Evaluation of Spinal Cord Blood Supply with Hyperspectral Imaging of the Paraspinous Musculature During Staged Endovascular Repair of Thoracoabdominal Aortic Aneurysm: A Sub-Study of the Prospective Multicenter PAPA-ARTiS Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Methodology of Conducted Research Procedures
2.3. Statistical Analysis
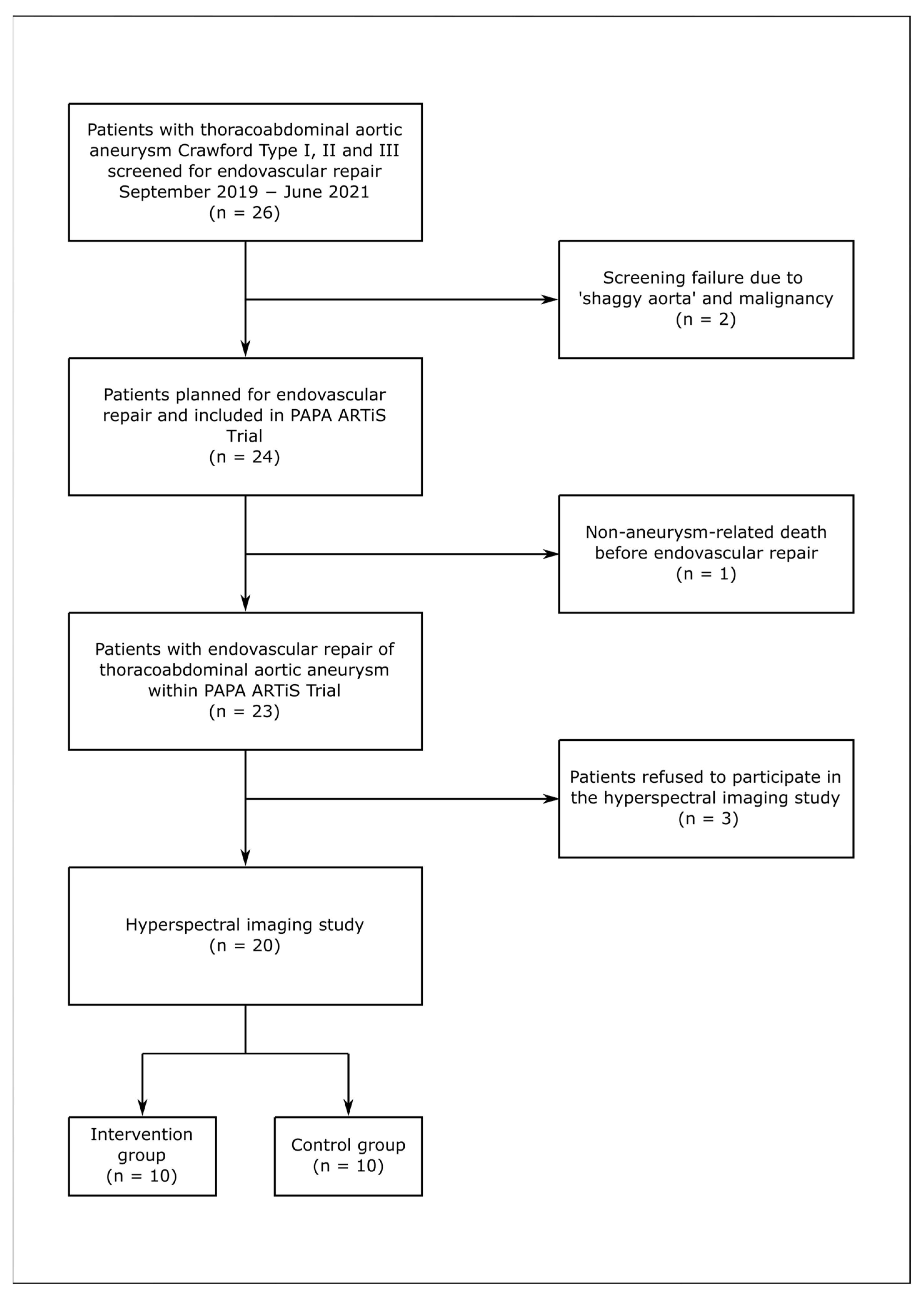
3. Results
3.1. Study Population
3.2. Aneurysm Characteristics
3.3. Control Group
3.4. Intervention Group
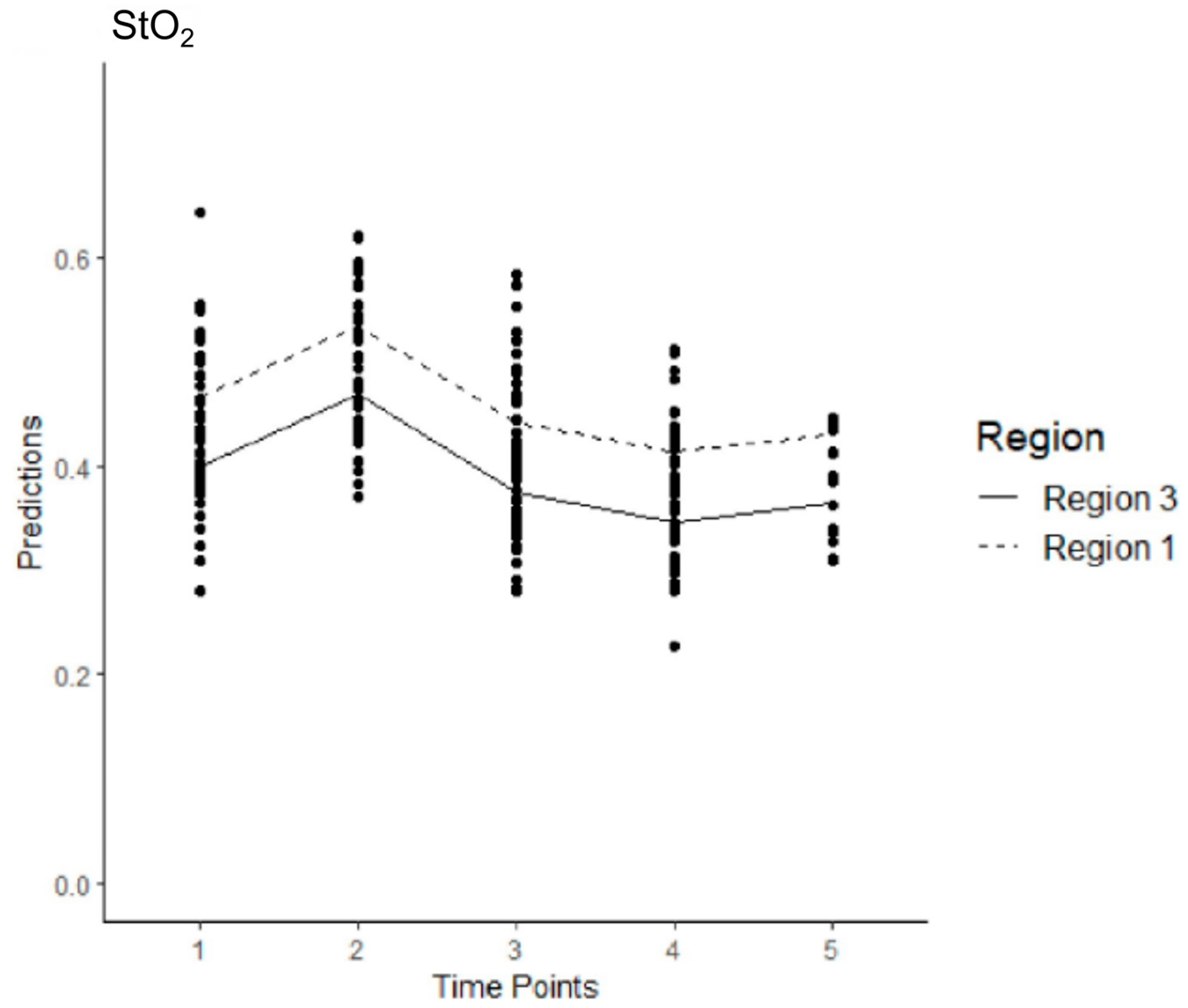
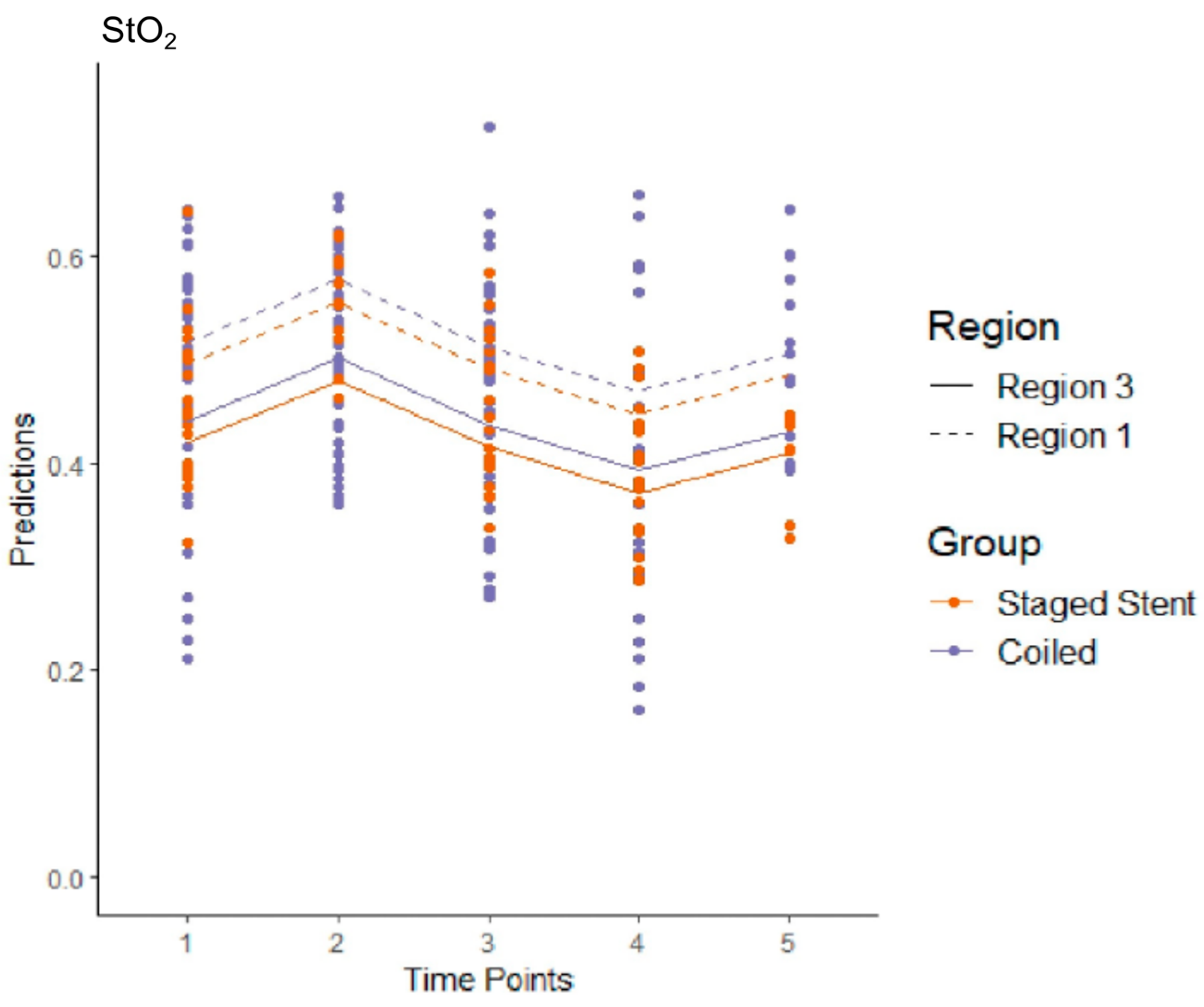
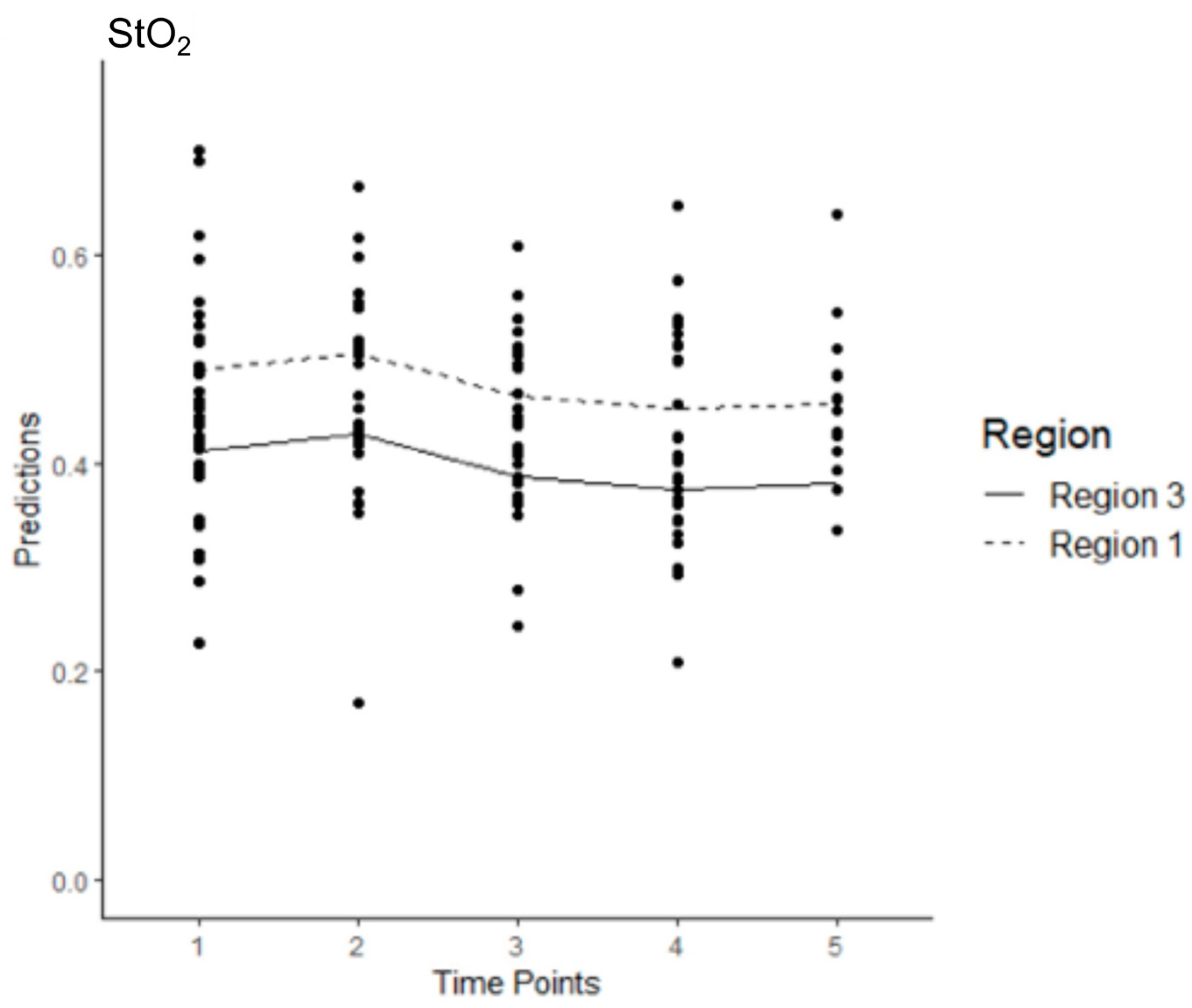
3.5. Model 1
3.6. Model 2
3.7. Model 3
3.8. Model 4
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Aortic Repair |
| CN | Collateral Network |
| CTA | Computer Tomography Angiography |
| ER | Endovascular Repair |
| HSI | Hyperspectral Imaging |
| MIS2ACE | Minimally Invasive Segmental Artery Coil Embolization |
| NIRS | Near-Infrared Spectroscopy |
| NIR PI | Near-Infrared Perfusion Index |
| SA | Segmental Artery |
| SCI | Spinal Cord Ischemia |
| StO2 | Tissue Oxygenation |
| TAAA | Thoracoabdominal Aortic Aneurysm |
| THI | Tissue Hemoglobin Index |
| TWI | Tissue Water Index |
References
- Stoecker, J.B.; Wang, G.J. Epidemiology of thoracoabdominal aortic aneurysms. Semin. Vasc. Surg. 2021, 34, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Kim, K.; Lindsay, M.E.; MacGillivray, T.; Isselbacher, E.M.; Cambria, R.P.; Sundt, T.M. Risk of Rupture or Dissection in Descending Thoracic Aortic Aneurysm. Circulation 2015, 132, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Ellahi, A.; Shaikh, F.N.; Kashif, H.; Khan, H.; Ali, E.; Nasim, B.; Adil, M.; Huda, Z.; Liaquat, A.; Arshad, M.S. Effectiveness of endovascular repair versus open surgery for the treatment of thoracoabdominal aneurysm: A systematic review and meta analysis. Ann. Med. Surg. 2022, 81, 104477. [Google Scholar] [CrossRef]
- Miranda, V.; Sousa, J.; Mansilha, A. Spinal cord injury in endovascular thoracoabdominal aortic aneurysm repair: Prevalence, risk factors and preventive strategies. Int. Angiol. 2018, 37, 112–126. Available online: https://www.minervamedica.it/index2.php?show=R34Y2018N02A0112 (accessed on 18 March 2025). [CrossRef] [PubMed]
- Bisdas, T.; Panuccio, G.; Sugimoto, M.; Torsello, G.; Austermann, M. Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2015, 61, 1408–1416. [Google Scholar] [CrossRef]
- Rizza, A.; Trimarchi, G.; Di Sibio, S.; Bastiani, L.; Murzi, M.; Palmieri, C.; Foffa, I.; Berti, S. Preliminary Outcomes of Zone 2 Thoracic Endovascular Aortic Repair Using Castor Single-Branched Stent Grafts: A Single-Center Experience. J. Clin. Med. 2023, 12, 7593. [Google Scholar] [CrossRef]
- Griepp, E.B.; Di Luozzo, G.; Schray, D.; Stefanovic, A.; Geisbüsch, S.; Griepp, R.B. The anatomy of the spinal cord collateral circulation. Ann. Cardiothorac. Surg. 2012, 1, 350. [Google Scholar]
- Etz, C.D.; Kari, F.A.; Mueller, C.S.; Brenner, R.M.; Lin, H.M.; Griepp, R.B. The collateral network concept: Remodeling of the arterial collateral network after experimental segmental artery sacrifice. J. Thorac. Cardiovasc. Surg. 2011, 141, 1029–1036. [Google Scholar] [CrossRef]
- O’Callaghan, A.; Mastracci, T.M.; Eagleton, M.J. Staged endovascular repair of thoracoabdominal aortic aneurysms limits incidence and severity of spinal cord ischemia. J. Vasc. Surg. 2015, 61, 347–354.e1. [Google Scholar] [CrossRef]
- Branzan, D.; Etz, C.D.; Moche, M.; Von Aspern, K.; Staab, H.; Fuchs, J.; Bergh, F.T.; Scheinert, D.; Schmidt, A. Ischaemic preconditioning of the spinal cord to prevent spinal cord ischaemia during endovascular repair of thoracoabdominal aortic aneurysm: First clinical experience. EuroIntervention 2018, 14, 828–835. [Google Scholar] [CrossRef]
- Tenorio, E.R.; Ribeiro, M.S.; Banga, P.V.; Mendes, B.C.; Kärkkäinen, J.; DeMartino, R.R.; Oderich, G.S. Prospective Assessment of a Protocol Using Neuromonitoring, Early Limb Reperfusion, and Selective Temporary Aneurysm Sac Perfusion to Prevent Spinal Cord Injury During Fenestrated-branched Endovascular Aortic Repair. Ann. Surg. 2022, 276, e1028–e1034. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.J.; Mess, W.; Mochtar, B.; Nijenhuis, R.J.; van Eps, R.G.S.; Schurink, G.W.H. The value of motor evoked potentials in reducing paraplegia during thoracoabdominal aneurysm repair. J. Vasc. Surg. 2006, 43, 239–246. [Google Scholar] [CrossRef]
- von Aspern, K.; Haunschild, J.; Hoyer, A.; Luehr, M.; Bakhtiary, F.; Misfeld, M.; Mohr, F.W.; Etz, C.D. Non-invasive spinal cord oxygenation monitoring: Validating collateral network near-infrared spectroscopy for thoracoabdominal aortic aneurysm repair. Eur. J. Cardiothorac. Surg. 2016, 50, 675–683. [Google Scholar] [CrossRef]
- von Aspern, K.; Haunschild, J.; Ziemann, M.; Misfeld, M.; Mohr, F.W.; Borger, M.A.; Etz, C.D. Evaluation of collateral network near- infrared spectroscopy during and after segmental artery occlusion in a chronic large animal model. J. Thorac. Cardiovasc. Surg. 2019, 158, 155–164.e5. [Google Scholar] [CrossRef]
- von Aspern, K.; Haunschild, J.; Heier, M.; Ossmann, S.; Mohr, F.W.; A Borger, M.; Etz, C.D. Experimental near-infrared spectroscopy- guided minimally invasive segmental artery occlusion. Eur. J. Cardiothorac. Surg. 2021, 60, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 10901. [Google Scholar] [CrossRef]
- Petroff, D.; Czerny, M.; Kölbel, T.; Melissano, G.; Lonn, L.; Haunschild, J.; Etz, C.D. Paraplegia prevention in aortic aneurysm repair by thoracoabdominal staging with ‘minimally invasive staged segmental artery coil embolisation’ (MIS2ACE): Trial protocol for a randomised controlled multicentre trial. BMJ Open 2019, 9, e025488. [Google Scholar] [CrossRef]
- Holmer, A.; Marotz, J.; Wahl, P.; Dau, M.; Kämmerer, P.W. Hyperspectral imaging in perfusion and wound diagnostics-methods and algorithms for the determination of tissue parameters. Biomed. Tech. 2018, 63, 547–556. [Google Scholar] [CrossRef] [PubMed]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Boezeman, R.P.; van Dongen, E.P.; Morshuis, W.J.; Sonker, U.; Boezeman, E.H.; Waanders, F.G.; de Vries, J.-P.P. Spinal Near-Infrared Spectroscopy Measurements During and After Thoracoabdominal Aortic Aneurysm Repair: A Pilot Study. Ann. Thorac. Surg. 2015, 99, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.; von Aspern, K.; Gudehus, S.; Luehr, M.; Girrbach, F.; Ender, J.; Borger, M.; Mohr, F. Near-infrared Spectroscopy Monitoring of the Collateral Network Prior to, During, and After Thoracoabdominal Aortic Repair: A Pilot Study. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Kari, F.A.; Mueller, C.S.; Silovitz, D.; Brenner, R.M.; Lin, H.M.; Griepp, R.B. The collateral network concept: A reassessment of the anatomy of spinal cord perfusion. J. Thorac. Cardiovasc. Surg. 2011, 141, 1020–1028. [Google Scholar] [CrossRef] [PubMed]


| Intervention Group (n = 10) | Control Group (n = 10) | Total (n = 20) | ||
|---|---|---|---|---|
| Variables | No. (%) | No. (%) | No. (%) | |
| Sex | ||||
| Male | 5 (50) | 6 (60) | 11 (55) | |
| Female | 5 (50) | 4 (40) | 9 (45) | |
| Age (years) | ||||
| Mean ± SD | 68.3 ± 8.4 | 64.6 ± 11.4 | 66.5 ± 10.2 | |
| Median (range) | 70.5 (51–80) | 65.5 (42–81) | 67.0 (42–81) | |
| History of hypertension | 10 (100) | 9 (90) | 19 (95) | |
| COPD | 3 (30) | 3 (30) | 6 (30) | |
| Smoker | 7 (70) | 9 (90) | 16 (80) | |
| Coronary heart disease | 3 (30) | 3 (30) | 6 (30) | |
| Diabetes mellitus | 0 (0) | 2 (20) | 2 (10) | |
| Renal insufficiency | ||||
| GFR < 60 mL/min/1.73 m2 | 3 (30) | 5 (50) | 8 (40) | |
| Mean GFR ± SD | 72.1 ± 18.6 | 58.7 ± 26.2 | 65.4 ± 23.2 | |
| Hyperlipidemia | 7 (70) | 5 (50) | 12 (60) | |
| Body mass index (BMI) | ||||
| Mean ± SD | 27.3 ± 4.9 | 29.3 ± 6.8 | 28.5 ± 6.0 | |
| Median (range) | 26.3 (21.5–40.6) | 25.2 (23.5–41.9) | 25.8 (21.5–41.9) | |
| ASA Classification | ||||
| II | 2 (20) | 2 (20) | 4 (20) | |
| III | 6 (60) | 6 (60) | 12 (60) | |
| IV | 2 (20) | 2 (20) | 4 (20) | |
| Crawford Classification | ||||
| Type II | 9 (90) | 8 (80) | 17 (85) | |
| Type III | 1 (10) | 2 (20) | 3 (15) | |
| Maximal Aortic Diameter, mm | ||||
| Mean ± SD | 58.5 ± 11.0 | 61.0 ± 8.8 | 59.7± 9.8 | |
| Previous Aortic Repair | 5 (50) | 8 (80) | 13 (65) | |
| Ascending Aortic Repair | 3 (30) | 5 (50) | 8 (40) | |
| ET | 1 (10) | 3 (30) | 4 (20) | |
| FET | 2 (20) | 4 (40) | 6 (30) | |
| TEVAR | 1 (10) | 1 (10) | 2 (10) | |
| EVAR | 1 (10) | 1 (10) | 2 (10) | |
| Etiology | ||||
| Degenerative | 6 (60) | 7 (70) | 13 (65) | |
| Post-dissection | 4 (40) | 3 (30) | 7 (35) | |
| Segmental Artery to be covered by the stentgraft | ||||
| Total | 159 | 188 | 347 | |
| Mean ± SD | 15.9 ± 5.3 | 18.8 ± 4.6 | 17.3 ± 5.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winther, B.; Branzan, D.; Etz, C.D.; Geisler, A.A.; Steiner, S.; Winther, H.; Meixner, R.; Jiménez-Muñoz, M.; Köhler, H.; Scheinert, D.; et al. Evaluation of Spinal Cord Blood Supply with Hyperspectral Imaging of the Paraspinous Musculature During Staged Endovascular Repair of Thoracoabdominal Aortic Aneurysm: A Sub-Study of the Prospective Multicenter PAPA-ARTiS Trial. J. Clin. Med. 2025, 14, 3188. https://doi.org/10.3390/jcm14093188
Winther B, Branzan D, Etz CD, Geisler AA, Steiner S, Winther H, Meixner R, Jiménez-Muñoz M, Köhler H, Scheinert D, et al. Evaluation of Spinal Cord Blood Supply with Hyperspectral Imaging of the Paraspinous Musculature During Staged Endovascular Repair of Thoracoabdominal Aortic Aneurysm: A Sub-Study of the Prospective Multicenter PAPA-ARTiS Trial. Journal of Clinical Medicine. 2025; 14(9):3188. https://doi.org/10.3390/jcm14093188
Chicago/Turabian StyleWinther, Birte, Daniela Branzan, Christian D. Etz, Antonia Alina Geisler, Sabine Steiner, Hinrich Winther, Raphael Meixner, Marina Jiménez-Muñoz, Hannes Köhler, Dierk Scheinert, and et al. 2025. "Evaluation of Spinal Cord Blood Supply with Hyperspectral Imaging of the Paraspinous Musculature During Staged Endovascular Repair of Thoracoabdominal Aortic Aneurysm: A Sub-Study of the Prospective Multicenter PAPA-ARTiS Trial" Journal of Clinical Medicine 14, no. 9: 3188. https://doi.org/10.3390/jcm14093188
APA StyleWinther, B., Branzan, D., Etz, C. D., Geisler, A. A., Steiner, S., Winther, H., Meixner, R., Jiménez-Muñoz, M., Köhler, H., Scheinert, D., & Schmidt, A. (2025). Evaluation of Spinal Cord Blood Supply with Hyperspectral Imaging of the Paraspinous Musculature During Staged Endovascular Repair of Thoracoabdominal Aortic Aneurysm: A Sub-Study of the Prospective Multicenter PAPA-ARTiS Trial. Journal of Clinical Medicine, 14(9), 3188. https://doi.org/10.3390/jcm14093188






