Abstract
Background: Peripheral arterial disease (PAD) is associated with cardiovascular events in patients with acute myocardial infarction (AMI). However, there are limited reports regarding the association between PAD and bleeding events. In this study, we aimed to evaluate whether PAD is independently associated with an increased risk of major bleeding events, in addition to major adverse cardiovascular events (MACEs), in patients with AMI undergoing percutaneous coronary intervention (PCI). Methods: We included 1391 patients with AMI who underwent PCI and divided them into the PAD group (n = 210) and the non-PAD group (n = 1181). The primary endpoint was total bleeding events, defined as Bleeding Academic Research Consortium type 3/5. The secondary endpoint was MACE, defined as the composite of all-cause death, non-fatal myocardial infarction, and hospitalization for heart failure. Results: The median follow-up duration was 653 days. Total bleeding events were more frequently observed in the PAD group than in the non-PAD group (24.8% vs. 11.3%, p < 0.001). The multivariate Cox hazard analysis confirmed that PAD was significantly associated with total bleeding events (HR 1.509; 95% CI 1.056–2.156, p = 0.024) as well as MACEs (HR 2.152; 95% CI 1.510–3.066, p < 0.001) after controlling for confounding factors. Conclusions: PAD was independently associated with a higher risk of major bleeding and cardiovascular events in patients with AMI undergoing PCI. These findings suggest that PAD should be recognized as a critical factor in risk stratification for AMI and may affect individualized bleeding risk management strategies in patients with AMI.
1. Introduction
Acute myocardial infarction (AMI) remains a critical health issue globally and is characterized by significant morbidity and mortality [1,2]. The development of primary percutaneous coronary intervention (PCI) has revolutionized the treatment of AMI, resulting in better clinical outcomes [3]. However, the success of primary PCI is sometimes tempered by the occurrence of complications such as bleeding, which can significantly impact all-cause mortality [4,5].
Bleeding events after PCI are common and associated with increased mortality and poor clinical outcomes [6,7]. The incidence of bleeding events after PCI is influenced by the presence of high-bleeding risk factors. Recently, the Japanese Society of Cardiology published the Japanese version of the High Bleeding Risk criteria (J-HBR) [8]. One of the major criteria in the J-HBR is peripheral artery disease (PAD), which was not focused on in other risk criteria, such as the Academic Research Consortium for High Bleeding Risk (ARC-HBR) [9]. Although recent international studies, including the EPICOR Asia study, have investigated the long-term bleeding risk in patients with acute coronary syndrome, the association between PAD and bleeding has not been established in patients with AMI [10]. The J-MINUET registry reported that PAD was associated with worse clinical outcomes in patients with AMI [11]. Most of these studies primarily focused on ischemic outcomes, and few have comprehensively evaluated the association between PAD and bleeding in patients with AMI. The purpose of this study was to investigate the association between PAD and major bleeding in patients with AMI after primary PCI.
2. Materials and Methods
2.1. Study Design
We reviewed all patients with AMI treated at our institution (Saitama Medical Center, Jichi Medical University) from January 2015 to December 2022. The inclusion criterion was patients with AMI. The exclusion criteria were as follows: (1) patients without complete ankle–brachial index (ABI) measurement during hospitalization, (2) a second or more than two AMIs during the study period, (3) patients who underwent CABG during hospitalization, and (4) patients who did not undergo PCI to the culprit lesion of AMI. These criteria aimed to ensure a uniform study population for evaluating the relationship between PAD and bleeding. Patients without ABI data were excluded for diagnostic consistency, while those with CABG or those without PCI were excluded to reduce potential confounding factors.
We defined PAD as a history of surgery or EVT for PAD, ABI < 0.9, or inter-arm blood pressure difference (IABPD) ≥ 10 mmHg [12,13,14,15]. Patients with only symptoms suggestive of PAD were not classified as having PAD. The final study population was divided into a PAD group and a non-PAD group. The primary endpoint was total bleeding events, which is defined as type 3 or 5 bleeding events by the Bleeding Academic Research Consortium (BARC) [9]. BARC type 1, 2, and 4 bleeding were not included in total bleeding events. The secondary endpoint was major cardiovascular events (MACEs), which was defined as the composite of all-cause death, non-fatal myocardial infarction, and readmission for heart failure. Hospital records were used to obtain information regarding the clinical outcomes. The day of PCI was defined as the index day (day 1). The study patients were followed up until all-cause death or until the study end date (31 May 2023). This study was approved by the institutional review board of the Saitama Medical Center, Jichi Medical University (S22-074), and the need for written informed consent was waived because of the retrospective study design.
2.2. Definitions
AMI was defined according to the universal definition [16,17]. Diagnostic ST elevation was defined as new ST elevation at the J point in at least two contiguous leads of 2 mm (0.2 mV), and patients with ST elevation were diagnosed as having STEMI [18,19]. Definitions of hypertension, diabetes mellitus, and dyslipidemia are described elsewhere [20,21,22]. We used the laboratory data at admission [21]. Left ventricular ejection fraction (LVEF) was measured via transthoracic echocardiography during the index hospitalization [23]. We also calculated estimated glomerular filtration rate (eGFR) [24]. The initial and final thrombolysis in myocardial infarction (TIMI) flow grades were documented from invasive coronary angiography [25].
2.3. Statistical Analysis
Data are expressed as the median (Q1–Q3) or percentage. Categorical variables are presented as numbers (percentages) and were compared using Fisher’s exact test. The Shapiro–Wilk test was performed to examine whether the continuous variables were normally distributed or not. Because none of the variables were normally distributed, continuous variables were compared using the Mann–Whitney U test. Event-free survival curves were constructed using the Kaplan–Meier method, and statistical differences were examined with the log-rank test. We performed a multivariate Cox hazard analysis to investigate the association between PAD and total bleeding events or between PAD and MACEs after controlling for confounding factors. In the model, total bleeding events or MACEs were used as the dependent variable. Variables that were significantly different (p < 0.05) between the PAD and non-PAD groups were included as independent variables in the model. Variables with missing values were not included in the model. To avoid multicollinearity, similar variables were not entered simultaneously. Hazard ratios (HRs) and the 95% confidence intervals (CIs) were calculated. A p-value < 0.05 was considered statistically significant. Furthermore, we conducted propensity score matching as a supplemental analysis. A logistic regression analysis was performed to calculate the propensity score using the full database. In this model, PAD was set as a dependent variable, whereas age, sex, BMI, presence of diabetes mellitus, chronic renal failure on hemodialysis, history of PCI, history of CABG, history of stroke, hemoglobin level, STEMI, and LVEF were set as independent variables. For matching, the match tolerance was set as a width of 0.25 multiplied by the SD of the propensity score distribution. Case–control matching resulted in 202 fuzzy matches with maximized matching performance. Thus, these 202 pairs were used in the supplementary analysis. All analyses were performed using statistical software, SPSS 25/Windows (SPSS, Chicago, IL, USA).
3. Results
From January 2015 to December 2022, 2238 patients with AMI were admitted to our institution. After excluding 847 patients who met the exclusion criteria, the final study population consisted of 1391 patients with AMI, who were assigned to the PAD group (n = 210) or the non-PAD group (n = 1181) (Figure 1).
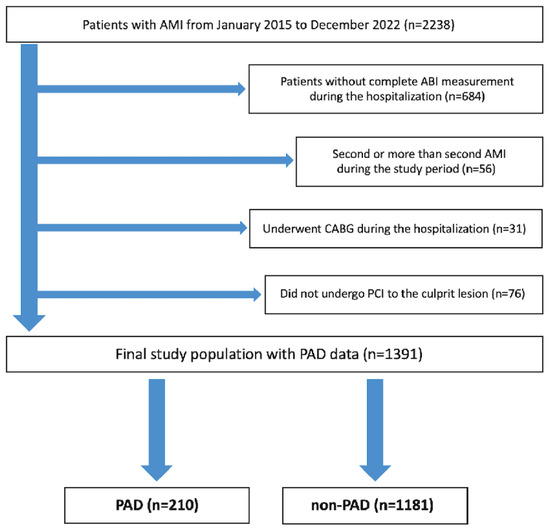
Figure 1.
Study flowchart. Abbreviations: AMI = acute myocardial infarction, ABI = ankle–brachial index, CABG = coronary artery bypass grafting, PCI = percutaneous coronary intervention, and PAD = peripheral arterial disease.
The comparison of patients’ characteristics between the two groups is shown in Table 1. Age was older and body mass index was lower in the PAD group compared to the non-PAD group. Anemia, hemodialysis, and history of cerebral infarction were more frequently observed in the PAD group than in the non-PAD group. The prevalence of STEMI was significantly lower in the PAD group than in the non-PAD group. Aspirin, thienopyridine, statin, and antihypertensive medications at admission were more frequently prescribed in the PAD group. Oral antidiabetics and insulin were also more prescribed in the PAD group. Table 2 shows the comparison of angiographic and procedural findings between the 2 groups. Triple vessel disease, left main disease, first TIMI flow grade 3, and chronic total occlusion in non-culprit arteries were more frequently observed in the PAD group than in the non-PAD group. Left main disease was also more frequently found in the PAD group.

Table 1.
Comparison of clinical characteristics between the PAD group and the non-PAD group.

Table 2.
Comparison of lesion and procedural characteristics between the PAD group and the non-PAD group.
Figure 2 shows the Kaplan–Meier curves between the two groups. The median follow-up duration was 653 (Q1: 259–Q3: 1404) days. Total bleeding events were more frequently observed in the PAD group compared to the non-PAD group (log-rank p < 0.001), and MACEs were also more frequently observed in the PAD group (log-rank p < 0.001). Table 3 shows the comparison of clinical outcomes between the two groups. A total of 186 bleeding events were observed during the follow-up duration. The incidence of total bleeding events was significantly higher in the PAD group than in the non-PAD group. In particular, the incidence of BARC type 3 bleeding events was significantly higher in the PAD group than in the non-PAD group, whereas there was no significant difference in BARC type 5 bleeding events between the 2 groups. The incidence of PCI access site-related bleeding events was significantly higher in the PAD group than in the non-PAD group. The incidence of MACEs was significantly higher in the PAD group than in the non-PAD group. The results of multivariate Cox hazard analysis are shown in Table 4. PAD was significantly associated with total bleeding events (HR 1.509, 95% CI 1.056–2.156, p = 0.024) and MACEs (HR 2.152, 95% CI 1.510–3.066, p < 0.001) after controlling for multiple confounding factors including age, gender, overweight (BMI ≥ 25), anemia, chronic renal failure on hemodialysis, previous myocardial infarction, previous cerebral infarction, CRP levels, STEMI, Killip class, diastolic blood pressure at admission, LVEF, use of NPPV, number of narrowed coronary arteries, first TIMI flow grade, use of drug-eluting stents, and catheter size.
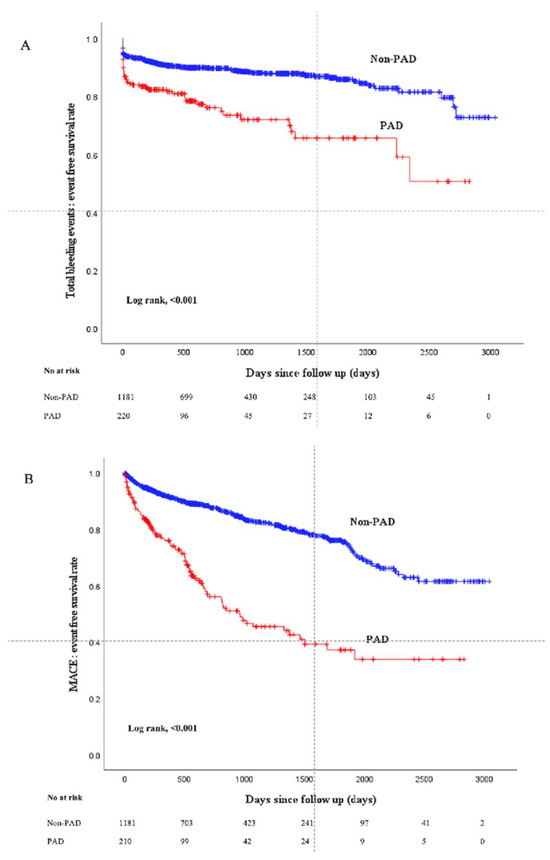
Figure 2.
Kaplan–Meier curves for total bleeding or MACE-free survival events-free survival between the PAD group and the non-PAD group. (A) Comparison of total bleeding events. (B) Comparison of MACEs. Abbreviations: MACEs = major cardiovascular events.

Table 3.
The comparison of clinical outcomes between the PAD group and the non-PAD group.

Table 4.
(a) Multivariate Cox hazard model to predict total bleeding events. (b) Multivariate Cox hazard model to predict MACEs.
Supplemental Tables S1–S3 show the comparison of clinical, lesion, and procedural outcomes between the matched PAD and matched non-PAD groups, respectively. Supplementary Figure S1 shows the Kaplan–Meier curves for bleeding (A) and MACEs (B) after propensity score matching. Total bleeding events were more frequently observed in the matched PAD group than in the matched non-PAD group without reaching statistical significance, whereas MACEs were more frequently observed in the matched PAD group.
4. Discussion
We included 1391 patients with AMI and divided them into the PAD group (n = 210) and the non-PAD group (n = 1181). We followed up the patients with a median duration of 653 days. Total bleeding events were more frequently observed in the PAD group than in the non-PAD group. The multivariate Cox hazard analysis revealed that PAD was significantly associated with total bleeding events (HR 1.509, 95% CI 1.056–2.156, p = 0.024) and MACEs (HR 2.152, 95% CI 1.510–3.066, p < 0.001) after controlling for multiple confounding factors. In the propensity score matching analysis, total bleeding events were more frequently observed in the matched PAD group than in the matched non-PAD group without reaching statistical significance, whereas MACEs were more frequently observed in the matched PAD group.
We should clarify the difference between the present study and previous studies. Saw et al. conducted a pooled analysis of eight randomized PCI trials and revealed a trend toward higher major bleeding in patients with PAD (4.5%) as compared to those without PAD (3.9%) (p = 0.06) [26]. Gupta et al. showed that a history of PAD was associated with ischemic and bleeding outcomes 2 years after successful PCI (HR 1.60%, 95% CI: 1.31–1.96; p < 0.0001) [27]. Bashar et al. also investigated the association between extracardiac vascular disease (ECVD), including PAD, and clinical outcomes after PCI and found that ECVD was associated with worse outcomes in patients undergoing PCI, including significantly higher rates of death and stroke [28]. Gao et al. investigated the impact of PAD on MACEs and bleeding in patients undergoing complex or non-complex PCI and found that PAD was associated with increased risk of bleeding regardless of procedural complexity [29]. Pinxterhuis et al. conducted a three-year pooled patient-level data analysis of two randomized PCI trials, including 5989 all-comer patients, and revealed that PCI patients with PAD had a significantly higher bleeding risk than PCI patients without PAD [30]. These studies did not focus on patients with AMI, whereas we focused on patients with AMI. Because patients with AMI have a greater risk of bleeding than patients with chronic coronary syndrome [31], it is important to elucidate the association between PAD and bleeding in patients with AMI who have undergone PCI.
In our study, PAD was significantly associated with MACEs, including all-cause death, non-fatal myocardial infarction, and readmission for heart failure. PAD is characterized by systemic atherosclerosis and extensive vascular damage, including the coronary arteries [32,33]. In patients with AMI, the presence of PAD, irrespective of symptoms, is strongly associated with an increased risk of MACEs, including all-cause death, myocardial infarction, and readmission for heart failure [12,34]. Patients with advanced PAD are more likely to be frail and undernourished [35]. Reduced physical activity and increased frailty might be associated with increased risk of MACEs [36].
We should discuss why PAD is associated with long-term bleeding in patients with AMI. Patients with PAD undergoing PCI for AMI are associated with long-term bleeding risks due to several interrelated factors. Among patients undergoing coronary stenting, those with PAD have more ischemic events, including revascularization, than those without PAD. Thus, patients with PAD are more likely to undergo multiple PCI procedures and require longer dual antiplatelet therapy. As a result, patients with PAD tend to have more bleeding events [37]. The systemic inflammation and endothelial dysfunction that are prevalent in PAD may further exacerbate bleeding risk, especially under antithrombotic treatment [38]. Moreover, patients with PAD often have comorbidities such as diabetes mellitus and hypertension, which contribute to an increased risk of bleeding [39]. Although PAD is a common disease worldwide, the optimal type, dose, and timing of antiplatelet and anticoagulant medications have not been determined. There are no uniform guidelines on this topic. In patients with PAD after revascularization, DAPT or the combination of aspirin and low-dose rivaroxaban may reduce the incidence of ischemic events but increase bleeding [40,41]. Therefore, patients with a high risk of bleeding should have an appropriate risk index.
The clinical implications of the present study should be noted. Since PAD in patients with AMI is associated with long-term bleeding events, it is important to recognize PAD as a risk factor for bleeding through routine measurement of ABI. It may be reasonable to include ABI in the standard practice for patients with AMI, because ABI is a non-invasive and non-expensive test. These high-risk patients should be carefully followed up by cardiologists. Careful follow-up may include close monitoring of hemoglobin levels and gastrointestinal symptoms. We should consider switching from DAPT to short DAPT in patients with PAD due to the increased risk of bleeding. Given the higher bleeding risk and lower thrombotic risk in Japanese and other East Asian populations compared to Western populations [42,43], the strategy to minimize bleeding risk is essential to improve the overall outcomes of AMI patients with PAD. Although the Japanese version of the HBR (J-HBR) includes PAD as one of the major criteria for bleeding [8], major bleeding risk criteria or bleeding risk score, including ARC-HBR and PRECISE DAPT, do not deem PAD as a significant risk factor [9,44]. Future bleeding risk criteria or revisions of major bleeding risk criteria, which would influence antiplatelet therapy decisions, may consider including PAD as one of the criteria.
There are several limitations to the present study. Since this study is a single-center, retrospective study, there is a potential for selection bias. To address this, we performed multivariable Cox proportional hazards regression analysis to adjust for known confounding variables. Furthermore, we also performed propensity score matching to adjust for potential confounding factors. Although the propensity score matching adjusted for clinical background, this matching reduced the study population significantly from 1391 to 404, which created a risk of beta-error. In the propensity score matched cohort, total bleeding events were not statistically significant between the matched PAD and non-PAD groups, which might be affected by beta error. Long-term bleeding events might have been influenced by post-discharge medications. Since our institution is a tertiary university hospital, most patients were referred to their local clinics after discharge. Because patients received their medications, including antiplatelet therapy, from these clinics, we were unable to obtain detailed information on post-discharge medications, including DAPT. Although the recommended DAPT duration was described in referral letters according to the guidelines, adherence to these recommendations was not systematically monitored. It is possible that some patients continued DAPT beyond the recommended period, which might have led to an increased incidence of bleeding events. This lack of information regarding post-discharge medications is a major limitation of this study. Frailty might be more prevalent in patients with PAD and could have affected clinical outcomes because frailty is closely associated with bleeding events [45]. However, since specific metrics to assess frailty were not available in our dataset, we could not evaluate or adjust for frailty in our multivariate analysis, which represents another important limitation. Because ABI and IABPD were measured in the physiological laboratory, the most severe patients, such as those with cardiogenic shock requiring mechanical support who could not be transported to the physiological laboratory, did not have their ABI and IABPD measured [46]. As a result, the most severely ill patients might not be included in our analysis, potentially limiting the generalizability of our findings. Furthermore, no imputation methods were used for missing data in this study.
5. Conclusions
In this study, PAD was significantly associated with major bleeding as well as adverse cardiovascular events in patients with AMI who underwent PCI. The presence of PAD needs to be recognized as a risk factor for bleeding in patients with AMI. The authors of future studies should validate our findings in broader cohorts and assess whether recognizing PAD as a bleeding risk can improve clinical outcomes in patients with AMI.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14093183/s1, Supplemental Table S1. Comparison of clinical characteristics between the matched PAD and matched non-PAD groups. Supplemental Table S2. Comparison of lesion and procedural characteristics between the matched PAD and matched non-PAD groups. Supplemental Table S3. Comparison of clinical outcomes between the matched PAD and matched non-PAD groups. Supplemental Figure S1. Kaplan-Meier curves for total bleeding or MACE-free survival events-free survival between the matched PAD and matched non-PAD groups.
Author Contributions
Conceptualization: S.B. and K.S.; data curation: S.B. and K.S.; formal analysis: S.B. and K.S.; investigation: S.B., K.S., H.J., Y.T., K.Y., T.T., M.H., T.K., Y.W., S.I., M.S. and H.F.; methodology: S.B. and K.S.; writing—original draft: S.B. and K.S.; writing—review and editing: S.B., K.S., H.J., Y.T., K.Y., T.T., M.H., T.K., Y.W., S.I., M.S. and H.F.; supervision: K.S. and H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Institutional Review Board of Jichi Medical University approved this study (S23-182) on 15 April 2024.
Informed Consent Statement
The need for written informed consent was waived because of the retrospective study design. Instead, an opt-out-style announcement of the outline of the present study was posted on the website of the Saitama Medical Center, Jichi Medical University.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mythili, S.; Malathi, N. Diagnostic markers of acute myocardial infarction. Biomed. Rep. 2015, 3, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Nishihira, K.; Nakai, M.; Kuriyama, N.; Kadooka, K.; Honda, Y.; Emori, H.; Yamamoto, K.; Nishino, S.; Kudo, T.; Ogata, K.; et al. Guideline-Directed Medical Therapy for Elderly Patients with Acute Myocardial Infarction Who Undergo Percutaneous Coronary Intervention-Insights from a Retrospective Observational Study. Circ. J. 2024, 88, 931–937. [Google Scholar] [CrossRef]
- Pappalardo, A.; Mamas, M.A.; Imola, F.; Ramazzotti, V.; Manzoli, A.; Prati, F.; El-Omar, M. Percutaneous coronary intervention of unprotected left main coronary artery disease as culprit lesion in patients with acute myocardial infarction. JACC Cardiovasc. Interv. 2011, 4, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.B.; Marso, S.P.; Pencina, M.; Stolker, J.M.; Kennedy, K.F.; Rihal, C.; Barsness, G.; Piana, R.N.; Goldberg, S.L.; Cutlip, D.E.; et al. Prognostic impact of periprocedural bleeding and myocardial infarction after percutaneous coronary intervention in unselected patients: Results from the EVENT (evaluation of drug-eluting stents and ischemic events) registry. JACC Cardiovasc. Interv. 2009, 2, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, I.C. Gastrointestinal bleeding after percutaneous coronary intervention: Not just a short-term complication but a long-term marker of mortality risk. Catheter. Cardiovasc. Interv. 2020, 95, E146–E147. [Google Scholar] [CrossRef]
- Marquis-Gravel, G.; Dalgaard, F.; Jones, A.D.; Lokhnygina, Y.; James, S.K.; Harrington, R.A.; Wallentin, L.; Steg, P.G.; Lopes, R.D.; Storey, R.F.; et al. Post-Discharge Bleeding and Mortality Following Acute Coronary Syndromes with or Without PCI. J. Am. Coll. Cardiol. 2020, 76, 162–171. [Google Scholar] [CrossRef]
- Kwok, C.S.; Tiong, D.; Pradhan, A.; Andreou, A.Y.; Nolan, J.; Bertrand, O.F.; Curzen, N.; Urban, P.; Myint, P.K.; Zaman, A.G.; et al. Meta-Analysis of the Prognostic Impact of Anemia in Patients Undergoing Percutaneous Coronary Intervention. Am. J. Cardiol. 2016, 118, 610–620. [Google Scholar] [CrossRef]
- Nakamura, M.; Kimura, K.; Kimura, T.; Ishihara, M.; Otsuka, F.; Kozuma, K.; Kosuge, M.; Shinke, T.; Nakagawa, Y.; Natsuaki, M.; et al. JCS 2020 Guideline Focused Update on Antithrombotic Therapy in Patients with Coronary Artery Disease. Circ. J. 2020, 84, 831–865. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef]
- Guan, S.; Xu, X.; Li, Y.; Li, J.; Guan, M.; Wang, X.; Jing, Q.; Huo, Y.; Han, Y. Impact of Diabetes Mellitus on Antithrombotic Management Patterns and Long-Term Clinical Outcomes in Patients with Acute Coronary Syndrome: Insights from the EPICOR Asia Study. J. Am. Heart Assoc. 2020, 9, e013476. [Google Scholar] [CrossRef]
- Akahori, H.; Masuyama, T.; Imanaka, T.; Nakao, K.; Ozaki, Y.; Kimura, K.; Ako, J.; Noguchi, T.; Suwa, S.; Fujimoto, K.; et al. Impact of peripheral artery disease on prognosis after myocardial infarction: The J-MINUET study. J. Cardiol. 2020, 76, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Association of Asymptomatic Low Ankle-Brachial Index with Long-Term Clinical Outcomes in Patients after Acute Myocardial Infarction. J. Atheroscler. Thromb. 2022, 29, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Hatori, M.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; et al. Association of Increased Inter-arm Blood Pressure Difference with Long-term Clinical Outcomes in Patients with Acute Myocardial Infarction Who Underwent Percutaneous Coronary Intervention. Intern. Med. 2024, 63, 1043–1051. [Google Scholar] [CrossRef]
- Shamaki, G.R.; Markson, F.; Soji-Ayoade, D.; Agwuegbo, C.C.; Bamgbose, M.O.; Tamunoinemi, B.M. Peripheral Artery Disease: A Comprehensive Updated Review. Curr. Probl. Cardiol. 2022, 47, 101082. [Google Scholar] [CrossRef] [PubMed]
- Slovut, D.P.; Lipsitz, E.C. Surgical technique and peripheral artery disease. Circulation 2012, 126, 1127–1138. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Sawano, M.; Yamaji, K.; Kohsaka, S.; Inohara, T.; Numasawa, Y.; Ando, H.; Iida, O.; Shinke, T.; Ishii, H.; Amano, T. Contemporary use and trends in percutaneous coronary intervention in Japan: An outline of the J-PCI registry. Cardiovasc. Interv. Ther. 2020, 35, 218–226. [Google Scholar] [CrossRef]
- Konoma, S.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Hatori, M.; Tamanaha, Y.; Kasahara, T.; Watanabe, Y.; Yamamoto, K.; et al. Impact of the Japanese Version of High Bleeding Risk Criteria on Clinical Outcomes in Patients with ST-segment Elevation Myocardial Infarction. J. Atheroscler. Thromb. 2024, 31, 917–930. [Google Scholar] [CrossRef]
- Hori, Y.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Association of peak C-reactive protein with long-term clinical outcomes in patients with ST-segment elevation myocardial infarction. Heart Vessel. 2023, 38, 764–772. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Yamamoto, K.; Tsukui, T.; Hatori, M.; Kasahara, T.; Ishibashi, S.; Watanabe, Y.; et al. Determinants of In-hospital Death in Non-ST-segment Elevation Myocardial Infarction with Triple-vessel Disease. Intern Med. 2024, 64, 993–999. [Google Scholar] [CrossRef]
- Yanase, T.; Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Tsukui, T.; Seguchi, M.; Wada, H.; Momomura, S.-I.; Fujita, H. Comparison of Clinical Characteristics of Acute Myocardial Infarction Between Young (<55 Years) and Older (55 to <70 Years) Patients. Int. Heart J. 2021, 62, 33–41. [Google Scholar] [PubMed]
- Sato, F.; Maeda, N.; Yamada, T.; Namazui, H.; Fukuda, S.; Natsukawa, T.; Nagao, H.; Murai, J.; Masuda, S.; Tanaka, Y.; et al. Association of Epicardial, Visceral, and Subcutaneous Fat with Cardiometabolic Diseases. Circ. J. 2018, 82, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, K.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Yamamoto, K.; Tsukui, T.; Hatori, M.; Kasahara, T.; Watanabe, Y.; Ishibashi, S.; et al. Comparison of clinical outcomes between proximal and non-proximal right coronary artery occlusion in patients with inferior ST-segment elevation myocardial infarction. J Cardiol. 2024, 85, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Hatori, M.; Tamanaha, Y.; Kasahara, T.; Watanabe, Y.; Yamamoto, K.; et al. Impact of Excessive Increase in Systolic Blood Pressure after Exercise on Clinical Outcomes in Patients with ST-Segment Elevation Myocardial Infarction. J. Clin. Med. 2023, 12, 6928. [Google Scholar] [CrossRef]
- Tsukui, T.; Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Seguchi, M.; Jinnouchi, H.; Wada, H.; Fujita, H. Factors associated with poor clinical outcomes of ST-elevation myocardial infarction in patients with door-to-balloon time <90 minutes. PLoS ONE 2020, 15, e0241251. [Google Scholar]
- Saw, J.; Bhatt, D.L.; Moliterno, D.J.; Brener, S.J.; Steinhubl, S.R.; Lincoff, A.M.; Tcheng, J.E.; Harrington, R.A.; Simoons, M.; Hu, T.; et al. The influence of peripheral arterial disease on outcomes: A pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J. Am. Coll. Cardiol. 2006, 48, 1567–1572. [Google Scholar] [CrossRef]
- Gupta, R.; Kirtane, A.J.; Ozan, M.O.; Witzenbichler, B.; Rinaldi, M.J.; Metzger, D.C.; Weisz, G.; Stuckey, T.D.; Brodie, B.R.; Mehran, R.; et al. Platelet Reactivity and Clinical Outcomes After Coronary Artery Implantation of Drug-Eluting Stents in Subjects with Peripheral Arterial Disease: Analysis From the ADAPT-DES Study (Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents). Circ Cardiovasc Interv. 2017, 10, e004904. [Google Scholar] [CrossRef]
- Bashar, H.; Matetić, A.; Curzen, N.; Mamas, M.A. Impact of extracardiac vascular disease on outcomes of 1.4 million patients undergoing percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2022, 100, 737–746. [Google Scholar] [CrossRef]
- Gao, M.; Oliva, A.; Sharma, R.; Kalaba, F.; Sartori, S.; Farhan, S.; Smith, K.; Vogel, B.; Krishnan, P.; Dangas, G.; et al. Impact of Peripheral Arterial Disease on Clinical Outcomes of Patients Undergoing Complex vs Noncomplex Percutaneous Coronary Intervention. Am. J. Cardiol. 2025, 247, 76–83. [Google Scholar] [CrossRef]
- Pinxterhuis, T.H.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; Schotborgh, C.E.; Anthonio, R.L.; Roguin, A.; Danse, P.W.; Benit, E.; Aminian, A.; et al. Risk of bleeding after percutaneous coronary intervention and its impact on further adverse events in clinical trial participants with comorbid peripheral arterial disease. Int. J. Cardiol. 2023, 374, 27–32. [Google Scholar] [CrossRef]
- Tung, Y.C.; See, L.C.; Chang, S.H.; Liu, J.R.; Kuo, C.T.; Chang, C.J. Impact of bleeding during dual antiplatelet therapy in patients with coronary artery disease. Sci. Rep. 2020, 10, 21345. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Dang, T.T.; Criqui, M.H. Peripheral arterial disease: Morbidity and mortality implications. Circulation 2006, 114, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.F.; Weinberg, M.D.; Olin, J.W. Peripheral artery disease. Part 1, clinical evaluation and noninvasive diagnosis. Nat. Rev. Cardiol. 2011, 8, 405–418. [Google Scholar] [CrossRef]
- Freisinger, E.; Malyar, N.M.; Reinecke, H. Peripheral artery disease is associated with high in-hospital mortality particularly in males with acute myocardial infarction in a nationwide real-world setting. Vasa 2016, 45, 169–174. [Google Scholar] [CrossRef]
- Ishii, H. Nutritional Status as a Predictor of Clinical Prognosis in Patients with Peripheral Artery Disease. J. Atheroscler. Thromb. 2020, 27, 132–133. [Google Scholar] [CrossRef]
- Singh, S.; Bailey, K.R.; Noheria, A.; Kullo, I.J. Frailty across the spectrum of ankle-brachial index. Angiology 2012, 63, 229–236. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Yeh, R.W.; Kereiakes, D.J.; Cutlip, D.E.; Steg, P.G.; Massaro, J.M.; Apruzzese, P.K.; Mauri, L.; on behalf of the Dual Antiplatelet Therapy Study Investigators. Extended Duration Dual Antiplatelet Therapy After Coronary Stenting Among Patients with Peripheral Arterial Disease: A Subanalysis of the Dual Antiplatelet Therapy Study. JACC Cardiovasc. Interv. 2017, 10, 942–954. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Beiswenger, A.C.; Jo, A.; Harth, K.; Kumins, N.H.; Shishehbor, M.H.; Kashyap, V.S. A systematic review of the efficacy of aspirin monotherapy versus other antiplatelet therapy regimens in peripheral arterial disease. J. Vasc. Surg. 2018, 67, 1922–1932.e6. [Google Scholar] [CrossRef]
- Mant, J. Rivaroxaban plus aspirin, compared with aspirin alone, reduced cardiovascular events in patients with stable peripheral or carotid artery disease, but increased the risk of major bleeding. BMJ Evid. Based Med. 2018, 23, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, K.W.; Palmerini, T.; Stone, G.W.; Lee, M.S.; Colombo, A.; Chieffo, A.; Feres, F.; Abizaid, A.; Bhatt, D.L.; et al. Racial Differences in Ischaemia/Bleeding Risk Trade-Off during Anti-Platelet Therapy: Individual Patient Level Landmark Meta-Analysis from Seven RCTs. Thromb. Haemost. 2019, 119, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Miyata, H.; Ueda, I.; Masoudi, F.A.; Peterson, E.D.; Maekawa, Y.; Kawamura, A.; Fukuda, K.; Roe, M.T.; Rumsfeld, J.S. An international comparison of patients undergoing percutaneous coronary intervention: A collaborative study of the National Cardiovascular Data Registry (NCDR) and Japan Cardiovascular Database-Keio interhospital Cardiovascular Studies (JCD-KiCS). Am. Heart J. 2015, 170, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.-K.; Kim, H.-S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef]
- Yao, J.; Chen, K.; He, Z.; Chen, D. Impact of frailty on outcomes of elderly patients with atrial fibrillation: A systematic review and meta-analysis. Pak. J. Med. Sci. 2025, 41, 891–901. [Google Scholar] [CrossRef]
- Nitta, M.; Nakano, S.; Kaneko, M.; Fushimi, K.; Hibi, K.; Shimizu, S. In-Hospital Mortality in Patients with Cardiogenic Shock Requiring Veno-Arterial Extracorporeal Membrane Oxygenation with Concomitant Use of Impella vs. Intra-Aortic Balloon Pump—A Retrospective Cohort Study Using a Japanese Claims-Based Database. Circ. J. 2024, 88, 1276–1285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).