Carcinoembryonic Antigen (CEA): Origin, Role in Oncology, and Concentrations in Serum and Peritoneal Fluid
Abstract
1. Introduction
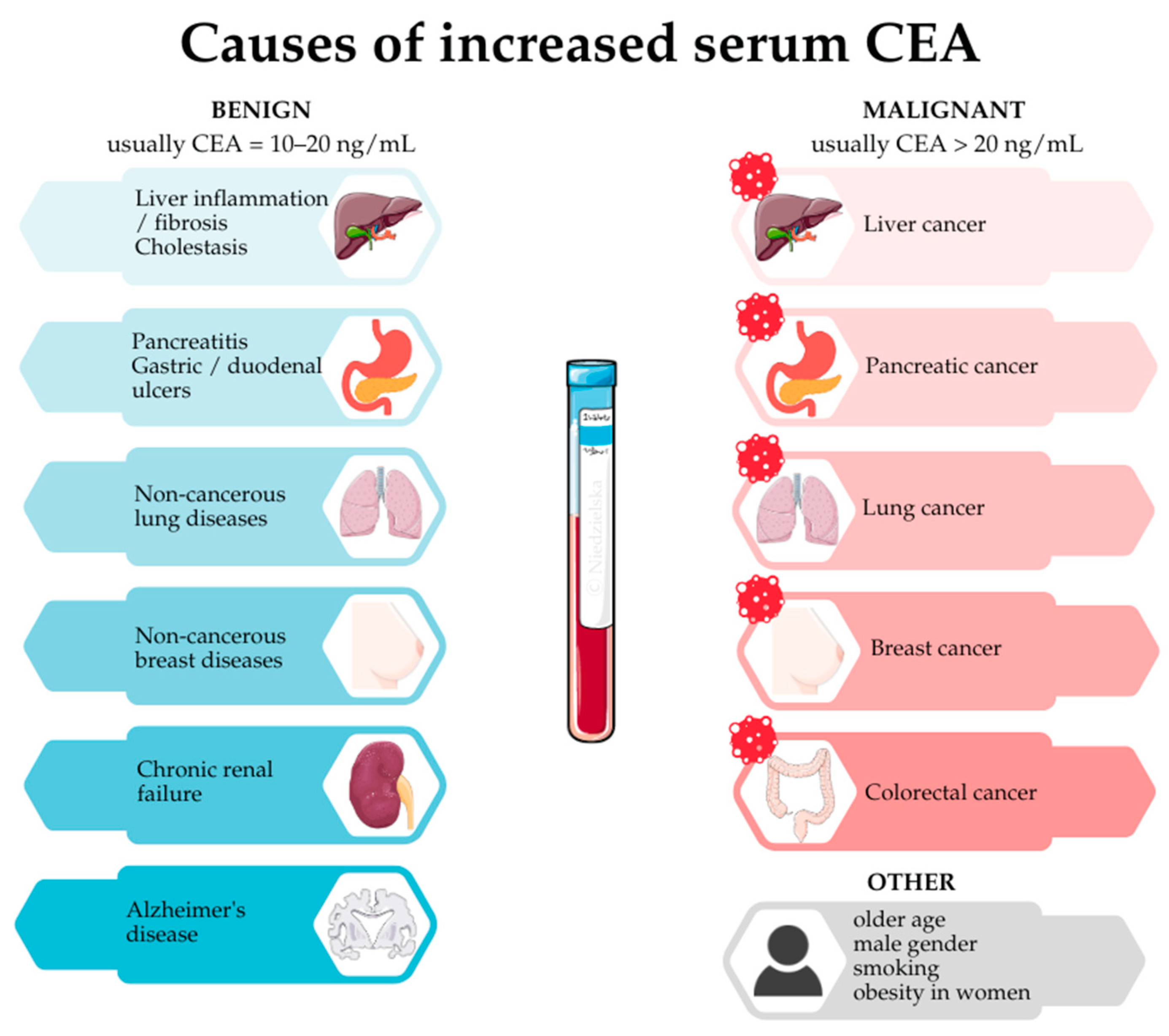
2. Cancer—Serum CEA
3. Cancer—Peritoneal Fluid CEA
4. Gynecological, Colorectal, and Gastric Cancers—Peritoneal Fluid CEA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha-fetoprotein |
| A/S | Ascites to serum concentration ratio |
| CA125 | Cancer antigen 125 |
| CA15-3 | Cancer antigen 15-3 |
| CA19-9 | Cancer antigen 19-9 |
| CA242 | Cancer antigen 242 |
| CA50 | Cancer antigen 50 |
| CA72-4 | Cancer antigen 72-4 |
| CEA | Carcinoembryonic antigen |
| CFS | Cancer-free survival time |
| CT | Computed tomography |
| DFS | Disease-free survival |
| ELISA | Enzyme-linked immunosorbent assay |
| FTO | Fat mass and obesity-associated protein |
| GPI-PLD | Glycosylphosphatidylinositol-specific phospholipase D |
| HCC | Hepatocellular carcinoma |
| HE4 | Human epididymis protein 4 |
| ISTA | Sphere turbidimetric assay |
| LRG1 | Leucine-rich alpha-2-glycoprotein 1 |
| mRNA | Messenger ribonucleic acid |
| NSCLC | Non-small cell lung cancer |
| OS | Overall survival time |
| PET-CT | Positron emission tomography-computed tomography |
| PIK3CB | Phosphatidylinositol-4,5-biphosphate 3-kinase catalytic subunit β |
| PIVKA-II | Protein induced by vitamin K absence-II |
| pCR | Pathological complete response |
| PRF | Peritoneal recurrence-free survival |
| qPCR | Real-time quantitative polymerase chain reaction |
| RT-PCR | Reverse-transcriptase polymerase chain reaction |
| TRCR | Transcription-reverse transcription concerted reaction |
References
- Kankanala, V.L.; Zubair, M.; Mukkamalla, S.K.R. Carcinoembryonic Antigen. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Adinolfi, M. Embryonic Antigens. In Encyclopedia of Immunology, 2nd ed.; Delves, P.J., Ed.; Elsevier: Oxford, UK, 1998; pp. 798–802. ISBN 978-0-12-226765-9. [Google Scholar]
- Ahadi, M.; Tehranian, S.; Memar, B.; Vossoughinia, H.; Salari, M.; Eskandari, E.; Farzanehfar, M.; Sadeghi, R. Diagnostic value of carcinoembryonic antigen in malignancy-related ascites: Systematic review and meta-analysis. Acta Gastro-Enterol. Belgica 2014, 77, 418–424. [Google Scholar]
- Gold, P.; Shuster, J.; Freedman, S.O. Carcinoembryonic antigen (CEA) in clinical medicine. Historical perspectives, pitfalls and projections. Cancer 1978, 42, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Zamcheck, N. Role of the liver in clearance and excretion of circulating carcinoembryonic antigen (CEA). Dig. Dis. Sci. 1983, 28, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Jessup, J.M.; Thomas, P. Carcinoembryonic antigen: Function in metastasis by human colorectal carcinoma. Cancer Metastasis Rev. 1989, 8, 263–280. [Google Scholar] [CrossRef]
- Morell, A.R. CEA Serum Levels in Non-Neoplastic Disease. Int. J. Biol. Markers 1992, 7, 160–166. [Google Scholar] [CrossRef]
- Naghibalhossaini, F.; Ebadi, P. Evidence for CEA release from human colon cancer cells by an endogenous GPI-PLD enzyme. Cancer Lett. 2006, 234, 158–167. [Google Scholar] [CrossRef]
- Sack, T.L.; Gum, J.R.; Low, M.G.; Kim, Y.S. Release of Carcinoembryonic Antigen from Human Colon Cancer Cells by Phosphatidylinositol-Specific Phospholipase C; American Society for Clinical Investigation: Ann Arbor, MI, USA, 1988; Available online: https://www.jci.org/articles/view/113636/pdf (accessed on 17 April 2025).
- Kinugasa, T.; Kuroki, M.; Yamanaka, T.; Matsuo, Y.; Oikawa, S.; Nakazato, H.; Matsuota, Y. Non-proteolytic release of carcinoembryonic antigen from normal human colonic epithelial cells cultured in collagen gel. Int. J. Cancer 1994, 58, 102–107. [Google Scholar] [CrossRef]
- Thomas, P.; Hems, D.A. The hepatic clearance of circulating native and asialo carcinoembryonic antigen by the rat. Biochem. Biophys. Res. Commun. 1975, 67, 1205–1209. [Google Scholar] [CrossRef]
- Hao, C.; Zhang, G.; Zhang, L. Serum CEA levels in 49 different types of cancer and noncancer diseases. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 213–227. ISBN 978-0-12-817738-9. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1877117318301595 (accessed on 2 December 2024).
- Tomasevic, Z.; Jelic, S.; Nikolic, L.; Filipovic, I.; Stamatovic, L.; Radosavljevic, D. Negative CEA values in Metastatic Colorectal Carcinoma and the Likelihood of Complete Chemotherapy Response. Int. J. Biol. Markers 2003, 18, 28–32. [Google Scholar] [CrossRef]
- Herbeth, B.; Bagrel, A. A study of factors influencing plasma CEA levels in an unselected population. Oncodev. Biol. Med. J. Int. Soc. Oncodev. Biol. Med. 1980, 1, 191–198. [Google Scholar]
- Faria, D.K.; Faria, C.S.; Doi, D.; Acencio, M.M.P.; Antonangelo, L. Hybrid panel of biomarkers can be useful in the diagnosis of pleural and peritoneal effusions. Clin. Chim. Acta 2019, 497, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, D.; Xiong, F.; Wang, Q.; Liu, W.; Chen, J.; Chen, Y. The emerging roles of CEACAM6 in human cancer (Review). Int. J. Oncol. 2024, 64, 27. [Google Scholar] [CrossRef] [PubMed]
- Bramswig, K.H.; Poettler, M.; Unseld, M.; Wrba, F.; Uhrin, P.; Zimmermann, W.; Zielinski, C.C.; Prager, G.W. Soluble Carcinoembryonic Antigen Activates Endothelial Cells and Tumor Angiogenesis. Cancer Res. 2013, 73, 6584–6596. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, R.; von Kleist, S. The carcinoembryonic antigen (CEA) modulates effector-target cell interaction by binding to activated lymphocytes. Int. J. Cancer 1996, 68, 457–463. [Google Scholar] [CrossRef]
- Wirth, T.; Soeth, E.; Czubayko, F.; Juhl, H. Inhibition of endogenous carcinoembryonic antigen (CEA) increases the apoptotic rate of colon cancer cells and inhibits metastatic tumor growth. Clin. Exp. Metastasis 2002, 19, 155–160. [Google Scholar] [CrossRef]
- Minami, S.; Furui, J.; Kanematsu, T. Role of carcinoembryonic antigen in the progression of colon cancer cells that express carbohydrate antigen. Cancer Res. 2001, 61, 2732–2735. [Google Scholar]
- Soeth, E.; Wirth, T.; List, H.J.; Kumbhani, S.; Petersen, A.; Neumaier, M.; Czubayko, F.; Juhl, H. Controlled ribozyme targeting demonstrates an antiapoptotic effect of carcinoembryonic antigen in HT29 colon cancer cells. Clin. Cancer Res. 2001, 7, 2022–2030. [Google Scholar]
- Yildirim, M.; Suren, D.; Yildiz, M.; Alikanoglu, A.S.; Kaya, V.; Doluoglu, S.G.; Aydin, O.; Yilmaz, N.; Sezer, C.; Karaca, M. Tumour Markers in Peritoneal Washing Fluid—Contribution to Cytology. Asian Pac. J. Cancer Prev. 2013, 14, 1027–1030. [Google Scholar] [CrossRef]
- Tomita, M.; Shimizu, T.; Matsuzaki, Y.; Hara, M.; Ayabe, T.; Onitsuka, T. Prognostic Significance of Carcinoembryonic Antigen Level in Pleural Lavage Fluid for Patients with Lung Adenocarcinoma. Ann. Thorac. Surg. 2005, 80, 276–281. [Google Scholar] [CrossRef]
- Saied, G.M.; El-Metenawy, W.H.; Elwan, M.S.; Dessouki, N.R. Urine carcinoembryonic antigen levels are more useful than serum levels for early detection of Bilharzial and non-Bilharzial urinary bladder carcinoma: Observations of 43 Egyptian cases. World J. Surg. Oncol. 2007, 5, 4. [Google Scholar] [CrossRef]
- Joshi, S.; Kallappa, S.; Kumar, P.; Shukla, S.; Ghosh, R. Simple diagnosis of cancer by detecting CEA and CYFRA 21-1 in saliva using electronic sensors. Sci. Rep. 2022, 12, 15315. [Google Scholar] [CrossRef] [PubMed]
- Sarandakou, A.; Phocas, I.; Botsis, D.; Rizos, D.; Trakakis, E.; Chryssikopoulos, A. Vaginal fluid and serum CEA, CA125 and SCC in normal conditions and in benign and malignant diseases of the genital tract. Acta Oncol. Stockh. Swed. 1997, 36, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Asseo, P.P.; Panidis, D.K.; Papaloucas, A.C. Carcinoembryonic antigen activity in human seminal plasma. Int. J. Fertil. Steril. 1986, 30, 53–56. [Google Scholar]
- Tuzun, Y.; Çelik, Y.; Bayan, K.; Yilmaz, S.; Dursun, M.; Canoruc, F. Correlation of Tumour Markers in Ascitic Fluid and Serum: Are Measurements of Ascitic Tumour Markers a Futile Attempt? J. Int. Med. Res. 2009, 37, 79–86. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Shinkins, B.; Pathiraja, I.; Roberts, N.W.; James, T.J.; Mallett, S.; Perera, R.; Primrose, J.N.; Mant, D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst. Rev. 2015, 12, 1–203. [Google Scholar] [CrossRef]
- Grunnet, M.; Sorensen, J.B. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; You, R.; Li, Y.; Pu, H.; Lei, M.; Fan, B.; Lv, J.; Liu, M.; Yan, G.; et al. Trajectory patterns and cumulative burden of CEA during follow-up with non-small cell lung cancer outcomes: A retrospective longitudinal cohort study. Br. J. Cancer 2024, 130, 1803–1808. [Google Scholar] [CrossRef]
- Verma, N.; Vinocha, A. Role of CA 19.9 and CEA in predicting diagnosis in hepatocellular carcinoma. J. Cancer Res. Ther. 2023, 19, 1356. [Google Scholar] [CrossRef]
- Qi, F.; Zhou, A.; Yan, L.; Yuan, X.; Wang, D.; Chang, R.; Zhang, Y.; Shi, F.; Han, X.; Hou, J.; et al. The diagnostic value of PIVKA-II, AFP, AFP-L3, CEA, and their combinations in primary and metastatic hepatocellular carcinoma. J. Clin. Lab. Anal. 2019, 34, e23158. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Wang, F.; Hao, M. CT combined with tumor markers in the diagnosis and prognosis of hepatocellular carcinoma. J. BUON 2018, 23, 985–991. [Google Scholar]
- Mi, J.; Zhang, H.; Cao, W.; Yuan, C. FTO, PIK3CB serve as potential markers to complement CEA and CA15-3 for the diagnosis of breast cancer. Medicine 2023, 102, e35361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, X.; Wang, W.; Chen, B.; Wang, L.; Zhang, N.; Wang, Z.; Yang, Q. Association of Preoperative Serum Levels of CEA and CA15-3 with Molecular Subtypes of Breast Cancer. Dis. Markers 2021, 2021, 5529106. [Google Scholar] [CrossRef] [PubMed]
- Uygur, M.M.; Gümüş, M. The utility of serum tumor markers CEA and CA 15–3 for breast cancer prognosis and their association with clinicopathological parameters. Cancer Treat. Res. Commun. 2021, 28, 100402. [Google Scholar] [CrossRef]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctol. 2019, 35, 294–305. [Google Scholar] [CrossRef]
- Rosati, G.; Ambrosini, G.; Barni, S.; Andreoni, B.; Corradini, G.; Luchena, G.; Daniele, B.; Gaion, F.; Oliverio, G.; Duro, M.; et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann. Oncol. 2016, 27, 274–280. [Google Scholar] [CrossRef]
- Primrose, J.N.; Perera, R.; Gray, A.; Rose, P.; Fuller, A.; Corkhill, A.; George, S.; Mant, D.; for the FACS Trial Investigators. Effect of 3 to 5 Years of Scheduled CEA and CT Follow-up to Detect Recurrence of Colorectal Cancer: The FACS Randomized Clinical Trial. JAMA 2014, 311, 263–270. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, J.; Chen, J.; Mei, S.; Wang, Z. Predictive Factors for Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1607–1611. [Google Scholar] [CrossRef]
- Hu, H.; Huang, J.; Lan, P.; Wang, L.; Huang, M.; Wang, J.; Deng, Y. CEA clearance pattern as a predictor of tumor response to neoadjuvant treatment in rectal cancer: A post-hoc analysis of FOWARC trial. BMC Cancer 2018, 18, 1145. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Schmidt, C.M.; Mao, X.; Irajizad, E.; Loftus, M.; Zhang, J.; Patel, N.; Vykoukal, J.; Dennison, J.B.; Long, J.P.; et al. Lead-time trajectory of CA19-9 as an anchor marker for pancreatic cancer early detection. Gastroenterology 2021, 160, 1373–1383.e6. [Google Scholar] [CrossRef]
- Meng, Q.; Shi, S.; Liang, C.; Liang, D.; Xu, W.; Ji, S.; Zhang, B.; Ni, Q.; Xu, J.; Yu, X. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: A systematic review and meta-analysis. OncoTargets Ther. 2017, 10, 4591–4598. [Google Scholar] [CrossRef]
- Simmons, A.R.; Fourkala, E.O.; Gentry-Maharaj, A.; Ryan, A.; Sutton, M.N.; Baggerly, K.; Zheng, H.; Lu, K.H.; Jacobs, I.; Skates, S.; et al. Complementary longitudinal serum biomarkers to CA125 for early detection of ovarian cancer. Cancer Prev. Res. 2019, 12, 391–400. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Chung, W.C.; Choi, S.; Jung, Y.D.; Lee, J.; Chae, S.Y.; Jun, K.-H.; Chin, H.-M. The Detection of Messenger RNA for Carcinoembryonic Antigen and Cytokeratin 20 in Peritoneal Washing Fluid in Patients with Advanced Gastric Cancer. Korean J. Gastroenterol. 2017, 69, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Trapé, J.; Sant, F.; Montesinos, J.; Arnau, A.; Sala, M.; Figols, C.; Franquesa, J.; Esteve-Valverde, E.; Pérez, R.; Aligué, J.; et al. Comparative Assessment of Two Strategies for Interpreting Tumor Markers in Ascitic Effusions. In Vivo 2020, 34, 715–722. [Google Scholar] [CrossRef]
- Benevolo, M.; Mottolese, M.; Cosimelli, M.; Tedesco, M.; Giannarelli, D.; Vasselli, S.; Carlini, M.; Garofalo, A.; Natali, P.G. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J. Clin. Oncol. 1998, 16, 3406–3411. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, D.H.; Gorden, D.L.; Lee, Y.S.; Sung, N.Y.; Park, G.-S.; Kim, H.J.; Kang, W.K.; Park, J.K.; Ahn, C.H.; et al. Prognostic Value of CEA and CA 19-9 Tumor Markers Combined with Cytology from Peritoneal Fluid in Colorectal Cancer. Ann. Surg. Oncol. 2009, 16, 861–870. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; He, X.; Ji, J.; Wang, G. Diagnostic values of carcinoembryonic antigen in predicting peritoneal recurrence after curative resection of gastric cancer: A meta-analysis. Ir. J. Med. Sci. 2014, 183, 557–564. [Google Scholar] [CrossRef]
- Du, L.; Wei, X.; Xiao, Z.; Wang, H.; Song, Y. Utility of ascitic tumor markers and adenosine deaminase for differential diagnosis of tuberculous peritonitis and peritoneal carcinomatosis. BMC Gastroenterol. 2022, 22, 423. [Google Scholar] [CrossRef]
- Liu, M. Diagnostic Algorithm Based on Ratio of Ascites-Serum Tumor Markers is Superior to Tumor Markers in the Differentiation of Benign Ascites from Malignant Ascites—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/38880300/ (accessed on 30 January 2025).
- Jain, T. Ascitic and serum levels of tumor biomarkers (CA 72-4, CA 19-9, CEA and CA 125) in discrimination of cause of ascites: A prospective study. Arq. Gastroenterol. 2022, 59, 198–203. [Google Scholar] [CrossRef]
- Gan, X.; Yang, J.; Wang, L.; Tan, B.; Wang, L. Application of PETCT imaging information combined with tumor markers in etiological screening of infectious and non-infectious ascites. J. Infect. Public Health 2020, 13, 1997–2000. [Google Scholar] [CrossRef]
- Song, S.E.; Choi, P.; Kim, J.H.; Jung, K.; Kim, S.E.; Moon, W.; Park, M.I.; Park, S.J. Diagnostic Value of Carcinoembryonic Antigen in Ascites for Colorectal Cancer with Peritoneal Carcinomatosis. Korean J. Gastroenterol. 2018, 71, 332–337. [Google Scholar] [CrossRef]
- Guller, U.; Zajac, P.; Schnider, A.; Bösch, B.; Vorburger, S.; Zuber, M.; Spagnoli, G.C.; Oertli, D.; Maurer, R.; Metzger, U.; et al. Disseminated Single Tumor Cells as Detected by Real-Time Quantitative Polymerase Chain Reaction Represent a Prognostic Factor in Patients Undergoing Surgery for Colorectal Cancer. Ann. Surg. 2002, 236, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Zhu, H.; Chen, M.; Wu, J.; Hu, R.; Tang, C. Prognostic Significance of Molecular Analysis of Peritoneal Fluid for Patients with Gastric Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0151608. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kanda, M.; Umeda, S.; Tanaka, C.; Kobayashi, D.; Hayashi, M.; Yamada, S.; Kodera, Y. The levels of SYT13 and CEA mRNAs in peritoneal lavages predict the peritoneal recurrence of gastric cancer. Gastric Cancer 2019, 22, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Soeno, T.; Nishizawa, N.; Washio, M.; Sakuraya, M.; Ushiku, H.; Niihara, M.; Hosoda, K.; Kumamoto, Y.; Naitoh, T.; et al. Prospective study to validate the clinical utility of DNA diagnosis of peritoneal fluid cytology test in Gastric cancer. Cancer Sci. 2021, 112, 1644–1654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedzielska, J.; Jastrzębski, T. Carcinoembryonic Antigen (CEA): Origin, Role in Oncology, and Concentrations in Serum and Peritoneal Fluid. J. Clin. Med. 2025, 14, 3189. https://doi.org/10.3390/jcm14093189
Niedzielska J, Jastrzębski T. Carcinoembryonic Antigen (CEA): Origin, Role in Oncology, and Concentrations in Serum and Peritoneal Fluid. Journal of Clinical Medicine. 2025; 14(9):3189. https://doi.org/10.3390/jcm14093189
Chicago/Turabian StyleNiedzielska, Julia, and Tomasz Jastrzębski. 2025. "Carcinoembryonic Antigen (CEA): Origin, Role in Oncology, and Concentrations in Serum and Peritoneal Fluid" Journal of Clinical Medicine 14, no. 9: 3189. https://doi.org/10.3390/jcm14093189
APA StyleNiedzielska, J., & Jastrzębski, T. (2025). Carcinoembryonic Antigen (CEA): Origin, Role in Oncology, and Concentrations in Serum and Peritoneal Fluid. Journal of Clinical Medicine, 14(9), 3189. https://doi.org/10.3390/jcm14093189





