Traumatic Vertebral Artery Injury: Diagnosis, Natural History, and Key Considerations for Management
Abstract
1. Introduction
2. Epidemiology
3. Associated Cervical Spine Trauma
4. VAI Screening
5. Ischemic Complications and Radiographic Outcomes
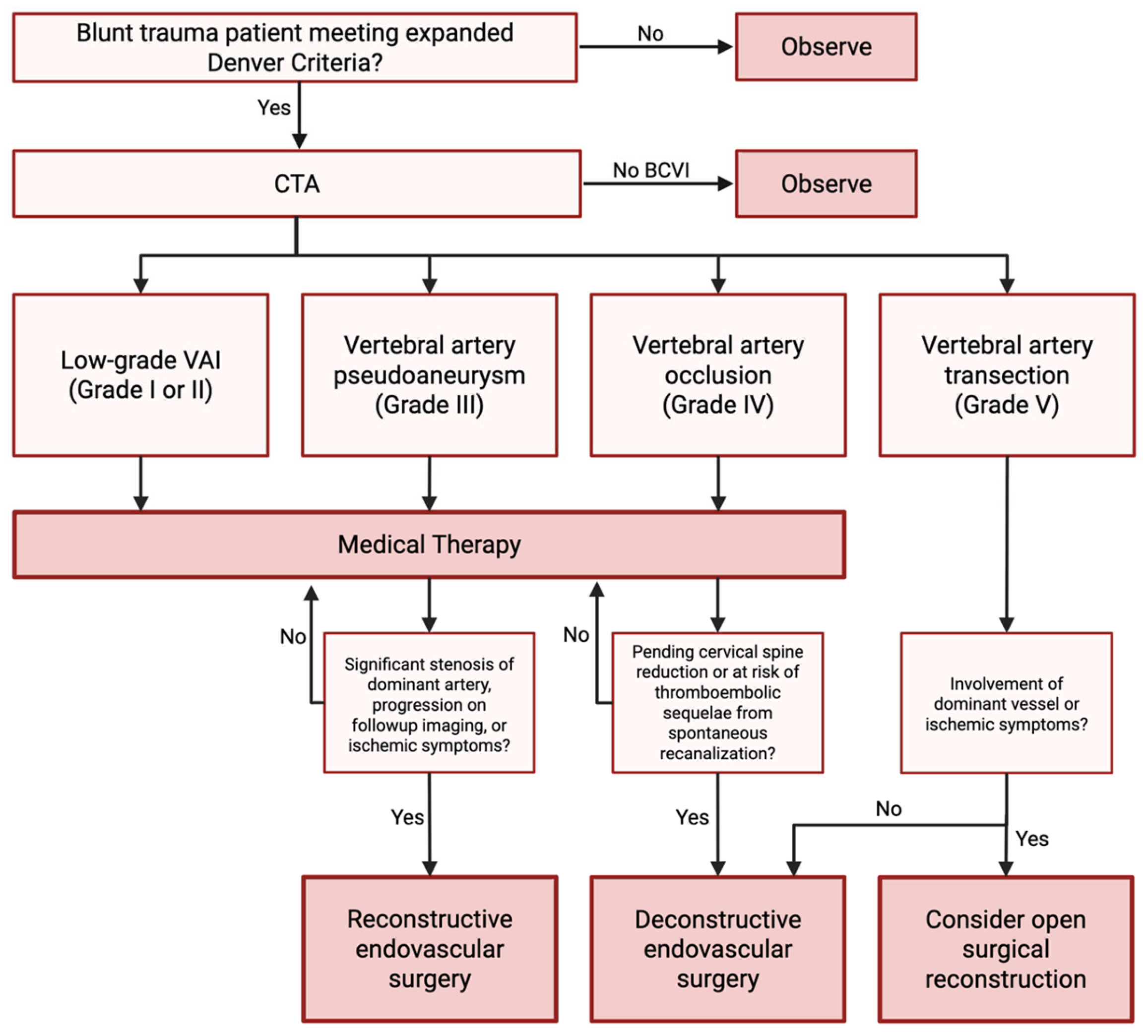
6. Management
6.1. Medical Management
| Study | AC Alone | AP Alone | ACAP | Other | Stroke | Therapy Duration | Note |
|---|---|---|---|---|---|---|---|
| Biffl 2000 [6] | 63% (24/38) | 16% (6/38) | 0% (0/38) | 21% (8/38) | 26% (10/38) | Not mentioned | BCVI |
| Callcut 2012 [92] | 30% (22/73) | 30% (22/73) | 1% (1/73) | 38% (28/73) | 27% (21/77) | Not mentioned | BCVI + Neurologically Injured Patients |
| Cothren 2009 [79] | 45% (192/422) | 21% (90/422) | 0% (0/422) | 33% (140/422) | 11% (45/422) | Not mentioned | BCVI |
| Miller 2001 [80] | 62% (31/50) | 26% (13/50) | 0% (0/50) | 12% (6/50) | 14% (7/50) | Not mentioned | VAI only |
| Stein 2009 [82] | 10% (15/147) | 36% (53/147) | 18% (27/147) | 35% (52/147) | 12% (18/147) | Not mentioned | BCVI |
6.2. Endovascular Management
6.3. Open Surgical Management
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAP | Anticoagulant and/or antiplatelet therapy |
| ASIA | American Spinal Injury Association |
| BCVI | Blunt cerebrovascular injury |
| CTA | Computed tomography angiography |
| DSA | Digital subtraction angiography |
| EAST | Eastern Association for the Surgery of Trauma |
| GCS | Glasgow Coma Scale |
| MRA | Magnetic resonance angiography |
| TBI | Traumatic brain injury |
| VAI | Vertebral artery injury |
| VAO | Vertebral artery occlusion |
References
- Biffl, W.L.; Moore, E.E.; Offner, P.J.; Brega, K.E.; Franciose, R.J.; Burch, J.M. Blunt carotid arterial injuries: Implications of a new grading scale. J. Trauma 1999, 47, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Foreman, P.M.; Griessenauer, C.J.; Chua, M.; Hadley, M.N.; Harrigan, M.R. Corrective spinal surgery may be protective against stroke in patients with blunt traumatic vertebral artery occlusion. J. Neurosurg. Spine 2015, 23, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, M.R. Ischemic stroke due to blunt traumatic cerebrovascular injury. Stroke 2020, 51, 353–360. [Google Scholar] [CrossRef]
- Sujijantarat, N.; Fathima, B.; Padmanaban, V.; Kosyakovsky, J.; Elsamadicy, A.A.; Haynes, J.O.; Koo, A.B.; Shankar, G.M.; Regenhardt, R.W.; Stapleton, C.J.; et al. Traumatic vertebral artery occlusion is associated with high rates of recanalization: Insights from a systematic review and meta-analysis. Neurosurg. Rev. 2025, 48, 306. [Google Scholar] [CrossRef]
- Michalopoulos, G.D.; Pennington, Z.; Bambakidis, P.; Alexander, A.Y.; Lakomkin, N.; Charalampous, C.; Sammak, S.E.; Hassett, L.C.; Graepel, S.; Meyer, F.B.; et al. Traumatic vertebral artery injury: Denver grade, bilaterality, and stroke risk. A systematic review and meta-analysis. J. Neurosurg. 2024, 140, 522–536. [Google Scholar] [CrossRef]
- Biffl, W.L.; Moore, E.E.; Elliott, J.P.; Ray, C.; Offner, P.J.; Franciose, R.J.; Brega, K.E.; Burch, J.M. The devastating potential of blunt vertebral arterial injuries. Ann. Surg. 2000, 231, 672–681. [Google Scholar] [CrossRef]
- Fleck, S.K.; Langner, S.; Baldauf, J.; Kirsch, M.; Rosenstengel, C.; Schroeder, H.W. Blunt craniocervical artery injury in cervical spine lesions: The value of ct angiography. Acta Neurochir. (Wien) 2010, 152, 1679–1686. [Google Scholar] [CrossRef]
- Temperley, H.C.; McDonnell, J.M.; O’Sullivan, N.J.; Waters, C.; Cunniffe, G.; Darwish, S.; Butler, J.S. The incidence, characteristics and outcomes of vertebral artery injury associated with cervical spine trauma: A systematic review. Glob. Spine J. 2023, 13, 1134–1152. [Google Scholar] [CrossRef]
- Egashira, S.; Tanaka, T.; Yamashiro, T.; Saito, S.; Abe, S.; Yoshimoto, T.; Fukuma, K.; Ishiyama, H.; Yamaguchi, E.; Hattori, Y.; et al. High pillow and spontaneous vertebral artery dissection: A case-control study implicating “shogun pillow syndrome”. Eur. Stroke J. 2024, 9, 501–509. [Google Scholar] [CrossRef]
- AlBayar, A.; Sullivan, P.Z.; Blue, R.; Leonard, J.; Kung, D.K.; Ozturk, A.K.; Chen, H.I.; Schuster, J.M. Risk of vertebral artery injury and stroke following blunt and penetrating cervical spine trauma: A retrospective review of 729 patients. World Neurosurg. 2019, 130, e672–e679. [Google Scholar] [CrossRef]
- Zygogiannis, K.; Benetos, I.S.; Evangelopoulos, D.S.; Koulalis, D.; Pneumaticos, S.G. Blunt traumatic vertebral artery injury after cervical fracture dislocation: A systematic review of the literature. Cureus 2024, 16, e65250. [Google Scholar] [CrossRef] [PubMed]
- Cothren, C.C.; Moore, E.E.; Biffl, W.L.; Ciesla, D.J.; Ray, C.E.; Johnson, J.L.; Moore, J.B.; Burch, J.M. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J. Trauma 2003, 55, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Du, P.Z.; Barton, D.; Bridge, N.; Ganapathy, V. Cervical fracture patterns associated with blunt cerebrovascular injures when utilizing computed tomographic angiography: A systematic review and meta-analysis. Spine J. 2022, 22, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kumar, A.; Gamangatti, S. Mechanism and patterns of cervical spine fractures-dislocations in vertebral artery injury. J. Craniovertebr. Junction Spine 2012, 3, 11–15. [Google Scholar]
- McKinney, A.; Ott, F.; Short, J.; McKinney, Z.; Truwit, C. Angiographic frequency of blunt cerebrovascular injury in patients with carotid canal or vertebral foramen fractures on multidetector ct. Eur. J. Radiol. 2007, 62, 385–393. [Google Scholar] [CrossRef]
- R, S.; Gem, K.; A, N.; E, A.M.; N, D. Vertebral artery injury in cervical spine fractures: A cohort study and review of the literature. Ulster Med. J. 2020, 89, 89–94. [Google Scholar]
- Willis, B.K.; Greiner, F.; Orrison, W.W.; Benzel, E.C. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery 1994, 34, 435–441; discussion 441–442. [Google Scholar] [CrossRef]
- Durand, D.; Wu, X.; Kalra, V.B.; Abbed, K.M.; Malhotra, A. Predictors of vertebral artery injury in isolated c2 fractures based on fracture morphology using ct angiography. Spine (Phila Pa 1976) 2015, 40, E713–E718. [Google Scholar] [CrossRef]
- Lockwood, M.M.; Smith, G.A.; Tanenbaum, J.; Lubelski, D.; Seicean, A.; Pace, J.; Benzel, E.C.; Mroz, T.E.; Steinmetz, M.P. Screening via ct angiogram after traumatic cervical spine fractures: Narrowing imaging to improve cost effectiveness. Experience of a level i trauma center. J. Neurosurg. Spine 2016, 24, 490–495. [Google Scholar] [CrossRef]
- Chung, D.; Sung, J.-K.; Cho, D.-C.; Kang, D.-H. Vertebral artery injury in destabilized midcervical spine trauma; predisposing factors and proposed mechanism. Acta Neurochir. (Wien) 2012, 154, 2091–2098; discussion 2098. [Google Scholar] [CrossRef]
- Gupta, R.; Siroya, H.L.; Bhat, D.I.; Shukla, D.P.; Pruthi, N.; Devi, B.I. Vertebral artery dissection in acute cervical spine trauma. J. Craniovertebr. Junction Spine 2022, 13, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Chikuda, H.; Inokuchi, K.; Ishii, K.; Kobayashi, A.; Kanai, H.; Miyoshi, K. Direct damage to a vertebral artery better predicts a vertebral artery injury than elongation in cervical spine dislocation. Acta Med. Okayama 2017, 71, 427–432. [Google Scholar] [PubMed]
- Oetgen, M.E.; Lawrence, B.D.; Yue, J.J. Does the morphology of foramen transversarium fractures predict vertebral artery injuries? Spine (Phila Pa 1976) 2008, 33, E957–E961. [Google Scholar] [CrossRef]
- Cloney, M.B.; Roumeliotis, A.G.; Azad, H.A.; Prasad, N.; Shlobin, N.A.; Hopkins, B.S.; Jahromi, B.S.; Potts, M.B.; Dahdaleh, N.S. Concomitant cervical spine fractures are the primary driver of disability after traumatic vertebral artery dissection: A case series of 123 patients. J. Craniovertebr. Junction Spine 2022, 13, 410–414. [Google Scholar] [CrossRef]
- Biffl, W.L.; Moore, E.E.; Offner, P.J.; Brega, K.E.; Franciose, R.J.; Elliott, J.P.; Burch, J.M. Optimizing screening for blunt cerebrovascular injuries. Am. J. Surg. 1999, 178, 517–522. [Google Scholar] [CrossRef]
- Kerwin, A.J.; Bynoe, R.P.; Murray, J.; Hudson, E.R.; Close, T.P.; Gifford, R.R.; Carson, K.W.; Smith, L.P.; Bell, R.M. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J. Trauma 2001, 51, 308–314. [Google Scholar] [CrossRef]
- Berne, J.D.; Norwood, S.H. Blunt vertebral artery injuries in the era of computed tomographic angiographic screening: Incidence and outcomes from 8,292 patients. J. Trauma 2009, 67, 1333–1338. [Google Scholar]
- Berne, J.D.; Reuland, K.S.; Villarreal, D.H.; McGovern, T.M.; Rowe, S.A.; Norwood, S.H. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J. Trauma 2006, 60, 1204–1209; discussion 1209–1210. [Google Scholar] [CrossRef]
- Biffl, W.L.; Egglin, T.; Benedetto, B.; Gibbs, F.; Cioffi, W.G. Sixteen-slice computed tomographic angiography is a reliable noninvasive screening test for clinically significant blunt cerebrovascular injuries. J. Trauma 2006, 60, 745–751; discussion 751–752. [Google Scholar] [CrossRef]
- Bub, L.D.; Hollingworth, W.; Jarvik, J.G.; Hallam, D.K. Screening for blunt cerebrovascular injury: Evaluating the accuracy of multidetector computed tomographic angiography. J. Trauma 2005, 59, 691–697. [Google Scholar]
- Eastman, A.L.; Chason, D.P.; Perez, C.L.; McAnulty, A.L.; Minei, J.P. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: Is it ready for primetime? J. Trauma Acute Care Surg. 2006, 60, 925. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.K.; Camacho, M.; Ivatury, R.R.; Davis, I.C.; Komorowski, D.J.; Leung, D.A.; Grizzard, J.D.; Aboutanos, M.B.; Duane, T.M.; Cockrell, C.; et al. Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: A note of caution. Ann. Surg. 2007, 246, 632–642; discussion 642–643. [Google Scholar] [CrossRef] [PubMed]
- Song, W.S.; Chiang, Y.H.; Chen, C.Y.; Lin, S.Z.; Liu, M.Y. A simple method for diagnosing traumatic occlusion of the vertebral artery at the craniovertebral junction. Spine (Phila Pa 1976) 1994, 19, 837–839. [Google Scholar] [CrossRef] [PubMed]
- Utter, G.H.; Hollingworth, W.; Hallam, D.K.; Jarvik, J.G.; Jurkovich, G.J. Sixteen-slice ct angiography in patients with suspected blunt carotid and vertebral artery injuries. J. Am. Coll. Surg. 2006, 203, 838–848. [Google Scholar] [CrossRef]
- Tobert, D.G.; Le, H.V.; Blucher, J.A.; Harris, M.B.; Schoenfeld, A.J. The clinical implications of adding ct angiography in the evaluation of cervical spine fractures: A propensity-matched analysis. J. Bone Joint Surg. Am. 2018, 100, 1490–1495. [Google Scholar] [CrossRef]
- Berne, J.D.; Cook, A.; Rowe, S.A.; Norwood, S.H. A multivariate logistic regression analysis of risk factors for blunt cerebrovascular injury. J. Vasc. Surg. 2010, 51, 57–64. [Google Scholar] [CrossRef]
- Drain, J.P.; Weinberg, D.S.; Ramey, J.S.; Moore, T.A.; Vallier, H.A. Indications for ct-angiography of the vertebral arteries after trauma. Spine (Phila Pa 1976) 2018, 43, E520–E524. [Google Scholar] [CrossRef]
- Shibahashi, K.; Hoda, H.; Ishida, T.; Motoshima, T.; Sugiyama, K.; Hamabe, Y. Derivation and validation of a quantitative screening model for blunt cerebrovascular injury. J. Neurosurg. 2021, 135, 1129–1138. [Google Scholar] [CrossRef]
- Bensch, F.V.; Varjonen, E.A.; Pyhältö, T.T.; Koskinen, S.K. Augmenting denver criteria yields increased bcvi detection, with screening showing markedly increased risk for subsequent ischemic stroke. Emerg. Radiol. 2019, 26, 365–372. [Google Scholar] [CrossRef]
- Emmett, K.P.; Fabian, T.C.; DiCocco, J.M.; Zarzaur, B.L.; Croce, M.A. Improving the screening criteria for blunt cerebrovascular injury: The appropriate role for computed tomography angiography. J. Trauma 2011, 70, 1058–1063; discussion 1063–1065. [Google Scholar] [CrossRef]
- Geddes, A.E.; Burlew, C.C.; Wagenaar, A.E.; Biffl, W.L.; Johnson, J.L.; Pieracci, F.M.; Campion, E.M.; Moore, E.E. Expanded screening criteria for blunt cerebrovascular injury: A bigger impact than anticipated. Am. J. Surg. 2016, 212, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.; Robertson, R.N.; Barton, B.M.; Cairns, M.A.; Webb, S.W. Blunt cerebrovascular injury in cervical spine fractures: Are more-liberal screening criteria warranted? Glob. Spine J. 2016, 6, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.E.; Ziemba-Davis, M.; Herrera, A.J. The limitations of using risk factors to screen for blunt cerebrovascular injuries: The harder you look, the more you find. World J. Emerg. Surg. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.A.; Cairns, A.L.; Shin, J.; Appelbaum, R.D.; Couture, D.E.; Nunn, A.M.; Miller, P.R.; Martin, R.S.; Carmichael, S.P. Above the clavicle: A simplified screening method for asymptomatic blunt cerebral vascular injury. Am. Surg. 2023, 89, 79–83. [Google Scholar] [CrossRef]
- Sinnathamby, M.; Rao, S.V.; Weber, D.G. Increased detection of blunt carotid and vertebral artery injury after implementation of diagnostic imaging pathway in level 1 trauma centre in western australia. Injury 2017, 48, 1917–1921. [Google Scholar] [CrossRef]
- Goyal, K.; Sunny, J.T.; Gillespie, C.S.; Wilby, M.; Clark, S.R.; Kaiser, R.; Fehlings, M.G.; Srikandarajah, N. A systematic review and meta-analysis of vertebral artery injury after cervical spine trauma. Glob. Spine J. 2024, 14, 1356–1368. [Google Scholar] [CrossRef]
- Karagiorgas, G.P.; Brotis, A.G.; Giannis, T.; Rountas, C.D.; Vassiou, K.G.; Fountas, K.N.; Kapsalaki, E.Z. The diagnostic accuracy of magnetic resonance angiography for blunt vertebral artery injury detection in trauma patients: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2017, 160, 152–163. [Google Scholar] [CrossRef]
- Friedman, D.; Flanders, A.; Thomas, C.; Millar, W. Vertebral artery injury after acute cervical spine trauma: Rate of occurrence as detected by mr angiography and assessment of clinical consequences. AJR Am. J. Roentgenol. 1995, 164, 443–447; discussion 448–449. [Google Scholar] [CrossRef][Green Version]
- Miller, P.R.; Fabian, T.C.; Croce, M.A.; Cagiannos, C.; Williams, J.S.; Vang, M.; Qaisi, W.G.; Felker, R.E.; Timmons, S.D. Prospective screening for blunt cerebrovascular injuries: Analysis of diagnostic modalities and outcomes. Ann. Surg. 2002, 236, 386–393; discussion 393–395. [Google Scholar] [CrossRef]
- Taneichi, H.; Suda, K.; Kajino, T.; Kaneda, K. Traumatically induced vertebral artery occlusion associated with cervical spine injuries: Prospective study using magnetic resonance angiography. Spine (Phila Pa 1976) 2005, 30, 1955–1962. [Google Scholar] [CrossRef]
- Weller, S.J.; Rossitch, E.; Malek, A.M. Detection of vertebral artery injury after cervical spine trauma using magnetic resonance angiography. J. Trauma 1999, 46, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Atsumi, T.; Araki, T.; Fuse, A.; Sato, H.; Kawai, M.; Yamamoto, Y. Significance of magnetic resonance imaging in the diagnosis of vertebral artery injury associated with blunt cervical spine trauma. J. Nippon Med. Sch. 2007, 74, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Iwasaki, H.; Sonekatsu, M.; Murata, S.; Kozaki, T.; Hashizume, H.; Tsutsui, S.; Takami, M.; Nagata, K.; Hira, K.; et al. Ultrasonography is an effective tool for the evaluation of traumatic vertebral artery injuries distal to fourth cervical vertebra in the emergency room. BMC Musculoskelet. Disord. 2023, 24, 314. [Google Scholar] [CrossRef] [PubMed]
- Purvis, D.; Aldaghlas, T.; Trickey, A.W.; Rizzo, A.; Sikdar, S. A novel decision tree approach based on transcranial doppler sonography to screen for blunt cervical vascular injuries. J. Ultrasound Med. 2013, 32, 1023–1031. [Google Scholar] [CrossRef]
- Grandhi, R.; Weiner, G.M.; Agarwal, N.; Panczykowski, D.M.; Ares, W.J.; Rodriguez, J.S.; Gelfond, J.A.; Myers, J.G.; Alarcon, L.H.; Okonkwo, D.O.; et al. Limitations of multidetector computed tomography angiography for the diagnosis of blunt cerebrovascular injury. J. Neurosurg. 2018, 128, 1642–1647. [Google Scholar] [CrossRef]
- Yi, Z.; Vankawala, J.; Koneru, M.; Oliveira, R.; Santucci, J.; Morse, C.; Ifrach, J.; Al-Atrache, Z.; Fox, N.M.; Goldenberg-Sandau, A.; et al. Change in management for digital subtraction angiography-identified false-positive traumatic vertebral artery injury. Interv Neuroradiol 2025, 15910199241312254. [Google Scholar] [CrossRef]
- Cothren, C.C.; Moore, E.E.; Ray, C.E.; Ciesla, D.J.; Johnson, J.L.; Moore, J.B.; Burch, J.M. Screening for blunt cerebrovascular injuries is cost-effective. Am. J. Surg. 2005, 190, 845–849. [Google Scholar] [CrossRef]
- Kaye, D.; Brasel, K.J.; Neideen, T.; Weigelt, J.A. Screening for blunt cerebrovascular injuries is cost-effective. J. Trauma 2011, 70, 1051–1056; discussion 1056–1057. [Google Scholar] [CrossRef]
- Tran, A.; Fernando, S.M.; Rochwerg, B.; Hawes, H.; Hameed, M.S.; Dawe, P.; Garraway, N.; Evans, D.C.; Kim, D.; Biffl, W.L.; et al. Prognostic factors associated with risk of stroke following blunt cerebrovascular injury: A systematic review and meta-analysis. Injury 2024, 55, 111319. [Google Scholar] [CrossRef]
- Harrigan, M.R.; Griffin, R.L.; Deveikis, J.P.; Prattipati, V.; Chimowitz, M.I.; Jansen, J.O. New ischemic lesions on brain magnetic resonance imaging in patients with blunt traumatic cerebrovascular injury. J. Trauma Acute Care Surg. 2020, 88, 796–802. [Google Scholar] [CrossRef]
- de Heredia, L.L.; Belci, M.; Briley, D.; Hughes, R.J.; McNeillis, B.; Meagher, T.M.; Yanny, S.; McKean, D. Posterior circulation infarction in patients with traumatic cervical spinal cord injury and its relationship to vertebral artery injury. Spinal Cord 2015, 53, 125–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torina, P.J.; Flanders, A.E.; Carrino, J.A.; Burns, A.S.; Friedman, D.P.; Harrop, J.S.; Vacarro, A.R. Incidence of vertebral artery thrombosis in cervical spine trauma: Correlation with severity of spinal cord injury. AJNR Am. J. Neuroradiol. 2005, 26, 2645–2651. [Google Scholar]
- Lichy, C.; Metso, A.; Pezzini, A.; Leys, D.; Metso, T.; Lyrer, P.; Debette, S.; Thijs, V.; Abboud, S.; Kloss, M.; et al. Predictors of delayed stroke in patients with cervical artery dissection. Int. J. Stroke 2015, 10, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Lytle, M.E.; West, J.; Burkes, J.N.; Beteck, B.; Fisher, T.; Daoud, Y.; Gable, D.R.; Shutze, W.P. Limited clinical relevance of vertebral artery injury in blunt trauma. Ann. Vasc. Surg. 2018, 53, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.R.; Fink, J.R.; Cohen, W.A. Cervical collaterals may protect against stroke after blunt vertebral artery injury. Emerg. Radiol. 2011, 18, 545–549. [Google Scholar] [CrossRef]
- Esposito, E.C.; Kufera, J.A.; Wolff, T.W.; Spalding, M.C.; Simpson, J.; Dunn, J.A.; Zier, L.; Burruss, S.; Kim, P.; Jacobson, L.E.; et al. Factors associated with stroke formation in blunt cerebrovascular injury: An east multicenter study. J. Trauma Acute Care Surg. 2022, 92, 347–354. [Google Scholar] [CrossRef]
- Biffl, W.L.; Ray, C.E.; Moore, E.E.; Franciose, R.J.; Aly, S.; Heyrosa, M.G.; Johnson, J.L.; Burch, J.M. Treatment-related outcomes from blunt cerebrovascular injuries: Importance of routine follow-up arteriography. Ann. Surg. 2002, 235, 699–706; discussion 706–707. [Google Scholar] [CrossRef]
- Wagenaar, A.E.; Burlew, C.C.; Biffl, W.L.; Beauchamp, K.M.; Pieracci, F.M.; Stovall, R.T.; Jurkovich, G.J.; Moore, E.E. Early repeat imaging is not warranted for high-grade blunt cerebrovascular injuries. J. Trauma Acute Care Surg. 2014, 77, 540–545, quiz 650. [Google Scholar] [CrossRef]
- Scott, W.W.; Sharp, S.; Figueroa, S.A.; Eastman, A.L.; Hatchette, C.V.; Madden, C.J.; Rickert, K.L. Clinical and radiological outcomes following traumatic grade 3 and 4 vertebral artery injuries: A 10-year retrospective analysis from a level i trauma center. The parkland carotid and vertebral artery injury survey. J. Neurosurg. 2015, 122, 1202–1207. [Google Scholar] [CrossRef]
- Nally, M.C.; Kling, C.; Hocking, K.M.; Lillemoe, H.; Boll, J.M.; Curci, J.A.; Garrard, C.L.; Naslund, T.C.; Valentine, R.J. Follow-up imaging of traumatic vertebral artery dissections is unnecessary in asymptomatic patients. J. Vasc. Surg. 2019, 69, 1704–1709. [Google Scholar] [CrossRef]
- Boggs, H.K.; Kiang, S.C.; Tran, Z.; Mukherjee, K.; Tomihama, R.T. Analysis of extracranial cerebrovascular injuries: Clinical predictors of management and outcomes. Ann. Vasc. Surg. 2024, 100, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.W.; Sharp, S.; Figueroa, S.A.; Madden, C.J.; Rickert, K.L. Clinical and radiological outcomes following traumatic grade 1 and 2 vertebral artery injuries: A 10-year retrospective analysis from a level 1 trauma center. J. Neurosurg. 2014, 121, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Indo, M.; Oya, S.; Shojima, M.; Inokuchi, K.; Yahata, T.; Sugiyama, S.; Matsui, T. Prevention of thromboembolic infarction after surgery for traumatic cervical fracture with vertebral artery occlusion by preoperative endovascular coil embolization. World Neurosurg. 2019, 129, e838–e844. [Google Scholar] [CrossRef]
- Asukai, M.; Ushirozako, H.; Suda, K.; Matsumoto Harmon, S.; Komatsu, M.; Minami, A.; Takahata, M.; Iwasaki, N.; Matsuyama, Y. Safety of early posterior fusion surgery without endovascular embolization for asymptomatic vertebral artery occlusion associated with cervical spine trauma. Eur. Spine J. 2022, 31, 3392–3401. [Google Scholar] [CrossRef] [PubMed]
- Veras, L.M.; Pedraza-Gutiérrez, S.; Castellanos, J.; Capellades, J.; Casamitjana, J.; Rovira-Cañellas, A. Vertebral artery occlusion after acute cervical spine trauma. Spine (Phila Pa 1976) 2000, 25, 1171–1177. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Mu, Z. Vertebral artery occlusion and recanalization after cervical facet dislocation. World Neurosurg. 2016, 95, 190–196. [Google Scholar] [CrossRef]
- Engelter, S.T.; Traenka, C.; Gensicke, H.; Schaedelin, S.A.; Luft, A.R.; Simonetti, B.G.; Fischer, U.; Michel, P.; Sirimarco, G.; Kägi, G.; et al. Aspirin versus anticoagulation in cervical artery dissection (treat-cad): An open-label, randomised, non-inferiority trial. Lancet Neurol. 2021, 20, 341–350. [Google Scholar] [CrossRef]
- Markus, H.S.; Levi, C.; King, A.; Madigan, J.; Norris, J. Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection. JAMA Neurol. 2019, 76, 657–664. [Google Scholar] [CrossRef]
- Cothren, C.C.; Biffl, W.L.; Moore, E.E.; Kashuk, J.L.; Johnson, J.L. Treatment for blunt cerebrovascular injuries: Equivalence of anticoagulation and antiplatelet agents. Arch. Surg. 2009, 144, 685–690. [Google Scholar] [CrossRef]
- Miller, P.R.; Fabian, T.C.; Bee, T.K.; Timmons, S.; Chamsuddin, A.; Finkle, R.; Croce, M.A. Blunt cerebrovascular injuries: Diagnosis and treatment. J Trauma 2001, 51, 279–285; discussion 285–286. [Google Scholar] [CrossRef]
- Pujari, A.; Ramos, C.R.; Nguyen, J.; Rajani, R.R.; Benarroch-Gampel, J. Pharmacologic therapy is not associated with stroke prevention in patients with isolated blunt vertebral artery injury. Ann. Vasc. Surg. 2021, 70, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.M.; Boswell, S.; Sliker, C.W.; Lui, F.Y.; Scalea, T.M. Blunt cerebrovascular injuries: Does treatment always matter? J Trauma 2009, 66, 132–143; discussion 143–144. [Google Scholar] [CrossRef] [PubMed]
- Zeineddine, H.A.; King, N.; Lewis, C.T.; Kole, M.J.; Kitagawa, R.; Dannenbaum, M.; Chen, P.R.; Day, A.L.; Blackburn, S. Blunt traumatic vertebral artery injuries: Incidence, therapeutic management, and outcomes. Neurosurgery 2022, 90, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, R.D.; Esposito, E.; Spaulding, M.C.; Simpson, J.P.; Dunn, J.; Zier, L.B.; Burruss, S.; Kim, P.P.; Jacobson, L.E.; Williams, J.M.; et al. Does treatment delay for blunt cerebrovascular injury affect stroke rate?: An east multicenter study. Injury 2022, 53, 3702–3708. [Google Scholar] [CrossRef]
- Camillo, F.X.; Mitchell, S.M. Cervical spine decompression and fusion outcomes in trauma patients actively receiving anticoagulation treatment for cerebrovascular injury: A retrospective comparative study. Int. J. Spine Surg. 2020, 14, 66–71. [Google Scholar] [CrossRef]
- Kim, D.Y.; Biffl, W.; Bokhari, F.; Brakenridge, S.; Chao, E.; Claridge, J.A.; Fraser, D.; Jawa, R.; Kasotakis, G.; Kerwin, A.; et al. Evaluation and management of blunt cerebrovascular injury: A practice management guideline from the eastern association for the surgery of trauma. J. Trauma Acute Care Surg. 2020, 88, 875–887. [Google Scholar] [CrossRef]
- Biffl, W.L.; Moore, E.E.; Ryu, R.K.; Offner, P.J.; Novak, Z.; Coldwell, D.M.; Franciose, R.J.; Burch, J.M. The unrecognized epidemic of blunt carotid arterial injuries: Early diagnosis improves neurologic outcome. Ann. Surg. 1998, 228, 462–470. [Google Scholar] [CrossRef]
- Eachempati, S.R.; Vaslef, S.N.; Sebastian, M.W.; Reed, R.L., 2nd. Blunt vascular injuries of the head and neck: Is heparinization necessary? J. Trauma 1998, 45, 997–1004. [Google Scholar] [CrossRef]
- Fabian, T.C.; Patton, J.H.; Croce, M.A.; Minard, G.; Kudsk, K.A.; Pritchard, F.E. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann. Surg. 1996, 223, 513–522; discussion 522–525. [Google Scholar] [CrossRef]
- Wahl, W.L.; Brandt, M.M.; Thompson, B.G.; Taheri, P.A.; Greenfield, L.J. Antiplatelet therapy: An alternative to heparin for blunt carotid injury. J. Trauma 2002, 52, 896–901. [Google Scholar] [CrossRef]
- Boggs, H.K.; Tomihama, R.T.; Tran, Z.; Mukherjee, K.; Turay, D.; Magtanong, E.; Pop, A.; Kiang, S.C. Medical management of traumatic vertebral artery injury is safe regardless of the severity of injury. Ann. Vasc. Surg. 2024, 101, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Callcut, R.A.; Hanseman, D.J.; Solan, P.D.; Kadon, K.S.; Ingalls, N.K.; Fortuna, G.R.; Tsuei, B.J.; Robinson, B.R. Early treatment of blunt cerebrovascular injury with concomitant hemorrhagic neurologic injury is safe and effective. J. Trauma Acute Care Surg. 2012, 72, 338–345; discussion 345–346. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Sui, M.; Xiao, W.; Sun, Z.; Bai, R.; Huang, C.; Hou, L. Individualized endovascular treatment of high-grade traumatic vertebral artery injury. Acta Neurochir. (Wien) 2014, 156, 1781–1788. [Google Scholar] [CrossRef]
- Cohen, J.E.; Gomori, J.M.; Rajz, G.; Rosenthal, G.; El Hassan, H.A.; Moscovici, S.; Itshayek, E. Vertebral artery pseudoaneurysms secondary to blunt trauma: Endovascular management by means of neurostents and flow diverters. J. Clin. Neurosci. 2016, 32, 77–82. [Google Scholar] [CrossRef]
- Pham, M.H.; Rahme, R.J.; Arnaout, O.; Hurley, M.C.; Bernstein, R.A.; Batjer, H.H.; Bendok, B.R. Endovascular stenting of extracranial carotid and vertebral artery dissections: A systematic review of the literature. Neurosurgery 2011, 68, 856–866; discussion 866. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Ahn, J.Y.; Han, I.B.; Chung, Y.S.; Hong, C.K.; Joo, J.Y. Therapeutic endovascular treatments for traumatic vertebral artery injuries. J. Trauma 2007, 62, 886–891. [Google Scholar] [CrossRef]
- Moon, K.; Albuquerque, F.C.; Cole, T.; Gross, B.A.; McDougall, C.G. Stroke prevention by endovascular treatment of carotid and vertebral artery dissections. J. Neurointerv. Surg. 2017, 9, 952–957. [Google Scholar] [CrossRef]
- Merrill, S.; Clifton, W.; Valero-Moreno, F.; Damon, A.; Rahmathulla, G. Vertebral artery injury with coinciding unstable cervical spine trauma: Mechanisms, evidence-based management, and treatment options. Cureus 2020, 12, e7225. [Google Scholar] [CrossRef]
- Isaji, T.; Ohshima, T.; Nakura, T.; Miyachi, S.; Joko, M.; Matsuo, N.; Kawaguchi, R.; Takayasu, M. Efficacy of endovascular proximal occlusion before direct reposition surgery of blunt cervical fracture with unilateral vertebral injury. NMC Case Rep. J. 2019, 6, 131–134. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kusakabe, K.; Nakao, S.; Hagihara, Y.; Matsuoka, T. Vertebral artery occlusion associated with blunt traumatic cervical spine injury. Acute Med. Surg. 2021, 8, e670. [Google Scholar] [CrossRef]
- Mwipatayi, B.P.; Jeffery, P.; Beningfield, S.J.; Motale, P.; Tunnicliffe, J.; Navsaria, P.H. Management of extra-cranial vertebral artery injuries. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Higashida, R.T.; Halbach, V.V.; Tsai, F.Y.; Norman, D.; Pribram, H.F.; Mehringer, C.M.; Hieshima, G.B. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: Results in 234 cases. AJR Am. J. Roentgenol. 1989, 153, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, D.; Theodorou, D.; Cornwell, E.; Berne, T.V.; Asensio, J.; Belzberg, H.; Velmahos, G.; Weaver, F.; Yellin, A. Evaluation of penetrating injuries of the neck: Prospective study of 223 patients. World J. Surg. 1997, 21, 41–47; discussion 47–48. [Google Scholar] [CrossRef] [PubMed]
- Berguer, R.; Flynn, L.M.; Kline, R.A.; Caplan, L. Surgical reconstruction of the extracranial vertebral artery: Management and outcome. J. Vasc. Surg. 2000, 31, 9–18. [Google Scholar] [CrossRef]
- Kieffer, E.; Praquin, B.; Chiche, L.; Koskas, F.; Bahnini, A. Distal vertebral artery reconstruction: Long-term outcome. J. Vasc. Surg. 2002, 36, 549–554. [Google Scholar] [CrossRef][Green Version]
| Grade | Definition |
|---|---|
| I | Dissection/luminal irregularity, with <25% stenosis |
| II | Dissection/intramural hematoma, with ≥25% stenosis, intraluminal thrombus, or raised intimal flap |
| III | Pseudoaneurysm |
| IV | Occlusion |
| V | Transection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teasdale, B.; Owolo, E.; Padmanaban, V.; Elsamadicy, A.A.; Amllay, A.; Shankar, G.M.; Krieger, P.P.; Regenhardt, R.W.; Hebert, R.M.; Stapleton, C.J.; et al. Traumatic Vertebral Artery Injury: Diagnosis, Natural History, and Key Considerations for Management. J. Clin. Med. 2025, 14, 3159. https://doi.org/10.3390/jcm14093159
Teasdale B, Owolo E, Padmanaban V, Elsamadicy AA, Amllay A, Shankar GM, Krieger PP, Regenhardt RW, Hebert RM, Stapleton CJ, et al. Traumatic Vertebral Artery Injury: Diagnosis, Natural History, and Key Considerations for Management. Journal of Clinical Medicine. 2025; 14(9):3159. https://doi.org/10.3390/jcm14093159
Chicago/Turabian StyleTeasdale, Ben, Edwin Owolo, Varun Padmanaban, Aladine A. Elsamadicy, Abdelaziz Amllay, Ganesh M. Shankar, Penina P. Krieger, Robert W. Regenhardt, Ryan M. Hebert, Christopher J. Stapleton, and et al. 2025. "Traumatic Vertebral Artery Injury: Diagnosis, Natural History, and Key Considerations for Management" Journal of Clinical Medicine 14, no. 9: 3159. https://doi.org/10.3390/jcm14093159
APA StyleTeasdale, B., Owolo, E., Padmanaban, V., Elsamadicy, A. A., Amllay, A., Shankar, G. M., Krieger, P. P., Regenhardt, R. W., Hebert, R. M., Stapleton, C. J., Rabinov, J. D., Matouk, C. C., Patel, A. B., & Sujijantarat, N. (2025). Traumatic Vertebral Artery Injury: Diagnosis, Natural History, and Key Considerations for Management. Journal of Clinical Medicine, 14(9), 3159. https://doi.org/10.3390/jcm14093159









