Finerenone: Potential Clinical Application Across the Spectrum of Cardiovascular Disease and Chronic Kidney Disease
Abstract
1. Introduction
2. Methods
3. CKD Associated with T2D Represents a Broad Spectrum of Patients
4. Defining CKD
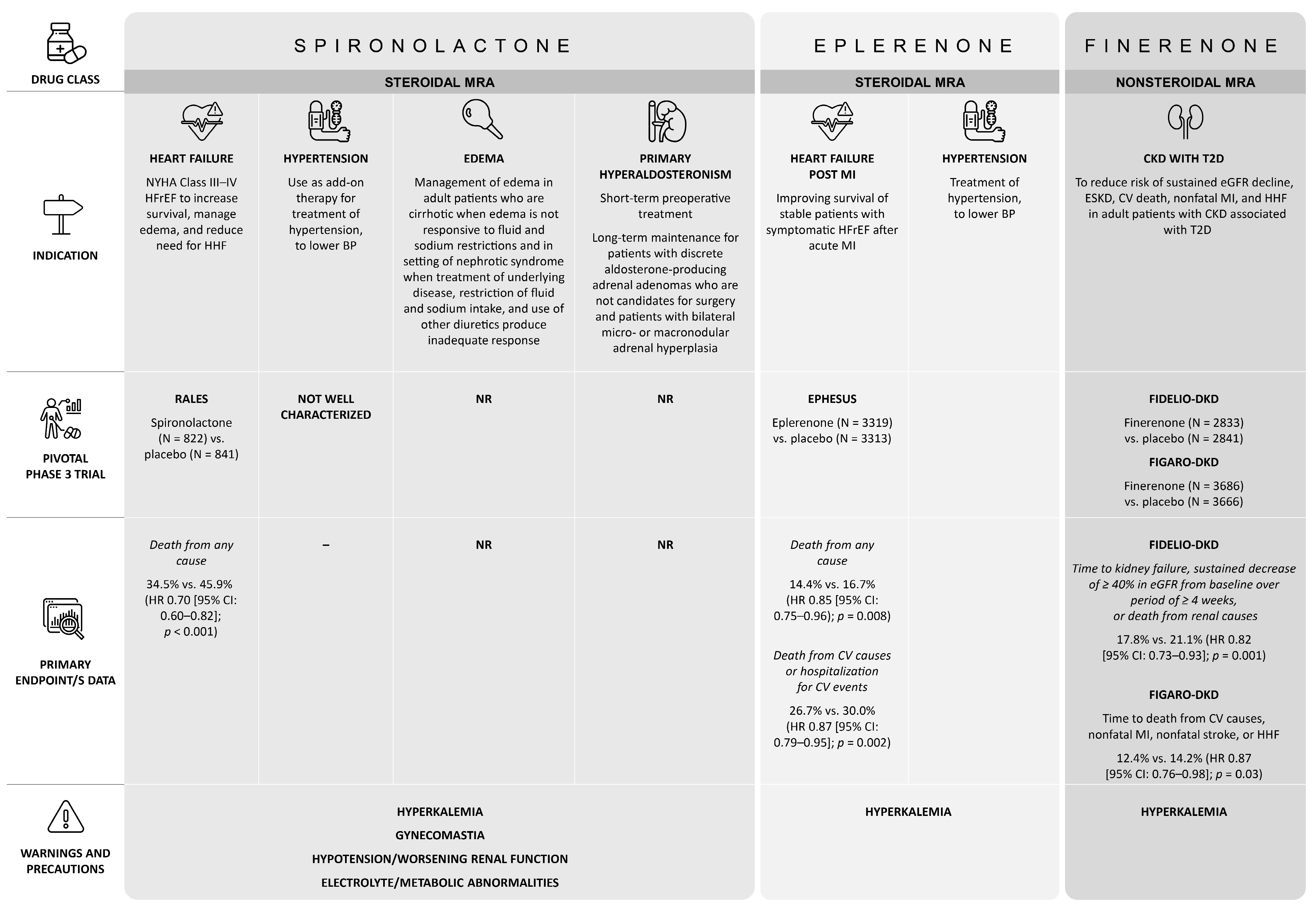
5. Finerenone Is a ns-MRA: Differences vs. Steroidal MRAs
6. The RAAS, Its Inhibition, and the Finerenone Phase 3 Clinical Trials
7. Potential Benefits of Finerenone Across the Spectrum of Patients with T2D and CKD
7.1. Patient Pretreatment Clinical Characteristics and Patient Subgroups in the Finerenone Phase 3 Clinical Trials
7.2. Pooling of Patient Data from the Finerenone Phase 3 Clinical Trials
7.3. CKD Risk Stages at Baseline in the Finerenone Phase 3 Clinical Trials
8. Cardiovascular Effects of Finerenone in CKD Associated with T2D
8.1. Systolic Blood Pressure
8.2. Atherosclerotic CV Disease
8.3. Risk of Heart Failure
8.4. Atrial Fibrillation
9. Finerenone Use in Clinical Practice: Primary Care
10. Finerenone Use in Clinical Practice: Serum Potassium Monitoring and Combination Therapy
11. Unexplored Patient Populations and Areas for Future Research
12. Discussion
13. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Majeed, M.S.; Ahmed, F.; Teeling, M.; Ahmed, F.W. The prevalence of chronic kidney disease and albuminuria in patients with type 1 and type 2 diabetes attending a single centre. Cureus 2022, 14, e32248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, Y.; Zou, Z.; Hong, P.; Zhuo, Y.; Xu, Y.; Wan, J. Risk factors for progression of CKD with and without diabetes. J. Diabetes Res. 2022, 2022, 9613062. [Google Scholar] [CrossRef]
- Pérez-Morales, R.E.; Del Pino, M.D.; Valdivielso, J.M.; Ortiz, A.; Mora-Fernández, C.; Navarro-González, J.F. Inflammation in diabetic kidney disease. World J. Diabetes 2014, 5, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Hofherr, A.; Williams, J.; Gan, L.M.; Söderberg, M.; Hansen, P.B.; Woollard, K.J. Targeting inflammation for the treatment of diabetic kidney disease: A five-compartment mechanistic model. BMC Nephrol. 2022, 23, 208. [Google Scholar] [CrossRef]
- Naaman, S.C.; Bakris, G.L. Diabetic nephropathy: Update on pillars of therapy slowing progression. Diabetes Care 2023, 46, 1574–1586. [Google Scholar] [CrossRef]
- Peti-Peterdi, J.; Kang, J.J.; Toma, I. Activation of the renal renin-angiotensin system in diabetes—New concepts. Nephrol. Dial. Transplant. 2008, 23, 3047–3049. [Google Scholar] [CrossRef] [PubMed]
- Schnaper, H.W. Remnant nephron physiology and the progression of chronic kidney disease. Pediatr. Nephrol. 2014, 29, 193–202. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-kidney-metabolic health: A presidential advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Yuan, J.; Zou, X.R.; Han, S.P.; Cheng, H.; Wang, L.; Wang, J.W.; Zhang, L.X.; Zhao, M.H.; Wang, X.Q.; C-STRIDE study group. Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: Results from the Chinese cohort study of chronic kidney disease (C-STRIDE). BMC Nephrol. 2017, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group, KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022, 102, S1–S127. [CrossRef]
- American Diabetes Association Professional Practice Committee, 11. Chronic kidney disease and risk management: Standards of care in diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S219–S230. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Ghanim, H.; Arora, P. Improving the residual risk of renal and cardiovascular outcomes in diabetic kidney disease: A review of pathophysiology, mechanisms, and evidence from recent trials. Diabetes Obes. Metab. 2022, 24, 365–376. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. KERENDIA (Finerenone). Prescribing Information. 2022. 27 October 2023. Available online: https://labeling.bayerhealthcare.com/html/products/pi/Kerendia_PI.pdf (accessed on 6 January 2025).
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2022. 31 October 2023. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 6 January 2025).
- Hoek, A.G.; Dal Canto, E.; Wenker, E.; Bindraban, N.; Handoko, M.L.; Elders, P.J.; Beulens, J.W. Epidemiology of heart failure in diabetes: A disease in disguise. Diabetologia 2024, 67, 574–601. [Google Scholar] [CrossRef]
- Piccirillo, F.; Liporace, P.; Nusca, A.; Nafisio, V.; Corlianò, A.; Magarò, F.; Antonelli Incalzi, R.; Ussia, G.P.; Grigioni, F. Effects of finerenone on cardiovascular and chronic kidney diseases: A new weapon against cardiorenal morbidity and mortality—A comprehensive review. J. Cardiovasc. Dev. Dis. 2023, 10, 236. [Google Scholar] [CrossRef]
- Carrero, J.J.; Yilmaz, M.I.; Lindholm, B.; Stenvinkel, P. Cytokine dysregulation in chronic kidney disease: How can we treat it? Blood Purif. 2008, 26, 291–299. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chronic Kidney Disease Basics. 2024. 21 August 2024. Available online: https://www.cdc.gov/kidney-disease/about/?CDC_AAref_Val=https://www.cdc.gov/kidneydisease/basics.html (accessed on 6 January 2025).
- Neuen, B.L.; Weldegiorgis, M.; Herrington, W.G.; Ohkuma, T.; Smith, M.; Woodward, M. Changes in GFR and albuminuria in routine clinical practice and the risk of kidney disease progression. Am. J. Kidney Dis. 2021, 78, 350–360.e1. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.A.; Seidu, S.; Zaccardi, F.; McCann, G.; Kadam, U.T.; Davies, M.J.; Lam, C.S.; Heerspink, H.L.; Khunti, K. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine 2021, 32, 100739. [Google Scholar] [CrossRef]
- Brenner, B.M.; Cooper, M.E.; De Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- EMPA-Kidney Collaborative Group. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Liu, W.; Yu, S. Nonsteroidal mineralocorticoid receptor antagonist eliciting cardiorenal protection is a new option for patients with chronic kidney disease. Kidney Dis. 2023, 9, 12–25. [Google Scholar] [CrossRef]
- Cannavo, A.; Bencivenga, L.; Liccardo, D.; Elia, A.; Marzano, F.; Gambino, G.; D’Amico, M.L.; Perna, C.; Ferrara, N.; Rengo, G.; et al. Aldosterone and mineralocorticoid receptor system in cardiovascular physiology and pathophysiology. Oxid. Med. Cell Longev. 2018, 2018, 1204598. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Agarwal, R.; Alpers, C.E.; Bakris, G.L.; Brosius, F.C.; Kolkhof, P.; Uribarri, J. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022, 102, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Xu, L.; Guo, M. The role of protein kinase C in diabetic microvascular complications. Front. Endocrinol. 2022, 13, 973058. [Google Scholar] [CrossRef]
- Mora-Fernández, C.; Domínguez-Pimentel, V.; de Fuentes, M.M.; Górriz, J.L.; Martínez-Castelao, A.; Navarro-González, J.F. Diabetic kidney disease: From physiology to therapeutics. J. Physiol. 2014, 592, 3997–4012. [Google Scholar] [CrossRef]
- Kolkhof, P.; Delbeck, M.; Kretschmer, A.; Steinke, W.; Hartmann, E.; Bärfacker, L.; Eitner, F.; Albrecht-Küpper, B.; Schäfer, S. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J. Cardiovasc. Pharmacol. 2014, 64, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Grune, J.; Beyhoff, N.; Smeir, E.; Chudek, R.; Blumrich, A.; Ban, Z.; Brix, S.; Betz, I.R.; Schupp, M.; Foryst-Ludwig, A.; et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone’s antifibrotic activity. Hypertension 2018, 71, 599–608. [Google Scholar] [CrossRef]
- Lattenist, L.; Lechner, S.M.; Messaoudi, S.; Le Mercier, A.; El Moghrabi, S.; Prince, S.; Bobadilla, N.A.; Kolkhof, P.; Jaisser, F.; Barrera-Chimal, J. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: Role of oxidative stress. Hypertension 2017, 69, 870–878. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Aldactone (Spironolactone). Prescribing Information. 2023. 11 October 2023. Available online: https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=520 (accessed on 6 January 2025).
- US Food and Drug Administration. Inspra (Eplerenone). Prescribing Information. 2020. 11 October 2023. Available online: https://labeling.pfizer.com/ShowLabeling.aspx?id=599 (accessed on 6 January 2025).
- Agarwal, R.; Anker, S.D.; Bakris, G.; Filippatos, G.; Pitt, B.; Rossing, P.; Ruilope, L.; Gebel, M.; Kolkhof, P.; Nowack, C.; et al. Investigating new treatment opportunities for patients with chronic kidney disease in type 2 diabetes: The role of finerenone. Nephrol. Dial. Transplant. 2022, 37, 1014–1023. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Amazit, L.; Le Billan, F.; Kolkhof, P.; Lamribet, K.; Viengchareun, S.; Fay, M.R.; Khan, J.A.; Hillisch, A.; Lombès, M.; Rafestin-Oblin, M.E.; et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J. Biol. Chem. 2015, 290, 21876–21889. [Google Scholar] [CrossRef]
- Yamaji, M.; Tsutamoto, T.; Kawahara, C.; Nishiyama, K.; Yamamoto, T.; Fujii, M.; Horie, M. Effect of eplerenone versus spironolactone on cortisol and hemoglobin A(1)(c) levels in patients with chronic heart failure. Am. Heart J. 2010, 160, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Puchades, M.J.; Garofalo, C.; Jongs, N.; D’Marco, L.; Andreucci, M.; De Nicola, L.; Gorriz, J.L. Albuminuria-lowering effect of dapagliflozin, eplerenone, and their combination in patients with chronic kidney disease: A randomized crossover clinical trial. J. Am. Soc. Nephrol. 2022, 33, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Ohtsu, H.; Uchida, S.; Kaname, S.; Arakawa, Y.; Fujita, T. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 944–953. [Google Scholar] [CrossRef]
- Epstein, M.; Williams, G.H.; Weinberger, M.; Lewin, A.; Krause, S.; Mukherjee, R.; Patni, R.; Beckerman, B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2006, 1, 940–951. [Google Scholar] [CrossRef]
- Davidson, M.B.; Wong, A.; Hamrahian, A.H.; Stevens, M.; Siraj, E.S. Effect of spironolactone therapy on albuminuria in patients with type 2 diabetes treated with angiotensin-converting enzyme inhibitors. Endocr. Pract. 2008, 14, 985–992. [Google Scholar] [CrossRef]
- Bianchi, S.; Bigazzi, R.; Campese, V.M. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006, 70, 2116–2123. [Google Scholar] [CrossRef]
- Chrysostomou, A.; Becker, G. Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N. Engl. J. Med. 2001, 345, 925–926. [Google Scholar] [CrossRef]
- Fountain, J.; Kaur, J.; Lappin, S. Physiology, Renin Angiotensin System. StatPearls. 31 July 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470410/ (accessed on 6 January 2025).
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Mancia, G.; Kreutz, R.; Bundy, J.D.; Williams, B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension blood pressure/hypertension guidelines: Comparisons, reflections, and recommendations. Circulation 2022, 146, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Vaidya, P. Angiotensin II Receptor Blockers. StatPearls. 2024. 31 July 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537027/ (accessed on 6 January 2025).
- National Institute of Diabetes and Digestive and Kidney Diseases. Angiotensin-Converting Enzyme Inhibitors. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2018. 21 August 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548912/ (accessed on 6 January 2025).
- Tuttle, K.R. Back to the future: Glomerular hyperfiltration and the diabetic kidney. Diabetes 2017, 66, 14–16. [Google Scholar] [CrossRef]
- Trimarchi, H. Mechanisms of podocyte detachment, podocyturia, and risk of progression of glomerulopathies. Kidney Dis. 2020, 6, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, G.; Viazzi, F.; De Cosmo, S.; Russo, G.; Fioretto, P.; Pontremoli, R. Blood pressure reduction and RAAS inhibition in diabetic kidney disease: Therapeutic potentials and limitations. J. Nephrol. 2020, 33, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef]
- Rossing, P.; Burgess, E.; Agarwal, R.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Gillard, P.; MacIsaac, R.J.; Wainstein, J.; et al. Finerenone in patients with chronic kidney disease and type 2 diabetes according to baseline HbA1c and insulin use: An analysis from the FIDELIO-DKD study. Diabetes Care 2022, 45, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Pitt, B.; Agarwal, R.; Farmakis, D.; Ruilope, L.M.; Rossing, P.; Bauersachs, J.; Mentz, R.J.; Kolkhof, P.; Scott, C.; et al. Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: A prespecified subgroup analysis of the FIDELIO-DKD trial. Eur. J. Heart Fail 2022, 24, 996–1005. [Google Scholar] [CrossRef]
- Filippatos, G.; Bakris, G.L.; Pitt, B.; Agarwal, R.; Rossing, P.; Ruilope, L.M.; Butler, J.; Lam, C.S.; Kolkhof, P.; Roberts, L.; et al. Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and type 2 diabetes. J. Am. Coll. Cardiol. 2021, 78, 142–152. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Agarwal, R.; Anker, S.D.; Filippatos, G.; Pitt, B.; Rossing, P.; Sarafidis, P.; Schmieder, R.E.; Joseph, A.; Rethemeier, N.; et al. Blood pressure and cardiorenal outcomes with finerenone in chronic kidney disease in type 2 diabetes. Hypertension 2022, 79, 2685–2695. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: Analyses from the FIGARO-DKD trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Pitt, B.; Anker, S.D.; Rossing, P.; Kovesdy, C.P.; Pecoits-Filho, R.; Pergola, P.; Joseph, A.; Lage, A.; Mentenich, N.; et al. Kidney outcomes with finerenone: An analysis from the FIGARO-DKD study. Nephrol. Dial. Transplant. 2023, 38, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; August, P.; Coats, A.J.; Januzzi, J.L.; Mankovsky, B.; Rossing, P.; Ruilope, L.M.; Pitt, B.; Sarafidis, P.; et al. Finerenone and effects on mortality in chronic kidney disease and type 2 diabetes: A FIDELITY analysis. Eur Heart J. Cardiovasc. Pharmacother. 2023, 9, 183–191. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Pitt, B.; Rossing, P.; Joseph, A.; Kolkhof, P.; Lambelet, M.; Lawatscheck, R.; Bakris, G.L.; Ruilope, L.M.; et al. Finerenone and heart failure outcomes by kidney function/albuminuria in chronic kidney disease and diabetes. JACC Heart Fail 2022, 10, 860–870. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Pitt, B.; McGuire, D.K.; Rossing, P.; Ruilope, L.M.; Butler, J.; Jankowska, E.A.; Michos, E.D.; Farmakis, D.; et al. Finerenone efficacy in patients with chronic kidney disease, type 2 diabetes and atherosclerotic cardiovascular disease. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 9, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Umpierrez, G.E.; Reddy, S.S.; McGill, J.B.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.J.; et al. American Association of Clinical Endocrinology clinical practice guideline: Developing a diabetes mellitus comprehensive care plan—2022 update. Endocr. Pract. 2022, 28, 923–1049, Erratum in Endocr Pract. 2023, 29, 80–81. [Google Scholar]
- Bakris, G.L.; Ruilope, L.M.; Anker, S.D.; Filippatos, G.; Pitt, B.; Rossing, P.; Fried, L.; Roy-Chaudhury, P.; Sarafidis, P.; Ahlers, C.; et al. A prespecified exploratory analysis from FIDELITY examined finerenone use and kidney outcomes in patients with chronic kidney disease and type 2 diabetes. Kidney Int. 2023, 103, 196–206. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Risk Factors for Chronic Kidney Disease. 2024. 21 August 2024. Available online: https://www.cdc.gov/kidney-disease/risk-factors/index.html (accessed on 6 January 2025).
- Centers for Disease Control and Prevention. Cardiovascular Diseases. 2021. 21 August 2024. Available online: https://www.cdc.gov/globalhealth/healthprotection/ncd/cardiovascular-diseases.html (accessed on 6 January 2025).
- Bramlage, P.; Lanzinger, S.; van Mark, G.; Hess, E.; Fahrner, S.; Heyer, C.H.; Friebe, M.; Seufert, J.; Danne, T.; Holl, R.W. Patient and disease characteristics of type-2 diabetes patients with or without chronic kidney disease: An analysis of the German DPV and DIVE databases. Cardiovasc. Diabetol. 2019, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar] [CrossRef]
- Kumar, U.; Wettersten, N.; Garimella, P.S. Cardiorenal syndrome: Pathophysiology. Cardiol. Clin. 2019, 37, 251–265. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. About Atrial Fibrillation. 2024. 20 August 2024. Available online: https://www.cdc.gov/heart-disease/about/atrial-fibrillation.html (accessed on 6 January 2025).
- Alonso, A.; Lopez, F.L.; Matsushita, K.; Loehr, L.R.; Agarwal, S.K.; Chen, L.Y.; Soliman, E.Z.; Astor, B.C.; Coresh, J. Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011, 123, 2946–2953. [Google Scholar] [CrossRef]
- Bohne, L.J.; Johnson, D.; Rose, R.A.; Wilton, S.B.; Gillis, A.M. The association between diabetes mellitus and atrial fibrillation: Clinical and mechanistic insights. Front. Physiol. 2019, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Seyed Ahmadi, S.; Svensson, A.M.; Pivodic, A.; Rosengren, A.; Lind, M. Risk of atrial fibrillation in persons with type 2 diabetes and the excess risk in relation to glycaemic control and renal function: A Swedish cohort study. Cardiovasc. Diabetol. 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. 2023. 18 October 2023. Available online: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease (accessed on 6 January 2025).
- Tangri, N.; Peach, E.J.; Franzén, S.; Barone, S.; Kushner, P.R. Patient management and clinical outcomes associated with a recorded diagnosis of stage 3 chronic kidney disease: The REVEAL-CKD study. Adv. Ther. 2023, 40, 2869–2885. [Google Scholar] [CrossRef]
- Van den Wyngaert, I.; Mamouris, P.; Vaes, B.; Van Pottelbergh, G. An exploration of under-registration of chronic kidney disease stages 3-5 in Belgian general practices using logistic regression. PLoS ONE 2022, 17, e0279291. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.D.; Blakeman, T. Chronic kidney disease: Identification and management in primary care. Pragmat. Obs. Res. 2016, 7, 21–32. [Google Scholar] [CrossRef]
- McGill, J.B.; Haller, H.; Roy-Chaudhury, P.; Cherrington, A.; Wada, T.; Wanner, C.; Ji, L.; Rossing, P. Making an impact on kidney disease in people with type 2 diabetes: The importance of screening for albuminuria. BMJ Open Diabetes Res. Care 2022, 10, e002806. [Google Scholar] [CrossRef]
- Crews, D.C.; Bello, A.K.; Saadi, G.; World Kidney Day Steering Committee. Burden, access and disparities in kidney disease. Clin. Kidney J. 2019, 12, 160–166. [Google Scholar] [CrossRef]
- Schernthaner, G.; Shehadeh, N.; Ametov, A.S.; Bazarova, A.V.; Ebrahimi, F.; Fasching, P.; Janež, A.; Kempler, P.; Konrāde, I.; Lalić, N.M.; et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 185. [Google Scholar] [CrossRef]
- Gardiner, F.W.; Nwose, E.U.; Bwititi, P.T.; Crockett, J.; Wang, L. Services aimed at achieving desirable clinical outcomes in patients with chronic kidney disease and diabetes mellitus: A narrative review. SAGE Open Med. 2017, 5, 2050312117740989. [Google Scholar] [CrossRef]
- Mendu, M.L.; Weiner, D.E. Health policy and kidney care in the United States: Core curriculum 2020. Am. J. Kidney Dis. 2020, 76, 720–730. [Google Scholar] [CrossRef]
- Farmer, R.E.; Beard, I.; Raza, S.I.; Gollop, N.D.; Patel, N.; Tebboth, A.; McGovern, A.P.; Kanumilli, N.; Ternouth, A. Prescribing in type 2 diabetes patients with and without cardiovascular disease history: A descriptive analysis in the UK CPRD. Clin. Ther. 2021, 43, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Ganz, M.; Ustyugova, A.; Sawalhi-Leckenby, N.; de Souza, S.; Zhang, L.; Gunnarsson, E.; Gao, R.; Desai, N.R. Utilization of glucose-lowering drugs in patients with T2DM and established CVD in US: A descriptive study using Optum Clinformatics data. J. Am. Coll. Cardiol. 2020, 75 (Suppl. 1), 2017. [Google Scholar] [CrossRef]
- Pantalone, K.M.; Misra-Hebert, A.D.; Hobbs, T.M.; Ji, X.; Kong, S.X.; Milinovich, A.; Weng, W.; Bauman, J.; Ganguly, R.; Burguera, B.; et al. Antidiabetic treatment patterns and specialty care utilization among patients with type 2 diabetes and cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Mahtta, D.; Ramsey, D.J.; Lee, M.T.; Chen, L.; Al Rifai, M.; Akeroyd, J.M.; Vaughan, E.M.; Matheny, M.E.; Santo, K.R.; Navaneethan, S.D.; et al. Utilization rates of SGLT2 inhibitors and GLP-1 receptor agonists and their facility-level variation among patients with atherosclerotic cardiovascular disease and type 2 diabetes: Insights from the Department of Veterans Affairs. Diabetes Care 2022, 45, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Fioretto, P.; Kovesdy, C.P.; Malyszko, J.; Pecoits-Filho, R.; Schnell, O.; Rossignol, P. Potassium management with finerenone: Practical aspects. Endocrinol. Diabetes Metab. 2022, 5, e360. [Google Scholar] [CrossRef]
- Agarwal, R.; Joseph, A.; Anker, S.D.; Filippatos, G.; Rossing, P.; Ruilope, L.M.; Pitt, B.; Kolkhof, P.; Scott, C.; Lawatscheck, R.; et al. Hyperkalemia risk with finerenone: Results from the FIDELIO-DKD trial. J. Am. Soc. Nephrol. 2022, 33, 225–237. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee, 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S239–S251. [Google Scholar] [CrossRef]
- Provenzano, M.; Rotundo, S.; Chiodini, P.; Gagliardi, I.; Michael, A.; Angotti, E.; Borrelli, S.; Serra, R.; Foti, D.; De Sarro, G.; et al. Contribution of Predictive and Prognostic Biomarkers to Clinical Research on Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 5846. [Google Scholar] [CrossRef]
- Chiu, N.; Aggarwal, R.; Bakris, G.L.; Pitt, B.; Bhatt, D.L. Generalizability of FIGARO-DKD and FIDELIO-DKD trial criteria to the US population eligible for finerenone. J. Am. Heart Assoc. 2022, 11, e025079. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.; Pitt, B.; Senni, M.; et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
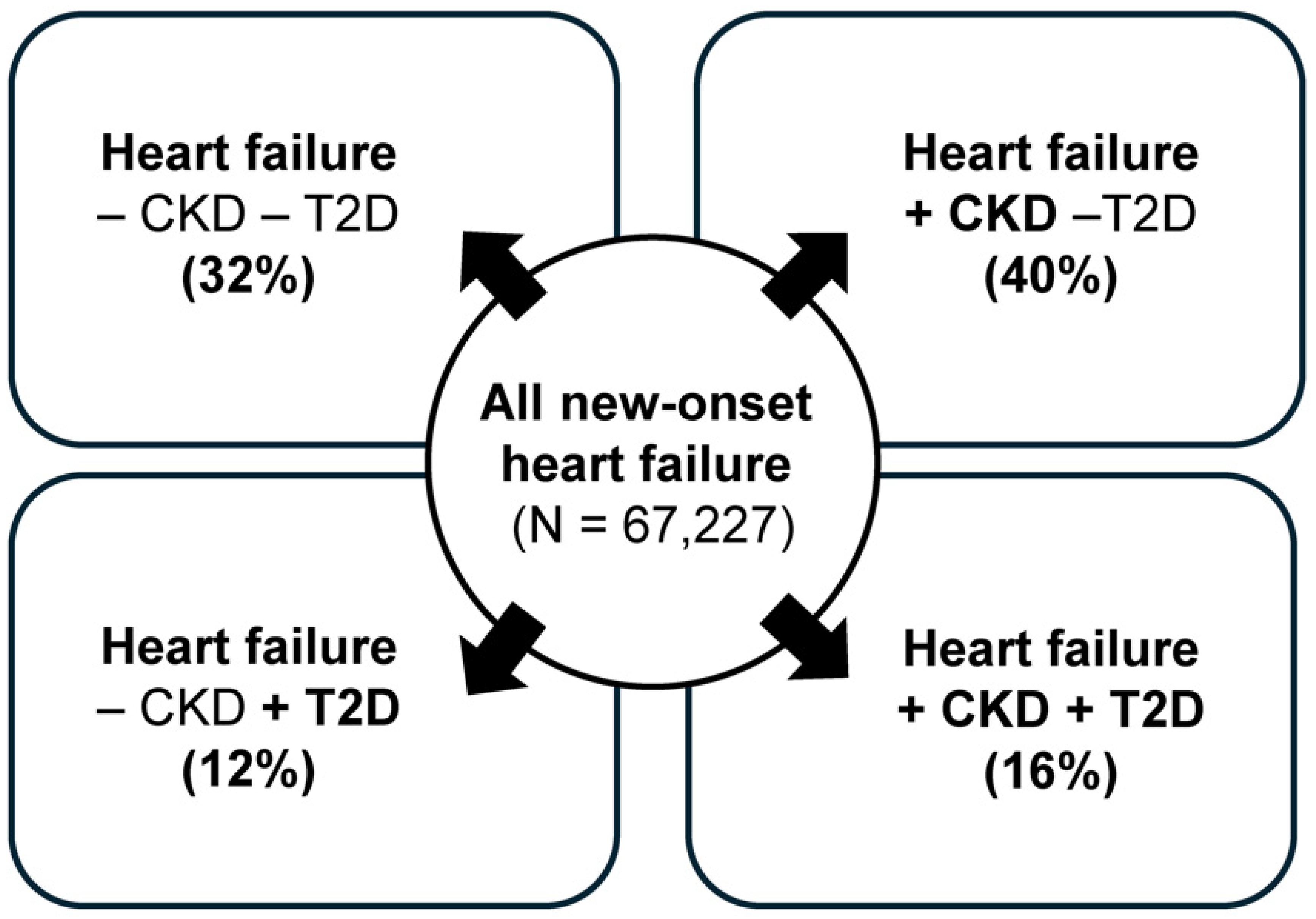
| Trial ID | Population | Treatment Arms | Median | Primary Endpoint | Key Secondary Endpoints | AEs of Special Interest |
|---|---|---|---|---|---|---|
| Follow-Up | (Finerenone vs. Placebo) | (Finerenone vs. Placebo) | (Finerenone vs. Placebo) | |||
| FIDELIO-DKD analyses | ||||||
| FIDELIO-DKD primary analysis [18] | T2D and CKD treated with an ACEi/ARB (max dose without unacceptable side effects) | Finerenone + ACEi/ARB (N = 2833) vs. placebo + ACEi/ARB (N = 2841) | 2.6 y | Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
| Hyperkalemia (related to study drug): 11.8% vs. 4.8% Serious hyperkalemia: 1.6% vs. 0.4% Permanent discontinuation because of hyperkalemia: 2.3% vs. 0.9% No sexual side effect reported |
| Prespecified subgroup analysis by history of HF [65] | History of HF With (n = 436) Without (n = 5238) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 2.6 y | History of HF Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| History of HF Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
| |
| Subgroup analysis by office SBP [67] | Baseline office SBP quartiles, mmHg Q1 (≤128.7; n = 1448) Q2 (>128.7 to ≤138.3; n = 1346) Q3 (>138.3 to ≤148.0; n = 1492) Q4 (>148.0; n = 1383) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 2.6 y | Baseline office SBP quartiles Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| Baseline office SBP quartiles Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
| |
| Subgroup analysis by history of AFF [66] | History of AFF With (n = 461) Without (n = 5213) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 2.6 y | History of AFF Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| History of AFF Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
| |
| Subgroup analysis by baseline HbA1c and insulin use [64] | Baseline HbA1c <7.5% (n = 2794) ≥7.5% (v = 2869) Baseline insulin use Yes (n = 2037) No (n = 3637) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 2.6 y | Baseline HbA1c Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| Baseline HbA1c Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
| |
| FIGARO-DKD analyses | ||||||
| FIGARO-DKD primary analysis [19] | T2D and CKD treated with an ACEi/ARB (max dose without unacceptable side effects) | Finerenone + ACEi/ARB (N = 3686) vs. placebo + ACEi/ARB (N = 3666) | 3.4 y | Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
| Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| Hyperkalemia (related to study drug): 6.5% vs. 3.1% Serious hyperkalemia: 0.7% vs. 0.1% No sexual side effect reported |
| Prespecified analysis of HF outcomes [68] | T2D and CKD treated with an ACEi/ARB (max dose without unacceptable side effects), without a history of symptomatic HFrEF | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 3.4 y | – | Risk for new-onset HF
| – |
| Exploratory, prespecified UACR subgroup analysis of kidney and CV composite endpoints [69] | UACR subgroups, mg/g 30 to <300 (n = 3414) ≥300 (v = 3729) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 3.4 y | UACR subgroups, mg/g Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
| UACR subgroups, mg/g Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| |
| FIDELITY analyses | ||||||
| FIDELITY [63] | T2D and CKD treated with an ACEi/ARB (max dose without unacceptable side effects) | Finerenone + ACEi/ARB (N = 6519) vs. placebo + ACEi/ARB (N = 6507) | 3.0 y | Time to kidney failure, a sustained decrease of ≥57% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| Time to kidney failure, a sustained decrease of ≥40% in eGFR from baseline over a period of ≥4 weeks, or death from renal causes
| Hyperkalemia (related to study drug): 8.8% vs. 3.8% Serious hyperkalemia: 1.1% vs. 0.2% Permanent discontinuation because of hyperkalemia: 1.7% vs. 0.6% No sexual side effect reported |
| Prespecified on-treatment analysis * [70] | T2D and CKD treated with an ACEi/ARB (max dose without unacceptable side effects) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 3.0 y | – | Risk for all-cause mortality
| – |
| Exploratory subgroup analysis of HF outcomes by eGFR/UACR [71] | UACR subgroups, mg/g < 300 (n = 4329) ≥300 (v = 8692) eGFR subgroups, mL/min/1.73 m2 ≥60 (v = 5195) < 60 (n = 7828) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 3.0 y | – | Overall | |
Time to first HHF
Time to first HHF
Time to first HHF
| ||||||
| Prespecified subgroup analysis by history of ASCVD [72] | History of ASCVD With (n = 5935) Without (n = 7091) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | 3.0 y | History of ASCVD Time to death from CV causes, nonfatal MI, nonfatal stroke, or HHF
| History of ASCVD Time to CV death or first HHF
| |
| Trial ID | Phase | Population (Estimated/Actual Enrollment) | Treatment Arms | Primary Endpoint | Study Start Date | Estimated Study Completion |
|---|---|---|---|---|---|---|
| CVD trials | ||||||
| REDEFINE-HF [NCT06008197] | 3 | Patients hospitalized with acute decompensated HF and mildly reduced or preserved LVEF (N = ~5200) | Finerenone vs. placebo | Total (first and subsequent) HHF, urgent visits for worsening HF, and CV deaths AEs leading to study drug discontinuation | November 2023 | April 2026 |
| CONFIRMATION-HF [NCT06024746] | 3 | Patients hospitalized for HF (N = ~1500) | Finerenone + empagliflozin vs. SOC (placebo) | Clinical benefit † SAEs or AEs leading to study drug discontinuation | February 2024 | August 2025 |
| FINALITY-HF [NCT06033950] | 3 | Patients with HFrEF who are intolerant or ineligible to receive treatment with sMRA (N = ~2600) | Finerenone vs. placebo | Time to first CV death or HF event SAEs or AEs leading to study drug discontinuation | June 2024 | November 2027 |
| FINEARTS-HF [NCT04435626] | 3 | Patients with HF and LVEF ≥40% (N = 6016) | Finerenone vs. placebo | CV deaths and HF events (first and recurrent) | September 2020 | July 2024 (completed) |
| Kidney disease trials | ||||||
| FIND-CKD [NCT05047263] | 3 | Nondiabetic CKD (N = 1574) | Finerenone vs. placebo | Change in eGFR | September 2021 | February 2026 |
| FINE-ONE [NCT05901831] | 3 | CKD associated with T1D (N = ~220) | Finerenone vs. placebo | Change in UACR | March 2024 | October 2025 |
| FIONA [NCT05196035] | 3 | Children with CKD (and proteinuria) (N = ~219) | Finerenone + ACEi/ARB vs. placebo + ACEi/ARB | Proportion with UPCR reduction of ≥ 30% | March 2022 | March 2027 |
| FIONA OLE [NCT05457283] | 3 (OLE) | Children with CKD (and proteinuria) who completed the FIONA study (N = ~100) | Finerenone + ACEi/ARB | TEAEs, change in serum K+ levels, change in SBP | November 2022 | September 2028 |
| CONFIDENCE [NCT05254002] | 2 | CKD associated with T2D (N = ~807) | Finerenone + placebo Empagliflozin + placebo Finerenone + empagliflozin + placebo (combination vs. single drug) | (1) Relative change from baseline in UACR at 180 days in combination therapy group vs. empagliflozin alone, and (2) relative change from baseline in UACR at 180 days in combination therapy group vs. finerenone alone | June 2022 | February 2025 (completed) |
| EFFEKTOR [NCT06059664] | 2 | Kidney transplant recipients (N = ~150) | Finerenone vs. placebo | Total number of participants who were eligible and enrolled in the main clinical trial and/or kidney biopsy substudy | November 2023 | December 2025 |
| FIVE-STAR [NCT05887817] | 4 | CKD associated with T2D (N = ~100) | Finerenone vs. placebo | Change in CAVI at 24 weeks after initiation of placebo or finerenone vs. baseline (CAVI is a physiological marker of arterial function) | September 2023 | July 2026 |
| Observational studies ‡ | ||||||
| FLAMINGO [NCT05640180] | Observational | CKD associated with T2D (N = ~3000) | Effects of treatment combination of finerenone + SGLT2is in routine medical care | (1) Time to first occurrence of the composite of onset of kidney failure, a sustained decrease of eGFR ≥40% from baseline over at least 4 weeks, or renal death; and (2) time to first occurrence of the composite endpoint of CV death or nonfatal CV event | November 2022 | December 2023 (completed) |
| FINE-REAL [NCT05348733] | Observational | Initiated treatment with finerenone for T2D and CKD (N = ~5500) | Finerenone | Description of clinical characteristics and treatment patterns | June 2022 | January 2028 |
| FIRST-2 [NCT05703880] | Observational | Initiated treatment with finerenone for T2D and CKD (N = ~10,000) | Finerenone | Description of clinical characteristics and treatment patterns | June 2023 | July 2024 (completed) |
| FINEGUST [NCT05526157] | Observational | Receiving or initiating treatment for CKD or T2D (N = ~50,000) | Finerenone, SGLT2is, GLP-1 RAs, sMRAs, ns-MRAs | Description of clinical characteristics and treatment patterns | October 2022 | September 2024 (completed) |
| RW study in HFrEF [NCT05974566] | Observational | HFrEF (N = ~60) | Finerenone | Change in serum NT-proBNP levels | August 2023 | October 2023 (not yet recruiting) |
| IN-REALITY [NCT06763146] | Observational | CKD and T2D (N = not provided) | Finerenone | Safety and tolerability of finerenone | May 2025 | October 2025 |
| FIRST 2.5 [NCT06608212] | Observational | CKD and T2D Finerenone use in routine medical care (US) N = ~150,000 | Finerenone | Time to composite CV outcome | October 2024 | June 2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, N.; Uppal, P.; Abedin, T.; Lala, A. Finerenone: Potential Clinical Application Across the Spectrum of Cardiovascular Disease and Chronic Kidney Disease. J. Clin. Med. 2025, 14, 3213. https://doi.org/10.3390/jcm14093213
Haq N, Uppal P, Abedin T, Lala A. Finerenone: Potential Clinical Application Across the Spectrum of Cardiovascular Disease and Chronic Kidney Disease. Journal of Clinical Medicine. 2025; 14(9):3213. https://doi.org/10.3390/jcm14093213
Chicago/Turabian StyleHaq, Nowreen, Pulkita Uppal, Taslova Abedin, and Anuradha Lala. 2025. "Finerenone: Potential Clinical Application Across the Spectrum of Cardiovascular Disease and Chronic Kidney Disease" Journal of Clinical Medicine 14, no. 9: 3213. https://doi.org/10.3390/jcm14093213
APA StyleHaq, N., Uppal, P., Abedin, T., & Lala, A. (2025). Finerenone: Potential Clinical Application Across the Spectrum of Cardiovascular Disease and Chronic Kidney Disease. Journal of Clinical Medicine, 14(9), 3213. https://doi.org/10.3390/jcm14093213





