Long-Term Outcomes After Arterial Switch Operation for dextro-Transposition of the Great Arteries—30-Year Single-Center Experience
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Management
2.2.1. Antenatal Management
2.2.2. Surgical Technique
2.2.3. Follow-Up Management
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Patient and Perioperative Characteristics
3.2. Early Outcome
3.3. Follow-Up
3.3.1. Survival
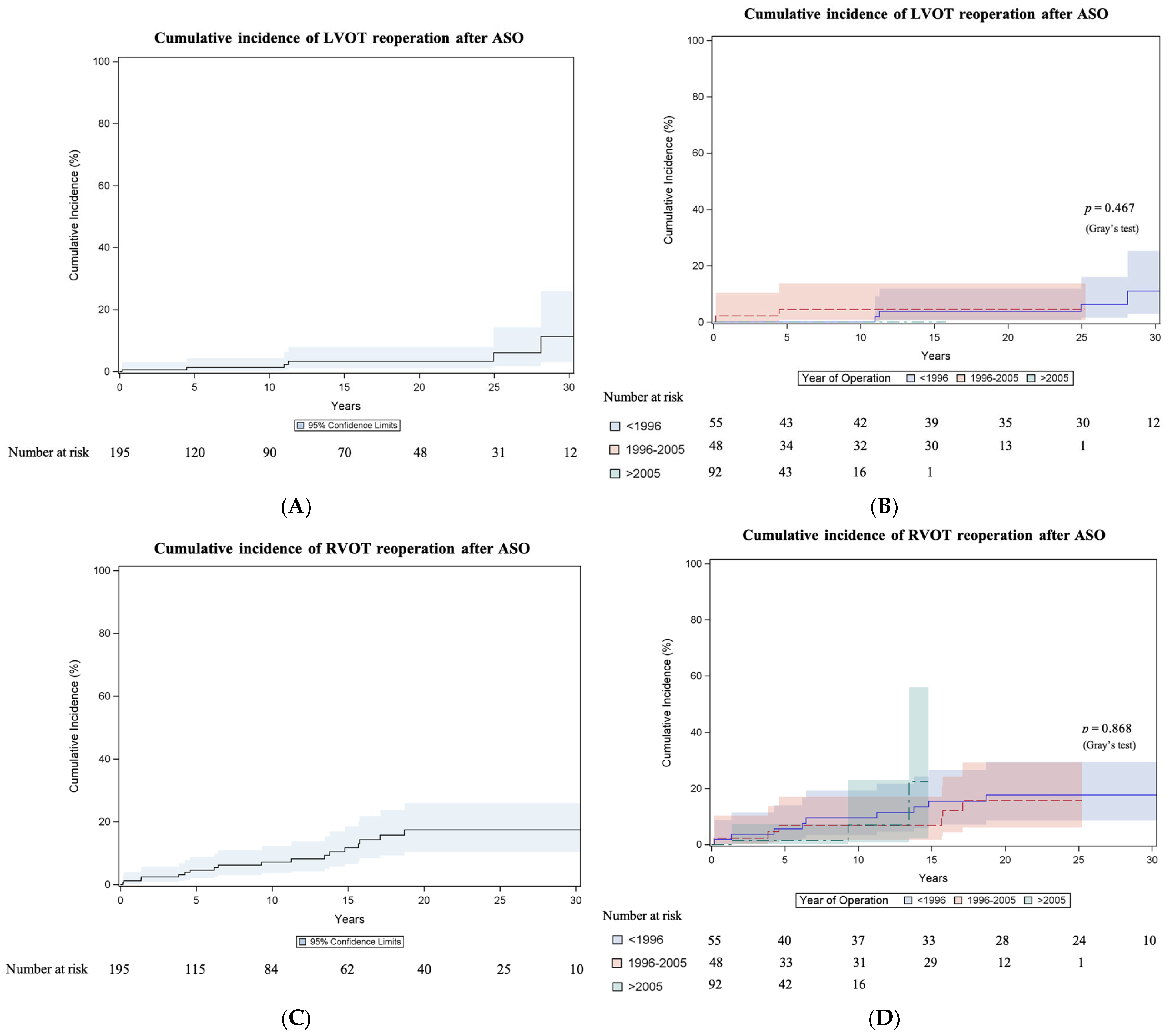
3.3.2. Left Ventricular Outflow Tract Reoperation
3.3.3. Right Ventricular Outflow Tract Reoperation
3.3.4. Right Ventricular Outflow Tract Percutaneous Reinterventions
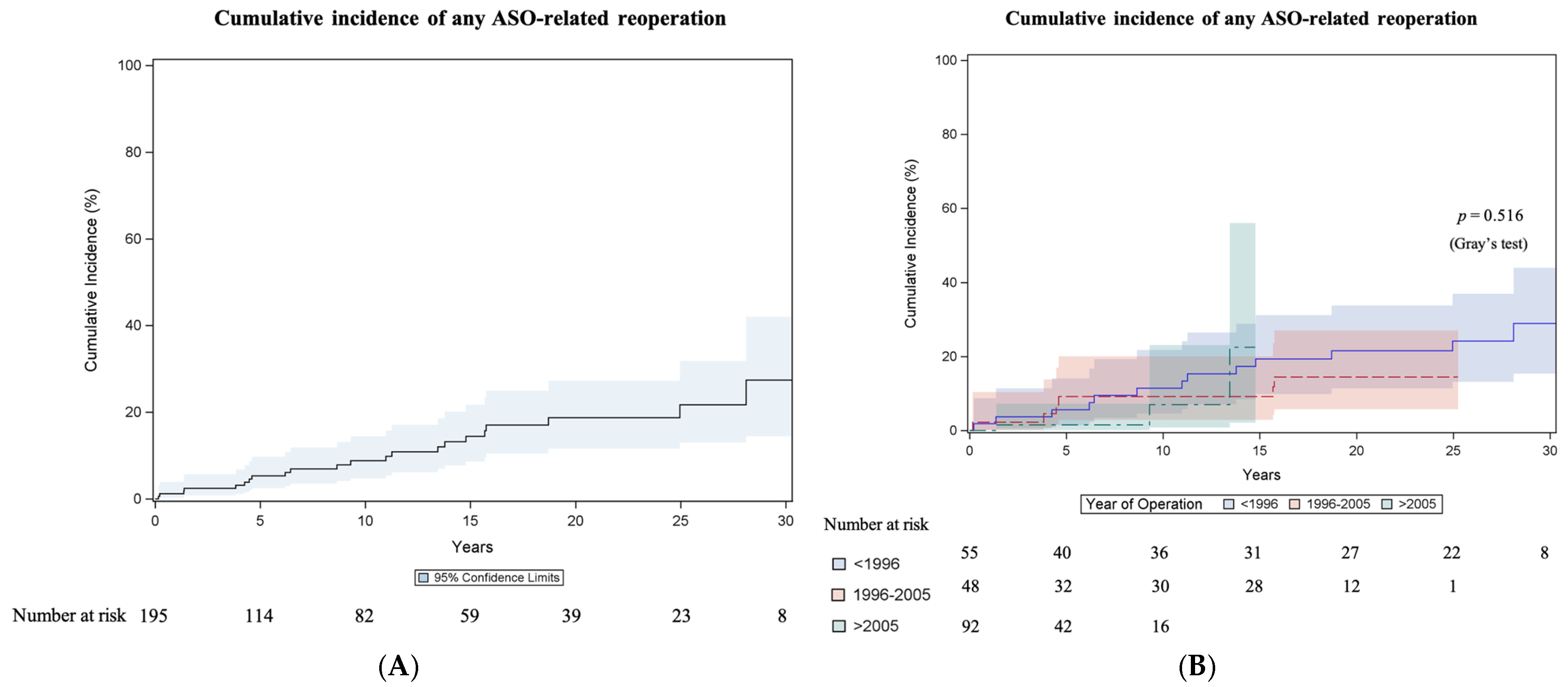
3.3.5. Any Reoperation Related to Arterial Switch Operation
3.3.6. Arrhythmias and Endocarditis
4. Discussion
4.1. Study Limitations
4.2. Future Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASO | Arterial switch operation |
| CI | Confidence interval |
| d-TGA | Dextro-transposition of the great arteries |
| IQR | Interquartile range |
| LVOT | Left ventricular outflow tract |
| RVOT | Right ventricular outflow tract |
References
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.-K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Jatene, A.D.; Fontes, V.; Paulista, P.; Souza, L.; Neger, F.; Galantier, M.; Sousa, J.; Zerbini, E. Anatomic correction of transposition of the great vessels. J. Thorac. Cardiovasc. Surg. 1976, 72, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.A.; Konstantinov, I.E. Arterial Switch Operation: Operative Approach and Outcomes. Ann. Thorac. Surg. 2019, 107, 302–310. [Google Scholar] [CrossRef]
- Senning, A. Surgical correction of transposition of the great vessels. Surgery 1959, 45, 966–980. [Google Scholar]
- Mustard, W.T.; Chute, A.L.; Keith, J.D.; Sirek, A.; Rowe, R.D.; Vlad, P. A surgical approach to transposition of the great vessels with extracorporeal circuit. Surgery 1954, 36, 31–51. [Google Scholar]
- Gittenberger-de Groot, A.C.; Koenraadt, W.M.C.; Bartelings, M.M.; Bökenkamp, R.; DeRuiter, M.C.; Hazekamp, M.G.; Bogers, A.J.J.C.; Quaegebeur, J.M.; Schalij, M.J.; Vliegen, H.W.; et al. Coding of coronary arterial origin and branching in congenital heart disease: The modified Leiden Convention. J. Thorac. Cardiovasc. Surg. 2018, 156, 2260–2269. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Fraser, C.D.; Chacon-Portillo, M.A.; Well, A.; Zea-Vera, R.; Binsalamah, Z.; Adachi, I.; Mery, C.M.; Heinle, J.S. Twenty-Three-Year Experience with the Arterial Switch Operation: Expectations and Long-Term Outcomes. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 292–299. [Google Scholar] [CrossRef]
- Fricke, T.A.; Buratto, E.; Weintraub, R.G.; Bullock, A.; Wheaton, G.; Grigg, L.; Disney, P.; D'Udekem, Y.; Brizard, C.P.; Konstantinov, I.E. Long-term outcomes of the arterial switch operation. J. Thorac. Cardiovasc. Surg. 2022, 163, 212–219. [Google Scholar] [CrossRef]
- Santens, B.; Van De Bruaene, A.; De Meester, P.; Gewillig, M.; Troost, E.; Claus, P.; Bogaert, J.; Budts, W. Outcome of arterial switch operation for transposition of the great arteries. A 35-year follow-up study. Int. J. Cardiol. 2020, 316, 94–100. [Google Scholar] [CrossRef]
- Vida, V.L.; Zanotto, L.; Zanotto, L.; Triglia, L.T.; Bellanti, E.; Castaldi, B.; Padalino, M.A.; Gasperetti, A.; Battista, F.; Varnier, M.; et al. Arterial switch operation for transposition of the great arteries: A single-centre 32-year experience. J. Card. Surg. 2019, 34, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- van der Palen, R.L.; Blom, N.A.; Kuipers, I.M.; Rammeloo, L.A.; Jongbloed, M.R.; Konings, T.C.; Bouma, B.J.; Koolbergen, D.R.; Hazekamp, M.G. Long-term outcome after the arterial switch operation: 43 years of experience. Eur. J. Cardiothorac. Surg. 2021, 59, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Morfaw, F.; Leenus, A.; Mbuagbaw, L.; Anderson, L.N.; Dillenburg, R.; Thabane, L. Outcomes after corrective surgery for congenital dextro-transposition of the arteries using the arterial switch technique: A scoping systematic review. Syst. Rev. 2020, 9, 231. [Google Scholar] [CrossRef]
- Nagata, H.; Glick, L.; Lougheed, J.; Grattan, M.; Mondal, T.; Thakur, V.; Schwartz, S.M.; Jaeggi, E. Prenatal Diagnosis of Transposition of the Great Arteries Reduces Postnatal Mortality: A Population-Based Study. Can. J. Cardiol. 2020, 36, 1592–1597. [Google Scholar] [CrossRef]
- van Velzen, C.L.; Haak, M.C.; Reijnders, G.; Rijlaarsdam, M.E.B.; Bax, C.J.; Pajkrt, E.; Hruda, J.; Galindo-Garre, F.; Bilardo, C.M.; De Groot, C.J.M.; et al. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound Ultrasound Obstet. Gynecol. 2015, 45, 320–325. [Google Scholar] [CrossRef]
- Bonnet, D.; Coltri, A.; Butera, G.; Fermont, L.; Le Bidois, J.; Kachaner, J.; Sidi, D. Detection of Transposition of the Great Arteries in Fetuses Reduces Neonatal Morbidity and Mortality. Circulation 1999, 99, 916–918. [Google Scholar] [CrossRef]
- Kumar, R.; Newburger, J.W.; Gauvreau, K.; A Kamenir, S.; Hornberger, L.K. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am. J. Cardiol. 1999, 83, 1649–1653. [Google Scholar] [CrossRef]
- Villafañe, J.; Lantin-Hermoso, M.R.; Bhatt, A.B.; Tweddell, J.S.; Geva, T.; Nathan, M.; Elliott, M.J.; Vetter, V.L.; Paridon, S.M.; Kochilas, L.; et al. D-transposition of the great arteries: The current era of the arterial switch operation. J. Am. Coll. Cardiol. 2014, 64, 498–511. [Google Scholar] [CrossRef]
- Huber, C.; Mimic, B.; Oswal, N.; Sullivan, I.; Kostolny, M.; Elliott, M.; de Leval, M.; Tsang, V. Outcomes and re-interventions after one-stage repair of transposition of great arteries and aortic arch obstruction. Eur. J. Cardiothoracic Surg. 2011, 39, 213–220. [Google Scholar] [CrossRef]
- Mohammadi, S.; Serraf, A.; Belli, E.; Aupecle, B.; Capderou, A.; Lacour-Gayet, F.; Martinovic, I.; Piot, D.; Touchot, A.; Losay, J.; et al. Left-sided lesions after anatomic repair of transposition of the great arteries, ventricular septal defect, and coarctation: Surgical factors. J. Thorac. Cardiovasc. Surg. 2004, 128, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.A.; Donaldson, S.; Schneider, J.R.; Menahem, S.; d’Udekem, Y.; Brizard, C.P.; Konstantinov, I.E. Outcomes of the arterial switch operation in patients with aortic arch obstruction. J. Thorac. Cardiovasc. Surg. 2020, 159, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Moe, T.G.; Bardo, D.M. Long-term Outcomes of the Arterial Switch Operation for d-Transposition of the Great Arteries. Prog. Cardiovasc. Dis. 2018, 61, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Vitanova, K.; von Ohain, J.P.; Ono, M.; Tanase, D.; Burri, M.; Lange, R.; Cleuziou, J. Incidence and Risk Factors for Right Ventricular Outflow Tract Obstruction after the Arterial Switch Operation. Thorac. Cardiovasc. Surg. 2019, 67, 037–043. [Google Scholar] [CrossRef]
- Lee, J.; Shahbah, D.A.; El-Said, H.; Rios, R.; Ratnayaka, K.; Moore, J. Pulmonary artery interventions after the arterial switch operation: Unique and significant risks. Congenit. Heart Dis. 2019, 14, 288–296. [Google Scholar] [CrossRef]
- Angeli, E.; Raisky, O.; Bonnet, D.; Sidi, D.; Vouhé, P.R. Late reoperations after neonatal arterial switch operation for transposition of the great arteries. Eur. J. Cardio-Thorac. Surg. 2008, 34, 32–36. [Google Scholar] [CrossRef]
- Al-Radi, O.O. Commentary: Heroes of the arterial switch operation in the 1980s. J. Thorac. Cardiovasc. Surg. 2020, 159, 617–618. [Google Scholar] [CrossRef]
- Khairy, P.; Clair, M.; Fernandes, S.M.; Blume, E.D.; Powell, A.J.; Newburger, J.W.; Landzberg, M.J.; Mayer, J.E., Jr. Cardiovascular Outcomes After the Arterial Switch Operation for D-Transposition of the Great Arteries. Circulation 2013, 127, 331–339. [Google Scholar] [CrossRef]

| Variable | 1985–1995 (n = 55) | 1996–2005 (n = 48) | 2006–2020 (n = 92) | p-Value |
|---|---|---|---|---|
| Number | 55 | 48 | 92 | |
| Male | 38 (69.1) | 36 (75) | 67 (72.8) | 0.783 |
| Age at ASO | 7 (5–10) | 6 (4–9) | 6 (3–8) | 0.092 |
| Premature birth (<37 week) | 3 (5.5) | 8 (16.7) | 5 (5.4) | 0.062 |
| Birthweight <2500 g | 2 (3.6) | 3 (6.3) | 3 (3.3) | 0.655 |
| Prenatal diagnosis of d-TGA | 0 (0) | 4 (8.3) | 23 (25) | <0.001 |
| Ventricular septal defect | 11 (20) | 18 (37.5) | 34 (37) | 0.067 |
| Coarctation of the aorta | 4 (7.3) | 7 (14.6) | 14 (15.2) | 0.371 |
| Hypoplastic aortic arch | 1 (1.8) | 4 (8.3) | 8 (8.7) | 0.234 |
| Coronary anomaly | 16 (29.1) | 9 (18.8) | 25 (27.2) | 0.437 |
| Intramural coronary artery | 0 (0) | 0 (0) | 8 (8.7) | 0.011 |
| Weight at ASO (kg) | 3.1 (2.6–3.6) | 3.2 (3.1–3.8) | 3.5 (3.1–3.8) | 0.035 |
| Height at ASO (cm) | 50 (48.7–51.3) | 51 (50–54) | 50 (49–53) | 0.080 |
| BSAHaycock at ASO | 0.21 (0.18–0.22) | 0.22 (0.21–0.24 | 0.22 (0.21–0.24) | 0.127 |
| Any previous cardiac intervention | 18 (32.7) | 41 (85.4) | 50 (54.3) | <0.001 |
| Rashkind procedure | 17 (30.9) | 36 (75) | 47 (51.1) | <0.001 |
| Persistent ductus arteriosus stent | 0 (0) | 0 (0) | 3 (3.3) | 0.334 |
| Cardiopulmonary bypass time | 154 (144–170) | 158 (140–190) | 161 (125–193) | 0.790 |
| Aortic cross clamp time | 89 (77–102) | 82 (66–99) | 98 (73–118) | 0.065 |
| Concomitant aortic arch surgery | 1 (1.8) | 4 (8.3) | 15 (16.3) | 0.014 |
| Variable | 1985–1995 (n = 55) | 1996–2005 (n = 48) | 2006–2020 (n = 92) | p-Value |
|---|---|---|---|---|
| Pacemaker implantation | 2 (3.6) | 1 (2.1) | 2 (2.2) | 0.847 |
| Ventilation time (days) * | 13 (8–100) | 8.5 (6–23) | 3 (2–5) | <0.001 |
| Intensive care unit stay (days) * | 19 (9–100) | 16 (9–26) | 7 (5–10) | <0.001 |
| Hospital stay (days) * | 50 (29–100) | 30 (22–51) | 16 (13–22) | <0.001 |
| Revision for bleeding | 1 (1.8) | 2 (4.2) | 2 (2.2) | 0.716 |
| Drainage for pericardial tamponade | 1 (1.8) | 5 (10.4) | 1 (1.1) | 0.019 |
| Revision coronary arteries/ischemia | 1 (1.8) | 0 | 1 (1.1) | >0.99 |
| Cardiopulmonary resuscitation | 5 (9.1) | 2 (4.2) | 2 (2.2) | 0.158 |
| Hemofiltration/dialysis | 1 (1.8) | 7 (14.6) | 10 (10.9) | 0.041 |
| Delayed sternal closure | 15 (27.3) | 22 (45.8) | 34 (37) | 0.154 |
| Extracorporeal membrane oxygenation | 7 (12.7) | 9 (18.8) | 0 (0) | <0.001 |
| Early in-hospital death | 9 (16.4) | 8 (16.7) | 0 (0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlein, J.; Ungerböck, C.; Tertschnig, D.; Kaider, A.; Karner, B.; Atteneder, C.; Urganci, E.; Werner, P.; Base, E.; Murin, P.; et al. Long-Term Outcomes After Arterial Switch Operation for dextro-Transposition of the Great Arteries—30-Year Single-Center Experience. J. Clin. Med. 2025, 14, 3160. https://doi.org/10.3390/jcm14093160
Schlein J, Ungerböck C, Tertschnig D, Kaider A, Karner B, Atteneder C, Urganci E, Werner P, Base E, Murin P, et al. Long-Term Outcomes After Arterial Switch Operation for dextro-Transposition of the Great Arteries—30-Year Single-Center Experience. Journal of Clinical Medicine. 2025; 14(9):3160. https://doi.org/10.3390/jcm14093160
Chicago/Turabian StyleSchlein, Johanna, Clemens Ungerböck, Daniela Tertschnig, Alexandra Kaider, Barbara Karner, Clemens Atteneder, Erhan Urganci, Paul Werner, Eva Base, Peter Murin, and et al. 2025. "Long-Term Outcomes After Arterial Switch Operation for dextro-Transposition of the Great Arteries—30-Year Single-Center Experience" Journal of Clinical Medicine 14, no. 9: 3160. https://doi.org/10.3390/jcm14093160
APA StyleSchlein, J., Ungerböck, C., Tertschnig, D., Kaider, A., Karner, B., Atteneder, C., Urganci, E., Werner, P., Base, E., Murin, P., & Zimpfer, D. (2025). Long-Term Outcomes After Arterial Switch Operation for dextro-Transposition of the Great Arteries—30-Year Single-Center Experience. Journal of Clinical Medicine, 14(9), 3160. https://doi.org/10.3390/jcm14093160







