Systematic Review of the Antitumor Activities and Mechanisms of Scorpion Venom on Human Breast Cancer Cells Lines (In Vitro Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
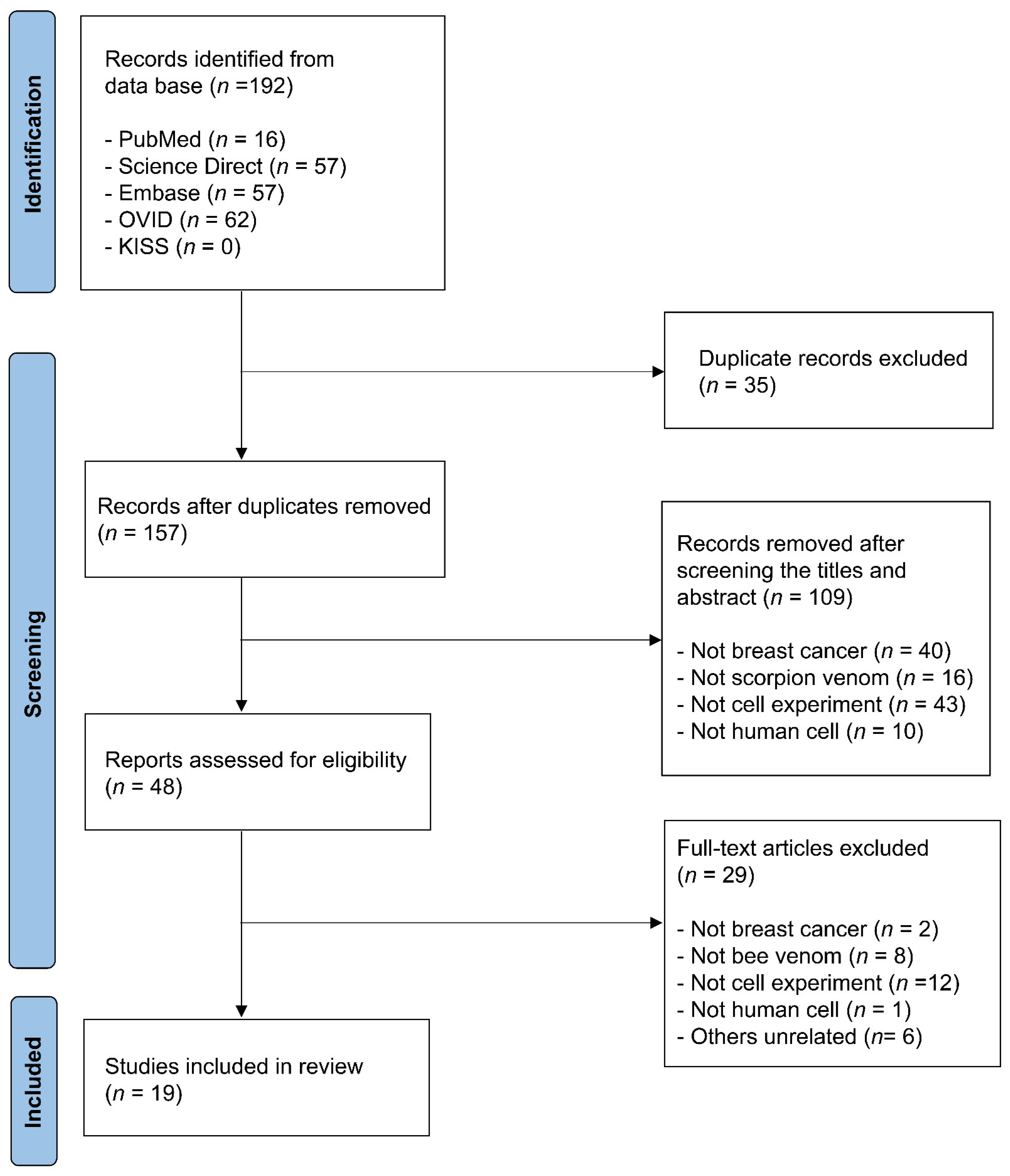
2.2. Study Selection
2.3. Data Extraction
3. Results
3.1. Analysis of Experimental Methods
3.2. Analysis of Experimental Results
3.2.1. Anticancer Activity
3.2.2. Anticancer Mechanism
- Apoptosis induction: Crude venom upregulates pro-apoptotic genes (e.g., Bax, Caspase-3) and downregulates anti-apoptotic genes (e.g., Bcl-2). DNA fragmentation, a hallmark of apoptosis, was consistently observed in venom-treated cells.
- Cell cycle regulation: Crude venom induces cell cycle arrest at multiple phases, including G0/G1, S, and G2/M, effectively inhibiting cancer cell proliferation and disrupting mitotic progression.
- Oxidative stress modulation: Crude venom alters oxidative stress markers such as NO and GSH, contributing to increased apoptosis and cellular damage in breast cancer cells.
- Signaling protein modulation: Crude venom influences key signaling proteins involved in cancer progression, including STAT3 and IL-6, thereby disrupting pathways essential for breast cancer cell growth and survival.
- Inhibition of cell proliferation: Crude venom reduces cell viability and suppresses mitotic activity, effectively inhibiting breast cancer cell proliferation.
- Apoptosis induction: Bioactive proteins such as chlorotoxin (CTX) and leptulipin enhance apoptotic processes by upregulating pro-apoptotic markers (e.g., FasL) and downregulating anti-apoptotic markers (e.g., Bcl-2). DNA fragmentation, a hallmark of apoptosis, was consistently observed in venom-treated cells, indicating effective initiation of programmed cell death. These findings highlight the ability of bioactive proteins to selectively induce apoptosis in breast cancer cells.
- Cell cycle regulation: Bioactive proteins induce cell cycle arrest at specific phases, such as G0/G1 and G2/M, by regulating cell cycle-related genes and proteins. This disruption inhibits cancer cell division and growth, emphasizing their potential to interfere with critical processes driving tumor proliferation.
- Inhibition of cell proliferation, migration, and invasion: Bioactive proteins reduce cancer cell proliferation by suppressing ERα, a key regulator in hormone-dependent breast cancer cells. Additionally, they inhibit cell migration and invasion by downregulating proteins such as MMP2 and VASP, essential for cellular motility and invasiveness. The disruption of these pathways underscores their therapeutic potential in limiting metastatic progression.
4. Discussion
4.1. Comparative Mechanistic Insights into Crude Venom and Bioactive Protein
4.2. Mechanistic Insights Based on Target Cells
4.2.1. Hormone Receptor-Positive Breast Cancer Cells (MCF-7, T47D)
4.2.2. Triple-Negative Breast Cancer Cells (MDA-MB-231, F3II)
4.2.3. HER2-Positive Breast Cancer Cells (SKBR3)
4.3. Limitations of the Study
4.4. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALDOA | Aldolas |
| CTX | Chlorotoxin |
| FaSL | Fas ligand |
| GSH | Glutathione |
| LDH | Lactate Dehydrogenase release assay |
| MeSH | Medical Subject Headings |
| MMP | Mitochondrial membrane potential |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide assay |
| NO | Nitrite oxide |
| NRU | Neutral Red Uptake assay |
| PKM | Pyruvate kinase |
| ROS | Reactive oxygen species |
| SERMs | Selective estrogen receptor modulators |
| SRB | Sulforhodamine B assay |
| XTT | 2,3-Bis(2-Methoxy-4-Nitro-5-Sulfophenyl)-5-[(Phenylamino)Carbonyl]-2H-Tetrazolium |
References
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.Y.; Sung, S.H.; Sung, H.K.; Park, J.K. Anticancer activity of bee venom components against breast cancer. Toxins 2022, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Eccles, S.A.; Aboagye, E.O.; Ali, S.; Anderson, A.S.; Armes, J.; Berditchevski, F.; Blaydes, J.P.; Brennan, K.; Brown, N.J.; Bryant, H.E.; et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013, 15, R92. [Google Scholar] [CrossRef]
- Arnold, M.; Karim-Kos, H.E.; Coebergh, J.W.; Byrnes, G.; Antilla, A.; Ferlay, J.; Renehan, A.G.; Forman, D.; Soerjomataram, I. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur. J. Cancer 2015, 51, 1164–1187. [Google Scholar] [CrossRef]
- Drigla, F.; Balacescu, O.; Visan, S.; Bisboaca, S.E.; Berindan-Neagoe, I.; Marghitas, L.A. Synergistic effects induced by combined treatments of aqueous extract of propolis and venom. Clujul Med. 2016, 89, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef]
- Eifel, P.; Axelson, J.A.; Costa, J.; Crowley, J.; Curran, W.J., Jr.; Deshler, A.; Fulton, S.; Hendricks, C.B.; Kemeny, M.; Kornblith, A.B.; et al. National Institutes of Health Consensus Development Conference Statement: Adjuvant therapy for breast cancer, November 1–3, 2000. J. Natl. Cancer Inst. 2001, 93, 979–989. [Google Scholar] [CrossRef]
- Daniluk, K.; Kutwin, M.; Grodzik, M.; Wierzbicki, M.; Strojny, B.; Szczepaniak, J.; Bałaban, J.; Sosnowska, M.; Chwalibog, A.; Sawosz, E.; et al. Use of selected carbon nanoparticles as melittin carriers for MCF-7 and MDA-MB-231 human breast cancer cells. Materials 2019, 13, 90. [Google Scholar] [CrossRef]
- Kshirsagar, A.S.; Wani, S.K. Health-related quality of life in patients with breast cancer surgery and undergoing chemotherapy in Ahmednagar district. J. Cancer Res. Ther. 2021, 17, 1335–1338. [Google Scholar] [CrossRef]
- Khamis, A.A.A.; Ali, E.M.M.; El-Moneim, M.A.A.; Abd-Alhaseeb, M.M.; El-Magd, M.A.; Salim, E.I. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharmacother. 2018, 105, 1335–1343. [Google Scholar] [CrossRef]
- Hwang, S.I.; Yoon, Y.J.; Sung, S.H.; Ha, K.T.; Park, J.K. Toxic animal-based medicinal materials can be effective in treating endometriosis: A scoping review. Toxins 2021, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B. Animal venoms have potential to treat cancer. Curr. Top. Med. Chem. 2018, 18, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.K. Use of animal venom peptides/toxins in cancer therapeutics. Curr. Trends Biomed. Eng. Biosci. 2018, 16, 555945. [Google Scholar] [CrossRef]
- Sarfo-Poku, C.; Eshun, O.; Lee, K.H. Medical application of scorpion venom to breast cancer: A mini-review. Toxicon 2016, 122, 109–112. [Google Scholar] [CrossRef]
- Heinen, T.E.; da Veiga, A.B.G. Arthropod venoms and cancer. Toxicon 2011, 57, 497–511. [Google Scholar] [CrossRef]
- Uzair, B.; Bint-e-Irshad, S.; Khan, B.A.; Azad, B.; Mahmood, T.; Rehman, M.U.; Braga, V.A. Scorpion venom peptides as a potential source for human drug candidates. Protein Pept. Lett. 2018, 25, 702–708. [Google Scholar] [CrossRef]
- Han, D.; Qi, H.; Huang, K.; Li, X.; Zhan, Q.; Zhao, J.; Hou, X.; Yang, X.; Kang, C.; Yuan, X. The effects of surface charge on the intra-tumor penetration of drug delivery vehicles with tumor progression. J. Mater. Chem. B 2018, 6, 3331–3339. [Google Scholar] [CrossRef]
- Nandhini, S.; Ilango, K. Development and characterization of a nano-drug delivery system containing vasaka phospholipid complex to improve bioavailability using quality by design approach. Res. Pharm. Sci. 2021, 16, 103–117. [Google Scholar] [CrossRef]
- Dueñas-Cuellar, R.A.; Santana, C.J.C.; Magalhães, A.C.M.; Pires, O.R., Jr.; Fontes, W.; Castro, M.S. Scorpion toxins and ion channels: Potential applications in cancer therapy. Toxins 2020, 12, 326. [Google Scholar] [CrossRef]
- Goudet, C.; Chi, C.W.; Tytgat, J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon 2002, 40, 1239–1258. [Google Scholar] [CrossRef]
- Ng, W.K.; Yazan, L.S.; Ismail, M. Thymoquinone from Nigella sativa was more potent than cisplatin in eliminating of SiHa cells via apoptosis with down-regulation of Bcl-2 protein. Toxicol. In Vitro 2011, 25, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Mikaelian, A.G.; Traboulay, E.; Zhang, X.M.; Yeritsyan, E.; Pedersen, P.L.; Ko, Y.H.; Matalka, K.Z. Pleiotropic anticancer properties of scorpion venom peptides: Rhopalurus princeps venom as an anticancer agent. Drug Des. Dev. Ther. 2020, 14, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer metabolism: Phenotype, signaling and therapeutic targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- D’Suze, G.; Rosales, A.; Salazar, V.; Sevcik, C. Apoptogenic peptides from Tityus discrepans scorpion venom acting against the SKBR3 breast cancer cell line. Toxicon 2010, 56, 1497–1505. [Google Scholar] [CrossRef]
- Erdeş, E.; Doğan, T.S.; Coşar, I.; Danışman, T.; Kunt, K.B.; Seker, T.; Yücel, M.; Ozen, C. Characterization of Leiurus abdullahbayrami (Scorpiones: Buthidae) venom: Peptide profile, cytotoxicity and antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 48. [Google Scholar] [CrossRef]
- Salama, W.M.; El-Nagga, S.A. Cytotoxic effect of Leurius quinquestratus (scorpion) venom in different human cancer cell lines in vitro. Trop. J. Pharm. Res. 2021, 20, 345–350. [Google Scholar] [CrossRef]
- Said, Y.M.; El-Gamel, N.E.A.; Ali, S.A.; Mohamed, A.F. Evaluation of human Wharton’s jelly-derived mesenchymal stem cells conditioning medium (hWJ-MSCs-CM) or scorpion venom breast cancer cell line in vitro. J. Gastrointest. Cancer 2022, 53, 888–901. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Islam, M.; Al-Zahrani, A.M. In vitro analysis of the anticancer properties of scorpion venom in colorectal and breast cancer cell lines. Oncol. Lett. 2016, 11, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; Shoukry, N.M.; Teleb, W.K.; Abdel-Daim, M.M.; Abdel-Rahman, M.A. In vitro and in vivo antitumor effects of the Egyptian scorpion Androctonus amoreuxi venom in an Ehrlich ascites tumor model. SpringerPlus 2016, 5, 570. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zhao, Y.; Yuan, J.; Mao, W. Inhibition effects of scorpion venom extracts (Buthus matensii Karsch) on the growth of human breast cancer MCF-7 cells. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 105–110. [Google Scholar] [CrossRef]
- Dezianian, S.; Zargan, J.; Goudarzi, H.R.; Haji Noormohamadi, A.; Mousavi, M.; Keshavarz Alikhani, H.; Johari, B. In-vitro study of Hottentotta schach crude venom anticancer effects on MCF-7 and Vero cell lines. Iran. J. Pharm. Res. 2020, 19, 192–202. [Google Scholar] [CrossRef]
- Díaz-García, A.; Ruiz-Fuentes, J.L.; Frión-Herrera, Y.; Yglesias-Rivera, A.; Garlobo, Y.R.; Sánchez, H.R.; Aurrecochea, J.C.R.; López Fuentes, L.X.L. Rhopalurus junceus scorpion venom induces antitumor effect in vitro and in vivo against a murine mammary adenocarcinoma model. Iran. J. Basic Med. Sci. 2019, 22, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, A.; Ruiz-Fuentes, J.L.; Rodríguez-Sánchez, H.; Fraga Castro, J.A.F. Rhopalurus junceus scorpion venom induces apoptosis in the triple negative human breast cancer cell line MDA-MB-231. J. Venom. Res. 2017, 8, 9–13. [Google Scholar] [PubMed]
- Al-Asmari, A.K.; Riyasdeen, A.; Abbasmanthiri, R.; Arshaduddin, M.; Al-Harthi, F.A. Scorpion (Androctonus bicolor) venom exhibits cytotoxicity and induces cell cycle arrest and apoptosis in breast and colorectal cancer cell lines. Indian. J. Pharmacol. 2016, 48, 537–543. [Google Scholar] [CrossRef]
- Zargan, J.; Sajad, M.; Umar, S.; Naime, M.; Ali, S.; Khan, H.A. Scorpion (Androctonus crassicauda) venom limits growth of transformed cells (SH-SY5Y and MCF-7) by cytotoxicity and cell cycle arrest. Exp. Mol. Pathol. 2011, 91, 447–454. [Google Scholar] [CrossRef]
- Zargan, J.; Umar, S.; Sajad, M.; Naime, M.; Ali, S.; Khan, H.A. Scorpion venom (Odontobuthus doriae) induces apoptosis by depolarization of mitochondria and reduces S-phase population in human breast cancer cells (MCF-7). Toxicol. In Vitro 2011, 25, 1748–1756. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion venom causes apoptosis by increasing reactive oxygen species and cell cycle arrest in MDA-MB-231 and HCT-8 cancer cell lines. J. Evid. Based Integr. Med. 2018, 23, 2156587217751796. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion venom causes upregulation of p53 and downregulation of Bcl-xL and BID protein expression by modulating signaling proteins ERK1/2 and STAT3, and DNA damage in breast and colorectal cancer cell lines. Integr. Cancer Ther. 2018, 17, 271–281. [Google Scholar] [CrossRef]
- Pedron, C.N.; Andrade, G.P.; Sato, R.H.; Torres, M.T.; Cerchiaro, G.; Ribeiro, A.O.; Oliveira, V.X., Jr. Anticancer activity of VmCT1 analogs against MCF-7 cells. Chem. Biol. Drug Des. 2018, 91, 588–596. [Google Scholar] [CrossRef]
- Rezaei, A.; Asgari, S.; Komijani, S.; Sadat, S.N.; Sabatier, J.M.; Nasrabadi, D.; Pooshang Bagheri, K.; Shahbazzadeh, D.; Akbari Eidgahi, M.R.; De Waard, M.; et al. Discovery of leptulipin, a new anticancer protein from the Iranian scorpion, Hemiscorpius Lepturus. Molecules 2022, 27, 2056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, K.; Han, S.; Tian, Y.H.; Hu, P.C.; Xu, X.L.; He, Y.Q.; Pan, W.T.; Gao, Y.; Zhang, Z.; et al. Chlorotoxin targets ERα/VASP signaling pathway to combat breast cancer. Cancer Med. 2019, 8, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gao, R.; Gopalakrishnakone, P. Isolation and characterization of a hyaluronidase from the venom of Chinese red scorpion Buthus martensi. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef]
- Song, X.; Zhang, G.; Sun, A.; Guo, J.; Tian, Z.; Wang, H.; Liu, Y. Scorpion venom component III inhibits cell proliferation by modulating NF-κB activation in human leukemia cells. Exp. Ther. Med. 2012, 4, 146–150. [Google Scholar] [CrossRef]
- Nosouhian, M.; Rastegari, A.A.; Shahanipour, K.; Ahadi, A.M.; Sajjadieh, M.S. Anticancer potentiality of Hottentotta saulcyi scorpion curd venom against breast cancer: An in vitro and in vivo study. Sci. Rep. 2024, 14, 24607. [Google Scholar] [CrossRef]
- Khalid, S.; Rehman, H.M.; Al-Qassab, Y.; Ahmad, I.; Fatima, T.; Mubasher, M.M.; Kalsoom, M.; Nadeem, T.; Bashir, H. Design and computational analysis of a novel Leptulipin-p28 fusion protein as a multitarget anticancer therapy in breast cancer. Toxicol. Res. 2024, 13, tfae174. [Google Scholar] [CrossRef]
- Seifi, R.; Ayat, H.; Ahadi, A.M. Design and construction of a chimeric peptide, MeICT/IMe-AGAP, from two anti-cancer toxins of Iranian Mesobuthus eupeus scorpion. Mol. Biol. Res. Commun. 2023, 12, 27–36. [Google Scholar] [CrossRef] [PubMed]
| Study ID | Author (Year) | Target Cells | Scorpion Species | Concentration | IC50 | Duration | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | D’Suze et al. (2010) [28] | SKBR3 | Tityus discrepans | 1, 5, 15, 30 µg/µL | n.r | 1, 2, 3, 4, 5, 6 h | 1. Cytotoxicity (MMT) | 1. Dose- and time-dependent increase |
| 2 | Erdeş et al. (2014) [29] | MCF-7 | Leiurus abdullahbayrami | 200 µg/mL | n.r | 24, 48 h | 1. Cytotoxicity (XTT) | 1. No effect |
| 3 | Salama et al. (2021) [30] | MCF-7 | Leiurus quinquestriatus | 0–50 µg/mL | 8.86 ± 0.7 µg/mL | 24 h | 1. Cytotoxicity (MMT) | 1. Dose-dependent increase |
| 4 | Said et al. (2022) [31] | MCF-7 | Leiurus quinquestriatus | 1–1000 µg/mL | 100 µg/mL | 24 h | 1. Cytotoxicity (MMT) | 1. Dose-dependent increase |
| 100 µg/mL | 1. Cell morphology | 1. Changed | ||||||
| 5 | Al-Asmari et al. (2016) [32] | MDA-MB-231 | Androctonus crassicauda (V1) | 50, 100 µg/mL | n.r | 24 h | 1. Cell motility 2. Colony formation | 1. Decrease (50 a/100 a) 2. Decrease (100 a) |
| Androctonus bicolor (V2) | 50, 100 µg/mL | n.r | 24 h | 1. Cell motility 2. Colony formation | 1. Decrease (50 a/100 a) 2. Decrease (100 a) | |||
| Leiurus quinquestriatus (V3) | 50, 100 µg/mL | n.r | 24 h | 1. Cell motility 2. Colony formation | 1. Decrease (50 a/100 a) 2. Decrease (50 a/100 a) | |||
| 6 | Salem et al. (2016) [33] | MCF-7 | Androctonus amoreuxi | 0.01, 0.1, 1, 10, 100 µg/mL | 0.61 µg/mL | 24, 48, 72 h | 1. Cytotoxicity (SRB) | 1. Increase |
| 7 | Li et al. (2014) [34] | MCF-7 | Buthus martensii Karsch | 100, 200, 400, 600, 800 µg/mL | n.r | 4, 12, 16, 24 h | 1. Cytotoxicity | 1. Dose- and time-dependent increase |
| 8 | Dezianian et al. (2020) [35] | MCF-7 | Hottentotta schach | 25, 50, 100, 200 µg/mL | n.r | 24 h | 1. Cytotoxicity (1) MTT (2) NRU | 1. (1) Increase (25 a/50 c/100 d/200 d) (2) Inhibited (25 a/50 e/100 e/200 e) |
| 100, 200 µg/mL | 1. Cell morphology | 1. Changed | ||||||
| 9 | Díaz-García et al. (2019) [36] | F3II cells | Rhopalurus junceus | 0.1, 0.5, 0.75, 1 mg/mL | 0.95 ± 0.17 mg/mL | 72 h | 1. Cytotoxicity (MTT) | 1. Increase (0.5 a/0.75 b/1 b) |
| 10 | Díaz-García et al. (2017) [37] | MDA-MB-231 | Rhopalurus junceus | 0.12, 0.25, 0.5, 0.75, 1 mg/mL | 0.75 mg/mL | 72 h | 1. Cytotoxicity (MTT) | 1. Increase (0.5 a/0.75 b/1 b) |
| 11 | Al-Asmari et al. (2016) [38] | MDA-MB-231 | Androctonus bicolor | 100–1000 µg/mL | 839 ± 8 µg/mL (24 h), 753 ± 6 µg/mL (48 h) | 24, 48 h | 1. Cytotoxicity (MTT) | 1. Dose- and time-dependent increase |
| 839 ± 8, 753 ± 6 µg/mL | 24, 48 h | 1. Cell morphology | 1. Changed a | |||||
| 12 | Zargan et al. (2011) (A) [39] | MCF-7 | Androctonus crassicauda | 10, 25, 50, 100, 200 μg/mL | 269 μg/mL | 24 h | 1. Cytotoxicity (1) MMT assay (2) LDH assay | 1. (1) Dose-dependent increase (20 a/200 c) (2) Significant release (50 b/100 c) |
| 50, 100 μg/mL | 1. Cell morphology | 1. Changed in a dose-dependent manner | ||||||
| 13 | Zargan et al. (2011) (B) [40] | MCF-7 | Odontobuthus doriae | 10, 25, 50, 100, 200 μg/mL | n.r | 24 h | 1. Cytotoxicity (1) MMT assay (2) LDH assay | 1. (1) Dose-dependent increase (10 b/20 c/50 c/100 c/200 c) (2) Significant release (10 a/100 c) |
| 50, 100 μg/mL | 1. Cell morphology | 1. Changed (50/100) | ||||||
| 14 | Al-Asmari et al. (2018) [41] | MDA-MB-231 | Androctonus crassicauda (V1) | 500–1000 μg/mL | 950 μg/mL 900 μg/mL | 24 h | 1. Cytotoxicity (MTT) | 1. Dose-dependent increase |
| Leiurus quinquestriatus (V2) | ||||||||
| 15 | Al-Asmari et al. [42] (2017) | MDA-MB-231 | Androctonus crassicauda (V1) | 80 μg/mL | n.r | 24, 48 h | 1. DNA damage | 1. Induced |
| Androctonus bicolor (V2) | ||||||||
| Leiurus quinquestriatus (V3) |
| Study ID | Author (Year) | Target Cells | Scorpion Species | Peptide | Concentration | IC50 | Duration | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|---|---|
| 16 | Pedron et al. (2018) [43] | MCF-7 | Vaejovis mexicanus smithi | VmCT1 | 0.09–50 µmol/L | n.r | 4, 24 h | 1. Cell viability | 1. Reduction (25 b/50 a at 4 h) |
| 1 | D’Suze et al. (2010) [28] | SKBR3 | Tityus discrepans | Neopladine 1,2 | 1, 5, 15, 30 µg/µL | n.r | 1, 2, 3, 4, 5, 6 h | 1. Cell morphology | 1. Changed |
| 2 | Erdeş et al. (2014) [29] | MCF-7 | Leiurus abdullahbayrami | Etoposide | 60 µM | n.r | 24, 48 h | 1. Cell viability | 1. Significant reduction (4 h b/48 h c) |
| 17 | Rezaei et al. (2022) [44] | MDA-MB-231 | Hemiscorpius Lepturus | Purified Leptulipin | 6.25, 12.5, 25, 50 µg/mL | 30 µg/mL | 24 h | 1. Cytotoxicity (1) MTT (2) LDH 2. Cell morphology | 1. (1) Increase (12.5 b/25 c/50 c) (2) Increase (25 c/50 d) 2. Changed |
| Study ID | Author, Year | Target Cells | Scorpion Species | Concentration | IC50 | Duration | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | D’Suze et al. (2010) [28] | SKBR3 | Tityus discrepans | 1, 5, 15, 30 µg/µL | n.r | 1, 2, 3, 4, 5, 6 h | 1. Pro-apoptotic protein 2. Anti-apoptotic gene | 1. FasL: Upregulated (n.r) 2. Bcl-2: Downregulated (n.r) |
| 4 | Said et al. (2022) [31] | MCF-7 | Leiurus quinquestriatus | 100 µg/mL | 100 µg/mL | 24 h | 1. Apoptosis 2. Cell cycle: S phase 3. Gene expression (1) Pro-apoptotic genes (2) Anti-apoptotic genes | 1. Significant elevation a 2. Significant elevation a 3. (1) Bax, Caspase-3, and Caspase-9: Upregulated a (2) Bcl-2, ALDOA, PKM: Downregulated a |
| 6 | Salem et al. (2016) [33] | MCF-7 | Androctonus amoreuxi | 0.5 µg/mL | 0.61 µg/mL | 24 h | 1. DNA fragmentation | 1. Induced |
| 7 | Li et al. (2014) [34] | MCF-7 | Buthus martensii Karsch | 600 µg/mL | n.r | 24 h | 1. Gene expression (1) Pro-apoptotic genes (2) Anti-apoptotic genes 2. Cell cycle (1) G0/G1 phase (2) G2/M phase (3) S phase 4) Cell cycle related protein | 1. (1) Caspase-3: Upregulated (n.r) (2) Bcl-2: Downregulated (n.r) 2. (1) Significant elevationa (2) No significant change (3) Significant reduction (4) Cyclin D1: Decrease (n.r) |
| 8 | Dezianian et al. (2020) [35] | MCF-7 | Hottentotta schach | 25, 50, 100, 200 µg/mL | n.r | 24 h | 1. Oxidative stress (1) NO (2) Catalase enzyme activity (3) GSH content 2. Apoptosis 3. Pro-apoptotic gene | 1. (1) Increase (25 a/50 c/100 d/200 d) (2) Decrease (25 c/50 d/100 d/200 d) (3) Decrease (25 d/50 d/100 d/200 d) 2. Significant elevation (50 d/100 d/200 d) 3. Capase-3: Upregulated (25 c/50 d/100 d/200 d) |
| 9 | Díaz-García et al. (2019) [36] | F3II cells | Rhopalurus junceus | 0.5 mg/mL | 0.95 ± 0.17 mg/mL | 24, 48 h | 1. Apoptosis 2. Gene expression (1) Pro-apoptotic genes (2) Anti-apoptotic genes | 1. Significant elevation: Early stage b, late stage a 2. (1) Upregulated: p53 (24 h b/48 h c), bax (48 h c), Caspase-3 (48 h c) (2) Downregulated: bcl-2 (24 h a/48 h c) |
| 10 | Díaz-García et al. (2017) [37] | MDA-MB-231 | Rhopalurus junceus | 0.375 mg/mL | 0.75 mg/mL | 48 h | 1. Apoptosis | 1. Significant elevation b |
| 0.375 mg/mL | 24, 48 h | 1. Gene expression (1) Pro-apoptotic genes (2) Anti-apoptotic genes | 1. (1) Upregulated: p53 (24 h a/48 h c), Bax (24 h b/48 h b), Puma (24 h b/48 h c), Noxa (24 h b/48 h c), Caspase-3 (24 h a/48 h a), p21 (24 h a/48 h a) (2) Downregulated a: Bcl-2 (48 h c), Bcl-xL (24 h a/48 h c) | |||||
| 11 | Al-Asmari et al. (2016) [38] | MDA-MB-231 | Androctonus bicolor | 839 ± 8, 753 ± 6 µg/mL | 839 ± 8 µg/mL (24 h), 753 ± 6 µg/mL (48 h) | 24, 48 h | 1. Cell cycle: G0/G1 phase | 1. Significant elevation (24 h a/48 h a) |
| 11 | Zargan et al. (2011) (A) [39] | MCF-7 | Androctonus crassicauda | 50, 100 μg/mL | 269 μg/mL | 24 h | 1. MMP 2. Oxidative stress: NO 3. Gene expression: Caspase-3 4. DNA fragmentation 5. Cell proliferation | 1. Significant increase (100 b) 2. Significant increase (100 b) 3. Significant increase (50 b, 100 b) 4. Induced (50) 5. Inhibited (50 b/100 c) |
| 13 | Zargan et al. (2011) (B) [40] | MCF-7 | Odontobuthus doriae | 50, 100 μg/mL | n.r | 24 h | 1. MMP 2. Oxidative stress (1) NO (2) GSH 3. Gene expression: Caspase-3 4. DNA fragmentation 5. Cell proliferation | 1. Significant increase (50 a/100 c) 2. (1) Significant increase (50 b/100 c) (2) Significant decrease (50 c/100 c) 3. Significant increases (50 a/100 c) 4. Induced (50/100) 5. Inhibited (50 a/100 c) |
| 14 | Al-Asmari et al. (2018) [41] | MDA-MB-231 | Androctonus crassicauda (V1) | 950 μg/mL | 950 μg/mL | 24, 48 h | 1. ROS 2. Cell cycle: G2/M phase 3. Morphological assessment | 1. Significant increase (24 h a) 2. Significant elevation (24 h a/48 h a) 3. Significant change (24 h a/48 h a) |
| Leiurus quinquestriatus (V2) | 900 μg/mL | 900 μg/mL | ||||||
| 15 | Al-Asmari et al. (2017) [42] | MDA-MB-231 | Androctonus crassicauda (V1) | 80 μg/mL | n.r | 24, 48 h | 1. DNA damage | 1. Induced |
| 22 h | 1. Cell invasion | 1. Inhibited a | ||||||
| 12 h | 1. Gene expression (1) Pro-apoptotic genes (2) Anti-apoptotic genes 2. Signaling protein 3. Cytokine | 1. (1) p53: Upregulateda (2) Bcl-xL, BID: Downregulated a 2. STAT3, RhoC: Inhibited a, Erk1/2: n.s 3. IL-6: Decreaseda | ||||||
| Androctonus bicolor (V2) | 24, 48 h | 1. DNA damage | 1. Induced | |||||
| 22 h | 1. Cell invasion | 1. Inhibiteda | ||||||
| 12 h | 1. Gene expression (1) Pro-apoptotic genes (2) Anti-apoptotic genes 2. Signaling protein 3. Cytokine | 1. (1) p53: Upregulated a (2) Bcl-xL, BID: Downregulated a 2. STAT3, RhoC, Erk1/2: Inhibited a 3. IL-6: Decreased a | ||||||
| Leiurus quinquestriatus (V3) | 24, 48 h | 1. DNA damage | 1. Induced | |||||
| 22 h | 1. Cell invasion | 1. Inhibited a | ||||||
| 12 h | 1. Gene expression (1) Pro-apoptotic genes (2) Anti-apoptotic genes 2. Signaling protein 3. Cytokine | 1. (1) p53: Upregulated a (2) Bcl-xL, BID: Downregulated a 2. STAT3, RhoC, Erk1/2: Inhibited a 3. IL-6: Decreased (n.s) |
| Study ID | Author, Year | Target Cells | Scorpion Species | Proteins | Concentration | IC50 | Duration | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|---|---|
| 1 | D’Suze et al. (2010) [28] | SKBR3 | Tityus discrepans | Neopladine 1,2 | 1, 5, 15, 30 µg/µL | n.r | 1, 2, 3, 4, 5, 6 h | 1. Apoptosis 2. Pro-apoptotic protein 3. Anti-apoptotic gene | 1. Elevation 2. FasL: Upregulated (n.r) 3. Bcl-2: Downregulated (n.r) |
| 18 | Wang et al. (2019) [45] | MCF-7 | Leiurus quinquestriatus | Chlorotoxin | 0.05, 0.5, 5 µmol/L | - | 12, 24, 48, 72 h | 1. Cell proliferation 2. Cell migration and invasion 3. ERα 4. MMP2 5. VASP | 1. Dose and time dependently inhibited a 2. Dose-dependently inhibited a at 24 h 3. Inhibited a 4. Inhibited a 5. Inhibited a |
| MDA-MB-231 | 1. Cell proliferation 2. Cell migration and invasion 3. ERα 4. MMP2 5. VASP | 1. Dose and time dependently inhibited a 2. Dose-dependently inhibited a at 24 h 3. Inhibited a 4. Inhibited a 5. Inhibited a | |||||||
| T47D | 1. Cell proliferation 2. Cell migration and invasion 3. ERα 4. MMP2 5. VASP | 1. Dose and time dependently inhibited a 2. Dose-dependently inhibited a at 36 h 3. Inhibited a 4. Inhibited a 5. Inhibited a | |||||||
| 17 | Rezaei et al. (2022) [44] | MDA-MB-231 | Hemiscorpius lepturus | Purified Leptulipin | 6.25, 12.5, 25, 50 µg/mL | 30 µg/mL | 24 h | 1. Cell cycle (1) G0/G1 phase (2) G2/M phase 2. Gene expression (1) Pro-apoptotic genes (2) Anti-apoptotic genes 3. DNA fragmentation | 1. (1) Increase c (2) Decrease b 2. (1) Upregulated: Bax d, Caspase-9 d (2) Downregulated: Bcl-2 c 3. Induced at 25 µg/mL |
| 19 | Feng et al. (2008) [46] | MDA-MB-231 | Buthus martensi karsch | BmHYA1 | 100U | - | 20 h | 1. Hyaluronan | 1. Reduction b |
| 12, 24, 48 h | 1. CD44v6 expression | 1. Downregulation after 48 h a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, N.-Y.; Sung, H.-K.; Park, J.-K. Systematic Review of the Antitumor Activities and Mechanisms of Scorpion Venom on Human Breast Cancer Cells Lines (In Vitro Study). J. Clin. Med. 2025, 14, 3181. https://doi.org/10.3390/jcm14093181
Kwon N-Y, Sung H-K, Park J-K. Systematic Review of the Antitumor Activities and Mechanisms of Scorpion Venom on Human Breast Cancer Cells Lines (In Vitro Study). Journal of Clinical Medicine. 2025; 14(9):3181. https://doi.org/10.3390/jcm14093181
Chicago/Turabian StyleKwon, Na-Yoen, Hyun-Kyung Sung, and Jang-Kyung Park. 2025. "Systematic Review of the Antitumor Activities and Mechanisms of Scorpion Venom on Human Breast Cancer Cells Lines (In Vitro Study)" Journal of Clinical Medicine 14, no. 9: 3181. https://doi.org/10.3390/jcm14093181
APA StyleKwon, N.-Y., Sung, H.-K., & Park, J.-K. (2025). Systematic Review of the Antitumor Activities and Mechanisms of Scorpion Venom on Human Breast Cancer Cells Lines (In Vitro Study). Journal of Clinical Medicine, 14(9), 3181. https://doi.org/10.3390/jcm14093181






