid-base behavior between intensive care unit survivors and nonsurvivors using both a physicochemical and a standard base excess approach: A prospective, observational study. J. Crit. Care 2009, 24, 477–483. [Google Scholar] [CrossRef]
- Durward, A.; Tibby, S.M.; Skellett, S.; Austin, C.; Anderson, D.; Murdoch, I.A. The strong ion gap predicts mortality in children following cardiopulmonary bypass surgery*. Pediatr. Crit. Care Med. 2005, 6, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Mæhle, K.; Haug, B.; Flaatten, H.; Nielsen, E.W. Metabolic alkalosis is the most common acid–base disorder in ICU patients. Crit. Care 2014, 18, 420. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.S. Acid–Base Disorders in Kidney Disease. Acid-Base Disorders; Springer International Publishing: Cham, Switzerland, 2020; pp. 171–183. [Google Scholar]
- Story, D.A.; Kellum, J.A. New aspects of acid-base balance in intensive care. Curr. Opin. Anaesthesiol. 2004, 17, 119–123. [Google Scholar] [CrossRef]
- Rocktaeschel, J.; Morimatsu, H.; Uchino, S.; Bellomo, R. Unmeasured anions in critically ill patients: Can they predict mortality?*. Crit. Care Med. 2003, 31, 2131–2136. [Google Scholar] [CrossRef]
| n = 172 | |
|---|---|
| Age, years | 69 [57–77] |
| Female sex, % (n) | 34.9 (60) |
| Weight, kg | 75 [63–88] |
| Body mass index, kg/m2 | 26 [23–29] |
| SOFA score | 3 [1–6] |
| APACHE III score | 9 [7–13] |
| Reason for ICU admission, % (n) | |
| Acute respiratory failure | 16.9 (29) |
| Sepsis | 12.8 (22) |
| Post-operative | 70.3 (121) |
| History of, % (n) | |
| Hypertension | 51.7 (89) |
| Chronic kidney disease | 4.7 (8) |
| Diabetes mellitus | 6.4 (11) |
| Liver disease | 16.3 (28) |
| Place prior to ICU admission, % (n) | |
| Emergency department | 19.2 (33) |
| Operating room | 70.3 (121) |
| Ward | 10.5 (18) |
| Diuretic home therapy, % (n) | 6.4 (11) |
| Hospital mortality, % (n) | 14.0 (24) |
| n = 172 | |
|---|---|
| Arterial pH | 7.38 [7.33–7.43] |
| PaCO2, mmHg | 42 [37–46] |
| [HCO3−], mMol/L | 24.5 [22.6–26.6] |
| Standard Base Excess, mMol/L | −0.6 [−3.0–1.7] |
| Apparent SID, mEq/L | 39.2 [37.5–41.8] |
| Effective SID, mEq/L | 35.7 [33.3–38.0] |
| SIG, mEq/L | 3.7 [1.8–5.7] |
| Lactate, mMol/L | 1.6 [1.2–2.4] |
| Albumin, g/dL | 3.2 [2.8–3.7] |
| Phosphate, mg/dL | 3.8 [3.2–4.4] |
| Sodium, mEq/L | 137 [135–139] |
| Potassium, mEq/L | 4.1 [3.8–4.5] |
| Calcium, mEq/L | 1.17 [1.14–1.20] |
| Magnesium, mEq/L | 2.2 [2.0–2.5] |
| Chloride, mEq/L | 104 [102–106] |
| pH < 7.35 32% (55) | 7.35 ≤ pH ≤ 7.45 51% (88) | pH > 7.45 17% (29) | p | |
|---|---|---|---|---|
| Plasmatic | ||||
| Arterial pH | 7.31 [7.28–7.33] | 7.39 [7.37–7.42] * | 7.48 [7.47–7.50] *° | <0.001 |
| PaCO2, mmHg | 47 [44–51] | 41 [38–44] * | 35 [33–37] *° | <0.001 |
| [HCO3−], mMol/L | 23.7 [21.4–25.5] | 24.6 [22.8–26.5] * | 26.3 [23.8–28.0] * | <0.001 |
| Standard Base Excess, mMol/L | −3.0 [−5.1–−0.8] | −0.4 [−2.2–1.7] * | 3.5 [ 0.1–4.5] *° | <0.001 |
| Apparent SID, mEq/L | 40.4 [38.2–42.2] | 38.8 [37.2–41.1] | 38.5 [37.1–41.7] | 0.112 |
| Effective SID, mEq/L | 34.6 [32.2–37.5] | 35.6 [33.6–38.2] | 37.5 [33.3–39.7] * | 0.024 |
| SIG, mEq/L | 5.4 [3.9–7.1] | 3.2 [1.4–4.6] * | 2.5 [1.2–5.0] * | <0.001 |
| Lactate, mMol/L | 1.7 [1.2–2.4] | 1.6 [1.1–2.4] | 1.3 [1.0–1.7] | 0.086 |
| Albumin, g/dL | 3.5 [3.0–3.7] | 3.2 [2.8–3.7] | 3.1 [2.7–3.4] * | 0.021 |
| Phosphate, mg/dL | 4.1 [3.6–4.7] | 3.8 [3.2–4.4] | 3.4 [2.6–4.0] * | 0.002 |
| Sodium, mEq/L | 138 [136–140] | 137 [135–138] * | 136 [134–141] | 0.033 |
| Potassium, mEq/L | 4.2 [4.0–4.7] | 4.2 [3.9–4.5] | 3.7 [3.5–4.0] *° | <0.001 |
| Calcium, mEq/L | 2.4 [2.3–2.4] | 2.3 [2.3–2.4] * | 2.3 [2.2–2.4] * | <0.001 |
| Magnesium, mEq/L | 1.8 [1.6–2.0] | 1.8 [1.6–2.1] | 1.7 [1.6–1.9] | 0.222 |
| Chloride, mEq/L | 104 [102–106] | 104 [102–106] | 104 [101–106] | 0.867 |
| Urinary | ||||
| Urine output, mL/kg/h | 0.6 [0.3–1.3] | 0.9 [0.5–1.6] | 0.8 [0.4–1.0] | 0.343 |
| Urinary pH | 6.5 [6.0–6.5] | 6.5 [6.5–6.5] | 6.5 [6.5–6.5] | 0.519 |
| Urinary SID, mEq/L | 56 [35–72] | 57 [36–77] | 59 [38–103] | 0.167 |
| Urinary sodium, mEq/L | 106 [70–150] | 128 [83–155] | 95 [68–143] | 0.131 |
| Urinary potassium, mEq/L | 44 [31–58] | 38 [24–58] | 40 [24–57] | 0.660 |
| Urinary chloride, mEq/L | 94 [48–126] | 105 [68–137] | 63 [26–115] ° | 0.009 |
| On ICU Admission | ||||
|---|---|---|---|---|
| κ = 0.08 | Standard Base Excess | |||
| Low | Normal | High | ||
| Strong Ion Difference | Low | 11 (19) | 22 (38) | 2 (3) |
| Normal | 10 (18) | 26 (44) | 6 (11) | |
| High | 3 (5) | 12 (21) | 8 (13) | |
| On Day One | ||||
|---|---|---|---|---|
| κ = 0.07 | Standard Base Excess | |||
| Low | Normal | High | ||
| Strong Ion Difference | Low | 5 (9) | 22 (39) | 8 (13) |
| Normal | 6 (10) | 28 (47) | 17 (29) | |
| High | 1 (2) | 3 (6) | 10 (18) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciabattoni, A.; Chiumello, D.; Mancusi, S.; Pozzi, T.; Monte, A.; Rocco, C.; Coppola, S. Acid–Base Status in Critically Ill Patients: Physicochemical vs. Traditional Approach. J. Clin. Med. 2025, 14, 3227. https://doi.org/10.3390/jcm14093227
Ciabattoni A, Chiumello D, Mancusi S, Pozzi T, Monte A, Rocco C, Coppola S. Acid–Base Status in Critically Ill Patients: Physicochemical vs. Traditional Approach. Journal of Clinical Medicine. 2025; 14(9):3227. https://doi.org/10.3390/jcm14093227
Chicago/Turabian StyleCiabattoni, Arianna, Davide Chiumello, Simone Mancusi, Tommaso Pozzi, Alessandro Monte, Cosmo Rocco, and Silvia Coppola. 2025. "Acid–Base Status in Critically Ill Patients: Physicochemical vs. Traditional Approach" Journal of Clinical Medicine 14, no. 9: 3227. https://doi.org/10.3390/jcm14093227
APA StyleCiabattoni, A., Chiumello, D., Mancusi, S., Pozzi, T., Monte, A., Rocco, C., & Coppola, S. (2025). Acid–Base Status in Critically Ill Patients: Physicochemical vs. Traditional Approach. Journal of Clinical Medicine, 14(9), 3227. https://doi.org/10.3390/jcm14093227







