Impact of Frailty on Surgical Outcomes in Nonacute Subdural Hematomas: A Nationwide Analysis of 251,597 Patients over 20 Years
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Demographics and Characteristics
3.2. Comorbidities and Healthcare Utilization
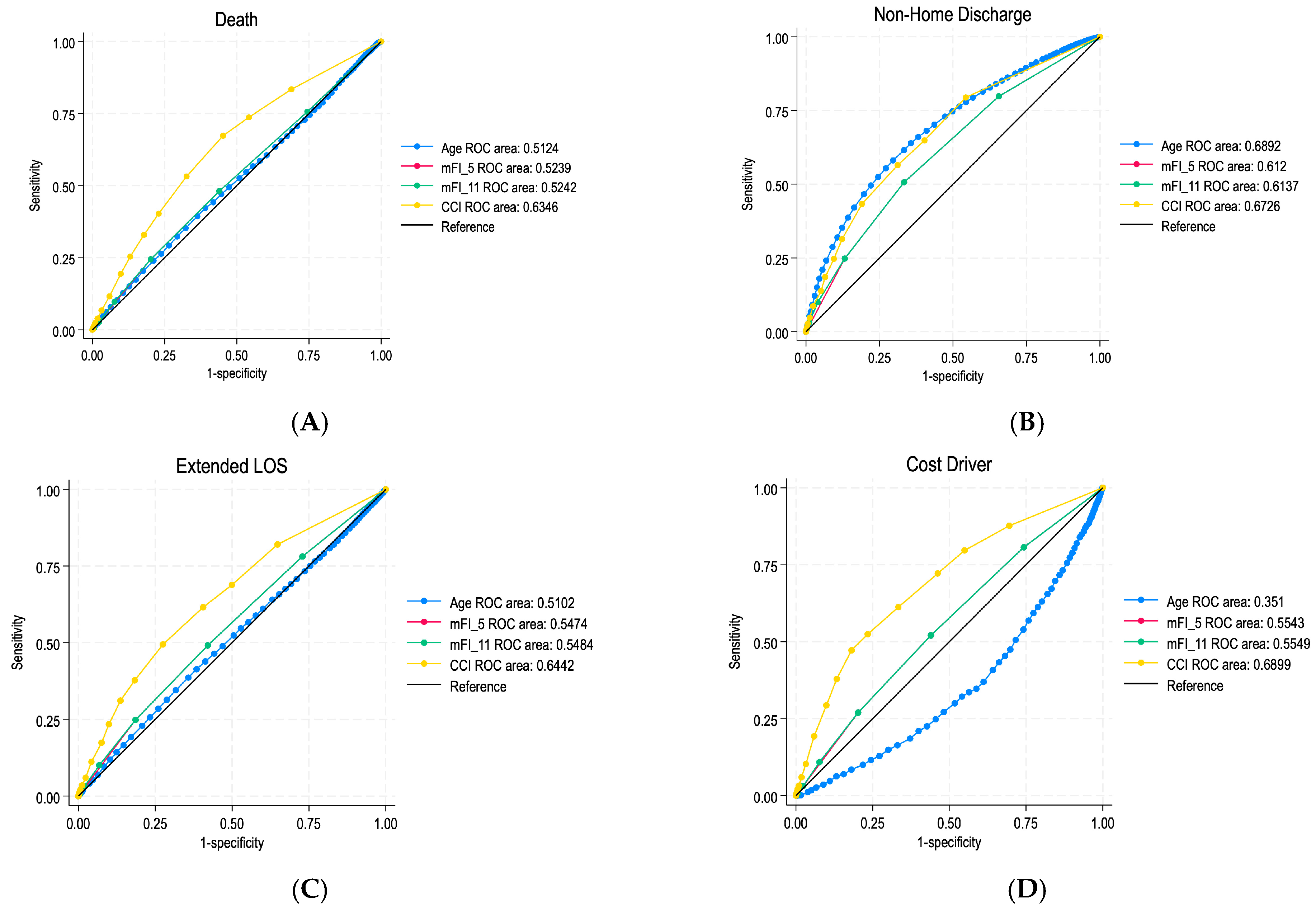
3.3. ROC Curve Predictive Value of Frailty Indices
3.4. Multivariable Analysis of Predictive Value of Frailty Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| naSDH | Nonacute Subdural Hematoma |
| mFI | Modified Frailty Index |
| mFI-5 | 5-Factor Modified Frailty Index |
| mFI-11 | 11-Factor Modified Frailty Index |
| CCI | Charlson Comorbidity Index |
| LOS | Length of Stay |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| ICD | International Classification of Diseases |
| NIS | Nationwide Inpatient Sample |
| ICD-10-CM | International Classification of Diseases, Tenth Revision, Clinical Modification |
| ICD-10-PCS | International Classification of Diseases, Tenth Revision, Procedure Coding System |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| OR | Odds Ratio |
| CI | Confidence Interval |
| IRB | Institutional Review Board |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| HCUP | Healthcare Cost and Utilization Project |
| SD | Standard Deviation |
References
- Joyce, E.; Bounajem, M.T.; Scoville, J.; Thomas, A.J.; Ogilvy, C.S.; Riina, H.A.; Tanweer, O.; Levy, E.I.; Spiotta, A.M.; Gross, B.A.; et al. Middle meningeal artery embolization treatment of nonacute subdural hematomas in the elderly: A multiinstitutional experience of 151 cases. Neurosurg. Focus 2020, 49, E5. [Google Scholar] [CrossRef] [PubMed]
- Rauhala, M.; Luoto, T.M.; Huhtala, H.; Iverson, G.L.; Niskakangas, T.; Öhman, J.; Helén, P. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J. Neurosurg. 2020, 132, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Ydreos, I.; Gavra, M.; Papadopoulos, E.K.; Banos, S.; Boviatsis, E.J.; Savvanis, G.; Stavrinou, L.C. Controversies in the Surgical Treatment of Chronic Subdural Hematoma: A Systematic Scoping Review. Diagnostics 2022, 12, 2060. [Google Scholar] [CrossRef]
- Sahyouni, R.; Goshtasbi, K.; Mahmoodi, A.; Tran, D.K.; Chen, J.W. Chronic Subdural Hematoma: A Historical and Clinical Perspective. World Neurosurg. 2017, 108, 948–953. [Google Scholar] [CrossRef]
- Mehta, V.; Harward, S.C.; Sankey, E.W.; Nayar, G.; Codd, P.J. Evidence based diagnosis and management of chronic subdural hematoma: A review of the literature. J. Clin. Neurosci. 2018, 50, 7–15. [Google Scholar] [CrossRef]
- Soleman, J.; Taussky, P.; Fandino, J.; Muroi, C. Evidence-Based Treatment of Chronic Subdural Hematoma. In Traumatic Brain Injury; Sadaka, F., Ed.; InTech: Houston, TX, USA, 2014. [Google Scholar] [CrossRef]
- Ducruet, A.F.; Grobelny, B.T.; Zacharia, B.E.; Hickman, Z.L.; DeRosa, P.L.; Anderson, K.; Sussman, E.; Carpenter, A.; Connolly, E.S. The surgical management of chronic subdural hematoma; discussion 169. Neurosurg. Rev. 2012, 35, 155–169. [Google Scholar] [CrossRef]
- Shimizu, K.; Sadatomo, T.; Hara, T.; Onishi, S.; Yuki, K.; Kurisu, K. Importance of frailty evaluation in the prediction of the prognosis of patients with chronic subdural hematoma. Geriatr. Gerontol. Int. 2018, 18, 1173–1176. [Google Scholar] [CrossRef]
- Sastry, R.A.; Pertsch, N.; Tang, O.; Shao, B.; Toms, S.A.; Weil, R.J. Frailty and Outcomes after Craniotomy or Craniectomy for Atraumatic Chronic Subdural Hematoma. World Neurosurg. 2021, 145, e242–e251. [Google Scholar] [CrossRef]
- Kesserwan, M.; Bergin, B.; Trivedi, A.; Shakil, H.; Martyniuk, A.; Takroni, R.; Kasper, E.; Engels, P.; Farrokhyar, F.; Sharma, S. Assessment of Frailty in Predicting Surgical Outcomes in Patients with Chronic Subdural Hematomas: Retrospective Chart Review. World Neurosurg. 2021, 146, e168–e174. [Google Scholar] [CrossRef]
- Blaauw, J.; Jacobs, B.; Hertog, H.M.D.; van der Gaag, N.A.; Jellema, K.; Dammers, R.; Kho, K.H.; Groen, R.J.M.; van der Naalt, J.; Lingsma, H.F. Mortality after chronic subdural hematoma is associated with frailty. Acta Neurochir. 2022, 164, 3133–3141. [Google Scholar] [CrossRef]
- Tracy, B.M.; Adams, M.A.; Schenker, M.L.; Gelbard, R.B. The 5 and 11 Factor Modified Frailty Indices are Equally Effective at Outcome Prediction Using TQIP. J. Surg. Res. 2020, 255, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar] [PubMed]
- Knopman, J.; Link, T.W.; Navi, B.B.; Murthy, S.B.; Merkler, A.E.; Kamel, H. Rates of Repeated Operation for Isolated Subdural Hematoma Among Older Adults. JAMA Netw. Open 2018, 1, e183737. [Google Scholar] [CrossRef]
- Jaffe, E.S.M.; Blattner, W.A.M.; Blayney, D.W.; Bunn, P.A.J.M.; Cossman, J.M.; Robert-Guroff, M.; Gallo, R.C.M. The pathologic spectrum of adult T-cell leukemia/lymphoma in the United States. Human T-cell leukemia/lymphoma virus-associated lymphoid malignancies. Am. J. Surg. Pathol. 1984, 8, 263–276. [Google Scholar] [CrossRef]
- McGraw, S.A.; Stone, E.J.; Osganian, S.K.; Elder, J.P.; Perry, C.L.; Johnson, C.C.; Parcel, G.S.; Webber, L.S.; Luepker, R.V. Design of Process Evaluation Within the Child and Adolescent Trial for Cardiovascular Health (CATCH). Health Educ. Q 1994, 21, S5–S26. [Google Scholar] [CrossRef]
- Sharma, M.; Dietz, N.; John, K.; Aljuboori, Z.; Wang, D.; Ugiliweneza, B.; Boakye, M.; Drazin, D. Impact of Surgical Approaches on Complications, Emergency Room Admissions, and Health Care Utilization in Patients Undergoing Lumbar Fusions for Degenerative Disc Diseases: A MarketScan Database Analysis. World Neurosurg. 2021, 145, e305–e319. [Google Scholar] [CrossRef]
- Tang, O.Y.; Bajaj, A.I.; Zhao, K.; Liu, J.K. Patient frailty association with cerebral arteriovenous malformation microsurgical outcomes and development of custom risk stratification score: An analysis of 16,721 nationwide admissions. Neurosurg. Focus 2022, 53, E14. [Google Scholar] [CrossRef]
- Davis, M.S.; Schmidt, C.J. The obnoxious and the nice: Some sociological consequences of two psychological types. Sociometry 1977, 40, 201–213. [Google Scholar] [CrossRef]
- Tang, O.Y.; Pugacheva, A.; Bajaj, A.I.; Perla, K.M.R.; Weil, R.J.; Toms, S.A. The National Inpatient Sample: A Primer for Neurosurgical Big Data Research and Systematic Review. World Neurosurg. 2022, 162, e198–e217. [Google Scholar] [CrossRef]
- Gutzwiller, F. Role of the practicing physician in prevention. Ther. Umsch. 1990, 47, 703–704. [Google Scholar]
- Dicpinigaitis, A.J.; McIntyre, M.K.; Al-Mufti, F.; Kazim, S.F.; Li, B.; Schmidt, M.H.; Gandhi, C.D.; Cole, C.D.; Bowers, C.A. Association of baseline frailty status with clinical outcome following aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2022, 31, 106394. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.H.; Hassab, T.; D’adamo, C.R.; Svoboda, S.; Demos, J.; Ahuja, V.; Katlic, M. Frailty is a stronger predictor than age for postoperative morbidity in Crohn’s disease. Surgery 2021, 170, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J.; et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Yanagawa, B.; An, K.R.; Arora, R.C.; Verma, S.; Friedrich, J.O.; on behalf of the Canadian Cardiovascular Surgery Meta-Analysis Working Group. Frailty and pre-frailty in cardiac surgery: A systematic review and meta-analysis of 66,448 patients. J. Cardiothorac. Surg. 2021, 16, 184. [Google Scholar] [CrossRef]
- Lin, H.-S.; Watts, J.N.; Peel, N.M.; Hubbard, R.E. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 157. [Google Scholar] [CrossRef]
- Robinson, T.N.; Wu, D.S.; Pointer, L.; Dunn, C.L.; Cleveland, J.C.; Moss, M. Simple frailty score predicts postoperative complications across surgical specialties. Am. J. Surg. 2013, 206, 544–550. [Google Scholar] [CrossRef]
- Larson, K.J.; Hamlin, R.J.; Sprung, J.; Schroeder, D.R.; Weingarten, T.N. Associations between Charlson Comorbidity Index and surgical risk severity and the surgical outcomes in advanced-age patients. Am. Surg. 2014, 80, 555–560. [Google Scholar] [CrossRef]
- Hall, D.E.; Arya, S.; Schmid, K.K.; Blaser, C.; Carlson, M.A.; Bailey, T.L.; Purviance, G.; Bockman, T.; Lynch, T.G.; Johanning, J. Development and Initial Validation of the Risk Analysis Index for Measuring Frailty in Surgical Populations. JAMA Surg. 2017, 152, 175–182. [Google Scholar] [CrossRef]
- Shotar, E.; Meyblum, L.; Premat, K.; Lenck, S.; Degos, V.; Grand, T.; Cortese, J.; Pouvelle, A.; Pouliquen, G.; Mouyal, S.; et al. Middle meningeal artery embolization reduces the post-operative recurrence rate of at-risk chronic subdural hematoma. J. Neurointerv. Surg. 2020, 12, 1209–1213. [Google Scholar] [CrossRef]
- Fleming, V.; Prasad, A.; Ge, C.; Crawford, S.; Meraj, S.; Hough, C.L.; Lo, B.; Carson, S.S.; Steingrub, J.; White, D.B.; et al. Prevalence and predictors of shared decision-making in goals-of-care clinician-family meetings for critically ill neurologic patients: A multi-center mixed-methods study. Crit. Care 2023, 27, 403. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Silvester, K.M.; Mohammed, M.A.; Harriman, P.; Girolami, A.; Downes, T.W. Timely care for frail older people referred to hospital improves efficiency and reduces mortality without the need for extra resources. Age Ageing 2014, 43, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.E.; Arya, S.; Schmid, K.K.; Carlson, M.A.; Lavedan, P.; Bailey, T.L.; Purviance, G.; Bockman, T.; Lynch, T.G.; Johanning, J.M. Association of a Frailty Screening Initiative with Postoperative Survival at 30, 180, and 365 Days. JAMA Surg. 2017, 152, 233–240. [Google Scholar] [CrossRef] [PubMed]
| Label | naSDH Patients |
|---|---|
| Total Number of Patients | 251,597 (100.0%) |
| Mean Age | 69.171 (14.666) |
| Age Group | |
| 18–29 | 4040 (1.6%) |
| 30–49 | 21,789 (8.7%) |
| 50–64 | 56,211 (22.3%) |
| 65–79 | 101,968 (40.5%) |
| 80+ | 67,589 (26.9%) |
| Sex | |
| Male | 166,334 (66.2%) |
| Female | 85,027 (33.8%) |
| Race | |
| White | 143,733 (67.5%) |
| Black | 30,142 (14.2%) |
| Hispanic | 20,596 (9.7%) |
| Asian | 10,020 (4.7%) |
| Native American | 1255 (0.6%) |
| Other | 7078 (3.3%) |
| Payer | |
| Medicare | 162,667 (64.8%) |
| Medicaid | 17,623 (7.0%) |
| Private | 54,665 (21.8%) |
| Self-Pay | 8048 (3.2%) |
| No Charge | 694 (0.3%) |
| Other | 7431 (3.0%) |
| Region of Hospital | |
| Northeast | 43,633 (17.3%) |
| Midwest | 51,733 (20.6%) |
| South | 97,169 (38.6%) |
| West | 59,062 (23.5%) |
| Location/Teaching Status of Hospital | |
| Rural | 7163 (2.9%) |
| Urban Non-teaching | 64,891 (25.9%) |
| Urban Teaching | 178,535 (71.2%) |
| Charlson Comorbidity Index | 3.842 (3.019) |
| Hospital Bed Size | |
| Small | 14,779 (5.9%) |
| Medium | 51,769 (20.7%) |
| Large | 184,040 (73.4%) |
| Median Household Income Quartile | |
| 1 | 58,528 (23.8%) |
| 2 | 60,256 (24.6%) |
| 3 | 60,845 (24.8%) |
| 4 | 65,800 (26.8%) |
| Length of Stay | 8.945 (8.481) |
| Total Charges | 92,443.182 (112,750.623) |
| Estimated Cost of Care | 26,536.995 (26,404.413) |
| Outcome | Robust | Pre-Frail | Frail | Severely Frail |
|---|---|---|---|---|
| Total Patients | 65,367 (26.0%) | 76,444 (30.4%) | 59,107 (23.5%) | 50,679 (20.1%) |
| Any Complication | 12,630 (19.3%) | 18,528 (24.2%) | 16,316 (27.6%) | 17,679 (34.9%) |
| Pulmonary | 8939 (13.7%) | 12,699 (16.6%) | 11,245 (19.0%) | 11,963 (23.6%) |
| Thromboembolic | 2181 (3.3%) | 3119 (4.1%) | 2558 (4.3%) | 2621 (5.2%) |
| Renal and Genitourinary | 2164 (3.3%) | 3786 (5.0%) | 3965 (6.7%) | 5250 (10.4%) |
| DVT | 2224 (3.4%) | 3183 (4.2%) | 2678 (4.5%) | 2666 (5.3%) |
| Sepsis | 711 (1.1%) | 1136 (1.5%) | 1200 (2.0%) | 1228 (2.4%) |
| Infected Wound | 1125 (1.7%) | 1033 (1.4%) | 688 (1.2%) | 553 (1.1%) |
| Cardiac | 1043 (1.6%) | 1583 (2.1%) | 1599 (2.7%) | 2451 (4.8%) |
| Outcome | mFI-5 | mFI-11 | CCI | Age |
|---|---|---|---|---|
| Died | 1.091 (1.048 to 1.135, <0.001) | 1.088 (1.058 to 1.119, <0.001) | 1.151 (1.140 to 1.163, <0.001) | 1.002 (0.999 to 1.006, p = 0.140) |
| Non-Home Discharge | 1.359 (1.326 to 1.394, <0.001) | 1.330 (1.305 to 1.355, <0.001) | 1.230 (1.219 to 1.242, <0.001) | 1.039 (1.037 to 1.041, p < 0.001) |
| Any Complication | 1.355 (1.323 to 1.389, <0.001) | 1.256 (1.235 to 1.278, <0.001) | 1.227 (1.218 to 1.237, <0.001) | 1.000 (0.998 to 1.002, p = 0.703) |
| Extended LOS | 1.147 (1.120 to 1.175, <0.001) | 1.150 (1.131 to 1.170, <0.001) | 1.182 (1.173 to 1.190, <0.001) | 1.001 (0.999 to 1.003, p = 0.338) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajjar, A.A.; Ramachandran, N.; Prabhala, T.; Chen, J.Y.; Custozzo, A.; Paul, A.R. Impact of Frailty on Surgical Outcomes in Nonacute Subdural Hematomas: A Nationwide Analysis of 251,597 Patients over 20 Years. J. Clin. Med. 2025, 14, 3176. https://doi.org/10.3390/jcm14093176
Gajjar AA, Ramachandran N, Prabhala T, Chen JY, Custozzo A, Paul AR. Impact of Frailty on Surgical Outcomes in Nonacute Subdural Hematomas: A Nationwide Analysis of 251,597 Patients over 20 Years. Journal of Clinical Medicine. 2025; 14(9):3176. https://doi.org/10.3390/jcm14093176
Chicago/Turabian StyleGajjar, Avi A., Nathan Ramachandran, Tarun Prabhala, John Y. Chen, Amanda Custozzo, and Alexandra R. Paul. 2025. "Impact of Frailty on Surgical Outcomes in Nonacute Subdural Hematomas: A Nationwide Analysis of 251,597 Patients over 20 Years" Journal of Clinical Medicine 14, no. 9: 3176. https://doi.org/10.3390/jcm14093176
APA StyleGajjar, A. A., Ramachandran, N., Prabhala, T., Chen, J. Y., Custozzo, A., & Paul, A. R. (2025). Impact of Frailty on Surgical Outcomes in Nonacute Subdural Hematomas: A Nationwide Analysis of 251,597 Patients over 20 Years. Journal of Clinical Medicine, 14(9), 3176. https://doi.org/10.3390/jcm14093176






