Assessment of Serum Cytokine Levels in Keratoconus Patients
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design and Settings
2.2. Participant Selection and Categorization
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Laboratory Investigations
2.3.1. Blood Sample Collection and Processing
2.3.2. Cytokines Measurement
2.4. Sample Size
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bui, A.D.; Truong, A.; Pasricha, N.D.; Indaram, M. Keratoconus diagnosis and treatment: Recent advances and future directions. Clin. Ophthalmol. 2023, 17, 2705–2718. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Cont. Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Fan, L.; Kandel, H.; Watson, S.L. Impacts of keratoconus on quality of life: A qualitative study. Eye 2024, 38, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.E.; Burdon, K.P. Genetic and environmental risk factors for keratoconus. Annu. Rev. Vis. Sci. 2020, 6, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fang, Q.Y.; Seth, I.; Baird, P.N.; Daniell, M.D.; Sahebjada, S. Non-genetic risk factors for keratoconus. Clin. Exp. Optom. 2023, 106, 362–372. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S.; Galvis, V.; Tello, A.; Rueda, D.; García, J.D. Genetics vs. chronic corneal mechanical trauma in the etiology of keratoconus. Exp. Eye Res. 2021, 202, 108328. [Google Scholar] [CrossRef]
- Crawford, A.Z.; Zhang, J.; Gokul, A.; McGhee, C.N.; Ormonde, S.E. The enigma of environmental factors in keratoconus. Asia Pac. J. Ophthalmol. 2020, 9, 549–556. [Google Scholar] [CrossRef]
- Navel, V.; Malecaze, J.; Pereira, B.; Baker, J.S.; Malecaze, F.; Sapin, V.; Chiambaretta, F.; Dutheil, F. Oxidative and antioxidative stress markers in keratoconus: A systematic review and meta-analysis. Acta Ophthalmol. 2021, 99, e777–e794. [Google Scholar] [CrossRef]
- Toprak, I.; Kucukatay, V.; Yildirim, C.; Kilic-Toprak, E.; Kilic-Erkek, O. Increased systemic oxidative stress in patients with keratoconus. Eye 2014, 28, 285–289. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, X.; Liu, Y.; Wang, P.; Li, X. Tear levels of inflammatory cytokines in keratoconus: A meta-analysis of case-control and cross-sectional studies. BioMed Res. Int. 2021, 2021, 6628923. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y. Topography-guided corneal surface laser ablation combined with simultaneous accelerated corneal collagen cross-linking for treatment of keratoconus. BMC Ophthalmol. 2021, 21, 286. [Google Scholar] [CrossRef] [PubMed]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Taheri, N.; Ahoor, M.H. Tear film inflammatory mediators in patients with keratoconus. Int. Ophthalmol. 2015, 35, 467–472. [Google Scholar] [CrossRef]
- Oltulu, R.; Katipoğlu, Z.; Gündoğan, A.O.; Mirza, E.; Belviranlı, S. Evaluation of inflammatory biomarkers in patients with keratoconus. Eur. J. Ophthalmol. 2022, 32, 154–159. [Google Scholar] [CrossRef]
- Nichani, P.A.; Solomon, B.; Trinh, T.; Mimouni, M.; Rootman, D.; Singal, N.; Chan, C.C. Investigating the role of inflammation in keratoconus: A retrospective analysis of 551 eyes. Eur. J. Ophthalmol. 2023, 33, 35–43. [Google Scholar] [CrossRef]
- Wisse, R.P.; Kuiper, J.J.; Gans, R.; Imhof, S.; Radstake, T.R.; Van der Lelij, A. Cytokine expression in keratoconus and its corneal microenvironment: A systematic review. Ocul. Surf. 2015, 13, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Taurone, S.; Ralli, M.; Plateroti, A.; Scorcia, V.; Greco, A.; Nebbioso, M.; Soda, G.; Artico, M.; Familiari, P.; Papa, V. Keratoconus: The possible involvement of inflammatory cytokines in its pathogenesis. An experimental study and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4478–4489. [Google Scholar] [PubMed]
- Khaled, M.L.; Helwa, I.; Drewry, M.; Seremwe, M.; Estes, A.; Liu, Y. Molecular and histopathological changes associated with keratoconus. BioMed Res. Int. 2017, 2017, 7803029. [Google Scholar] [CrossRef]
- Deshmukh, R.; Das, A.V.; Vaddavalli, P.K. Blinding associations of keratoconus. Indian J. Ophthalmol. 2023, 71, 1050–1051. [Google Scholar] [CrossRef]
- Mahmoud, M.M.I.; Hamdy, A.M.; Mohamed, A.B.i.; Diaa El Din, Y.A. An updated overview of keratoconus management. Egypt. J. Hosp. Med. 2022, 88, 2777–2780. [Google Scholar] [CrossRef]
- Gordon-Shaag, A.; Millodot, M.; Shneor, E.; Liu, Y. The genetic and environmental factors for keratoconus. BioMed Res. Int. 2015, 2015, 795738. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. Evolving concepts in etiopathogenesis of keratoconus: Is it quasi-inflammatory or inflammatory? Indian J. Ophthalmol. 2023, 71, 2609–2610. [Google Scholar] [CrossRef] [PubMed]
- Lema, I.; Durán, J.A. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology 2005, 112, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Ghosh, A.; Lim, R.R.; Subramani, M.; Mihir, K.; Ranganath, A.; Nagaraj, S.; Nuijts, R.M.; Beuerman, R.; Shetty, R. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest. Ophthalmol. Vis. Sci. 2015, 56, 738–750. [Google Scholar] [CrossRef]
- Shetty, R.; Deshmukh, R.; Ghosh, A.; Sethu, S.; Jayadev, C. Altered tear inflammatory profile in Indian keratoconus patients-The 2015 Col Rangachari Award paper. Indian J. Ophthalmol. 2017, 65, 1105–1108. [Google Scholar]
- Peyman, A.; Namgar, M.; Feizi, A.; Hakemi, M.G.; Nasab, F.H.; Pourazizi, M. Interleukin-6 and tumor necrosis factor-α levels in tear film of Keratoconus patients. J. Res. Med. Sci. 2021, 26, 75. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.; Nair, A.P.; Sahu, G.R.; Vaidya, T.; Shetty, R.; Khamar, P.; Mullick, R.; Gupta, S.; Dickman, M.M.; Nuijts, R.M. Keratoconus patients exhibit a distinct ocular surface immune cell and inflammatory profile. Sci. Rep. 2021, 11, 20891. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: Relevance in keratoconus. Clin. Exp. Optom. 2013, 96, 214–218. [Google Scholar] [CrossRef]
- Pinheiro-Costa, J.; Lima Fontes, M.; Luís, C.; Martins, S.; Soares, R.; Madeira, D.; Falcão-Reis, F.; Carneiro, Â. Serum inflammatory biomarkers are associated with increased choroidal thickness in keratoconus. Sci. Rep. 2023, 13, 10862. [Google Scholar] [CrossRef]
- Krok, M.; Wróblewska-Czajka, E.; Łach-Wojnarowicz, O.; Bronikowska, J.; Czuba, Z.P.; Wylęgała, E.; Dobrowolski, D. Analysis of cytokine and chemokine level in tear film in keratoconus patients before and after corneal cross-linking (CXL) treatment. Int. J. Mol. Sci. 2024, 25, 1052. [Google Scholar] [CrossRef]
- Jaskiewicz, K.; Maleszka-Kurpiel, M.; Michalski, A.; Ploski, R.; Rydzanicz, M.; Gajecka, M. Non-allergic eye rubbing is a major behavioral risk factor for keratoconus. PLoS ONE 2023, 18, e0284454. [Google Scholar] [CrossRef]
- García-Onrubia, L.; Mateos Olivares, M.; García-Vázquez, C.; Enríquez-de-Salamanca, A.; Cocho, L.; Herreras Cantalapiedra, J.M. Tear and plasma levels of cytokines in patients with uveitis: Search for active disease biomarkers. J. Clin. Med. 2022, 11, 7034. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Healthy (n = 20) | Progressive KC (n = 20) | Stable KC (n = 20) | p Value | |
|---|---|---|---|---|---|
| Age | 23.4 ± 4.18 | 22.15 ± 3.34 | 25.4 ± 3.97 | 0.033 | |
| Gender | |||||
| Male | 10 (50%) | 10 (50%) | 10 (50%) | 1 | |
| Female | 10 (50%) | 10 (50%) | 10 (50%) | ||
| Body mass index (kg/m2) | 25.69 ± 4.46 | 24.75 ± 3.62 | 28.20 ± 7.84 | 0.143 | |
| Smoking | 9 (45%) | 12 (63%) | 15 (75%) | 0.144 | |
| Occupation | |||||
| Employed | 10 (50%) | 4 (20%) | 8 (40%) | 0.134 | |
| Self-employed | 0 (0%) | 1 (5.0%) | 1 (5.0%) | ||
| Student | 10 (50%) | 11 (55%) | 6(30%) | ||
| Unemployed | 0 (0%) | 4 (20%) | 5(25%) | ||
| Healthy (n = 20) | Progressive KC (n = 20) | Stable KC (n = 20) | p Value | |
|---|---|---|---|---|
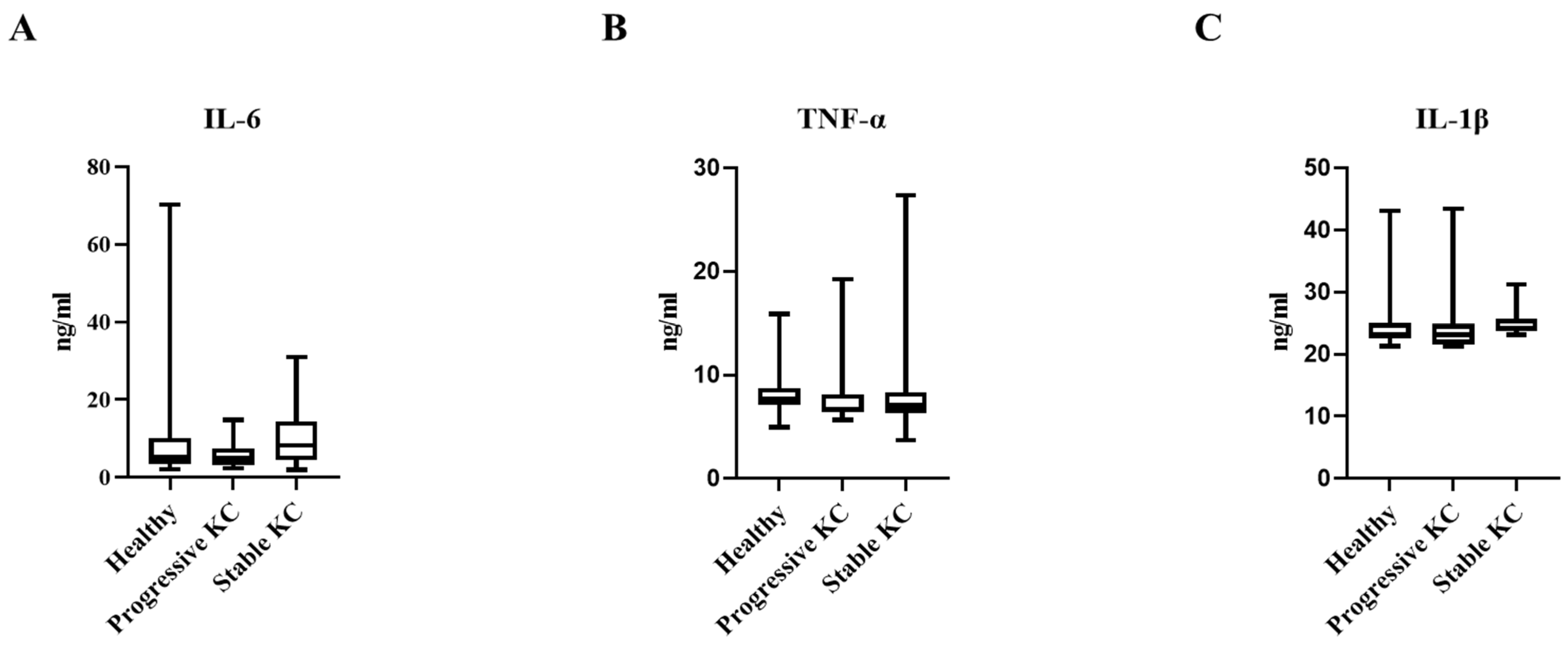
| IL-6 (ng/mL) | 5.12 (3.85–10.16) | 4.90 (3.46–6.9) | 8.18 (4.54–13.26) | 0.294 |
| TNF-α (ng/mL) | 8.22 ± 2.55 | 7.93 ± 3.17 | 8.57 ± 5.29 | 0.872 |
| IL-1B (ng/mL) | 24.78 ± 4.99 | 24.58 ± 4.99 | 25.18 ± 2.32 | 0.903 |
| Severity Category | IL-6 Level (ng/mL) | IL-1β Level (ng/mL) | TNF-α Level (ng/mL) | p Value |
|---|---|---|---|---|
| Mild | 9.02 ± 7.64 | 24.94 ± 4.42 | 8.90 ± 5.25 | 0.593 |
| Moderate | 7 ± 5.80 | 24.96 ± 2.83 | 6.94 ± 0.82 | 0.941 |
| Severe | 6.34 ± 3.33 | 24.25 ± 2.23 | 7.32 ± 0.69 | 0.546 |
| Cytokine | Spearman’s Correlation | p Value |
|---|---|---|
| IL-6 | −0.108 | 0.504 |
| TNF-α | −0.082 | 0.613 |
| IL-1β | 0.081 | 0.615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqudah, N.; Al-Azzam, N.; El Taani, L.; Sharayah, A.; Al Qudah, M.; Mhedat, K.; Tahat, S. Assessment of Serum Cytokine Levels in Keratoconus Patients. J. Clin. Med. 2025, 14, 3179. https://doi.org/10.3390/jcm14093179
Alqudah N, Al-Azzam N, El Taani L, Sharayah A, Al Qudah M, Mhedat K, Tahat S. Assessment of Serum Cytokine Levels in Keratoconus Patients. Journal of Clinical Medicine. 2025; 14(9):3179. https://doi.org/10.3390/jcm14093179
Chicago/Turabian StyleAlqudah, Noor, Nosayba Al-Azzam, Leen El Taani, Abdallah Sharayah, Mohammad Al Qudah, Khawlah Mhedat, and Suha Tahat. 2025. "Assessment of Serum Cytokine Levels in Keratoconus Patients" Journal of Clinical Medicine 14, no. 9: 3179. https://doi.org/10.3390/jcm14093179
APA StyleAlqudah, N., Al-Azzam, N., El Taani, L., Sharayah, A., Al Qudah, M., Mhedat, K., & Tahat, S. (2025). Assessment of Serum Cytokine Levels in Keratoconus Patients. Journal of Clinical Medicine, 14(9), 3179. https://doi.org/10.3390/jcm14093179






