Intravenous Administration of Remdesivir at the Acute Phase of SARS-CoV-2 Infection Is Associated with a Lower Prevalence of Post-COVID-19 Pain
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. General Effects of Remdesivir
4.2. Mechanisms Explaining the Effects of Remdesivir
4.3. Previous Pain Conditions
4.4. Antiviral Treatment and COVID-19 Severity
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zur, M.; Peselev, T.; Yanko, S.; Rotshild, V.; Matok, I. Efficacy and safety of antiviral treatments for symptomatic COVID-19 outpatients: Systematic review and network meta-analysis. Antiviral. Res. 2024, 221, 105768. [Google Scholar] [CrossRef] [PubMed]
- Rahmah, L.; Abarikwu, S.O.; Arero, A.G.; Essouma, M.; Jibril, A.T.; Fal, A.; Flisiak, R.; Makuku, R.; Marquez, L.; Mohamed, K.; et al. Oral antiviral treatments for COVID-19: Opportunities and challenges. Pharmacol. Rep. 2022, 74, 1255–1278. [Google Scholar] [CrossRef] [PubMed]
- Malin, J.J.; Suárez, I.; Priesner, V.; Fätkenheuer, G.; Rybniker, J. Remdesivir against COVID-19 and other viral diseases. Clin. Microbiol. Rev. 2021, 34, e00162-20. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/docs/default-source/coronaviruse/module-1-introduction-to-therapeutics-for-covid-19.pdf/ (accessed on 1 April 2024).
- Zadeh, F.; Wilson, D.; Agrawal, D. Long COVID: Complications, underlying mechanisms, and treatment strategies. Arch. Microbiol. Immunol. 2023, 7, 36–61. [Google Scholar]
- Fernández-de-las-Peñas, C. Long COVID: Current definition. Infection 2022, 50, 285–286. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global prevalence of post COVID-19 condition or long COVID: A meta-analysis and systematic review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-term sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Notarte, K.I.; Macasaet, R.; Velasco, J.V.; Catahay, J.A.; Therese Ver, A.; Chung, W.; Valera-Calero, J.A.; Navarro-Santana, M. Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: A systematic review and meta-analysis. J. Infect. 2024, 88, 77–88. [Google Scholar] [CrossRef]
- Rahmati, M.; Udeh, R.; Yon, D.K.; Lee, S.W.; Dolja-Gore, X.; McEVoy, M.; Kenna, T.; Jacob, L.; López Sánchez, G.F.; Koyanagi, A.; et al. A systematic review and meta-analysis of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection: A call to action for neurological, physical, and psychological sciences. J. Med. Virol. 2023, 95, e28852. [Google Scholar] [CrossRef]
- Sebők, S.; Gyires, K. Long COVID and possible preventive options. Inflammopharmacology 2023, 31, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Torres-Macho, J.; Catahay, J.A.; Macasaet, R.; Velasco, J.V.; Macapagal, S.; Caldararo, M.; Henry, B.M.; Lippi, G.; Franco-Moreno, A.; et al. Is antiviral treatment at the acute phase of COVID-19 effective for decreasing the risk of long-COVID? A systematic review. Infection 2024, 52, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Boglione, L.; Meli, G.; Poletti, F.; Rostagno, R.; Moglia, R.; Cantone, M.; Esposito, M.; Scianguetta, C.; Domenicale, B.; Di Pasquale, F.; et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: Does remdesivir have a protective effect? QJM 2022, 114, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Badenes Bonet, D.; Caguana Vélez, O.A.; Duran Jordà, X.; Comas Serrano, M.; Posso Rivera, M.; Admetlló, M.; Herranz Blasco, A.; Cuadrado Godia, E.; Marco Navarro, E.; Martin Ezquerra, G.; et al. Treatment of COVID-19 during the acute phase in hospitalized patients decreases post-acute sequelae of COVID-19. J. Clin. Med. 2023, 12, 4158. [Google Scholar] [CrossRef]
- Nevalainen, O.P.O.; Horstia, S.; Laakkonen, S.; Rutanen, J.; Mustonen, J.M.; Kalliala, I.E.; Ansakorpi, H.; Kreivi, H.R.; Kuutti, P.; Paajanen, J.; et al. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat. Commun. 2022, 13, 6152. [Google Scholar] [CrossRef]
- Fésü, D.; Bárczi, E.; Csoma, B.; Polivka, L.; Boga, M.; Horváth, G.; Varga, J.T.; Sebők, S.; Müller, V. Real-world evidence of remdesivir in formerly hospitalized COVID-19 patients: Patient-reported and functional outcomes. BMC Infect. Dis. 2025, 25, 43. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Navarro-Santana, M.; Plaza-Manzano, G.; Palacios-Ceña Arendt-Nielsen, L. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived to SARS-CoV-2 infection: A systematic review and meta-analysis. Pain 2022, 163, 1220–1231. [Google Scholar] [CrossRef]
- Kerzhner, O.; Berla, E.; Har-Even, M.; Ratmansky, M.; Goor-Aryeh, I. Consistency of inconsistency in long-COVID-19 pain symptoms persistency: A systematic review and meta-analysis. Pain Pract. 2024, 24, 120–159. [Google Scholar] [CrossRef]
- Perrot, S.; Cohen, M.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic secondary musculoskeletal pain. Pain 2019, 160, 77–82. [Google Scholar] [CrossRef]
- Herrmann-Lingen, C.; Buss, U.; Snaith, R.P. Hospital Anxiety and Depression Scale—Deutsche Version (HADS-D); Verlag Hans Huber: Bern, Switzerland, 2011. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Hedman, E.; Ljótsson, B.; Blom, K.; El Alaoui, S.; Kraepelien, M.; Rück, C.; Andersson, G.; Svanborg, C.; Lindefors, N.; Kaldo, V. Telephone versus internet administration of self-report measures of social anxiety, depressive symptoms, and insomnia: Psychometric evaluation of a method to reduce the impact of missing data. J. Med. Internet Res. 2013, 15, e229. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Rodríguez-Jiménez, J.; Palacios-Ceña, M.; de-la-Llave-Rincón, A.I.; Fuensalida-Novo, S.; Florencio, L.L.; Ambite-Quesada, S.; Ortega-Santiago, R.; Arias-Buría, J.L.; Liew, B.X.W.; et al. Psychometric properties of the Hospital Anxiety and Depression Scale (HADS) in previously hospitalized COVID-19 patients. Int. J. Environ. Res. Public Health 2022, 19, 9273. [Google Scholar] [CrossRef] [PubMed]
- Olssøn, I.; Mykletun, A.; Dahl, A.A. The Hospital Anxiety and Depression Rating Scale: A cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry 2005, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, N.; Soprun, L.; Lukashenko, M.; Ryabkova, V.; Fedotkina, T.V.; Churilov, L.P.; Shoenfeld, Y. New clinical phenotype of the Post-COVID syndrome: Fibromyalgia and joint hypermobility condition. Pathophysiology 2022, 29, 24–29. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Nijs, J.; Neblett, R.; Polli, A.; Moens, M.; Goudman, L.; Shekhar Patil, M.; Knaggs, R.D.; Pickering, G.; Arendt-Nielsen, L. Phenotyping post-COVID pain as a nociceptive, neuropathic, or nociplastic pain condition. Biomedicines 2022, 10, 2562. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Nijs, J.; Giordano, R.; Arendt-Nielsen, L. Precision management of post-COVID pain: An evidence and clinical-based approach. Eur. J. Pain 2023, 27, 1107–1125. [Google Scholar] [CrossRef]
- Liew, F.; Efstathiou, C.; Fontanella, S.; Richardson, M.; Saunders, R.; Swieboda, D.; Sidhu, J.K.; Ascough, S.; Moore, S.C.; Mohamed, N.; et al. PHOSP-COVID collaborative group; ISARIC investigators. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat. Immunol. 2024, 25, 607–621. [Google Scholar] [CrossRef]
- Cascella, M.; Del Gaudio, A.; Vittori, A.; Bimonte, S.; Del Prete, P.; Forte, C.A.; Cuomo, A.; De Blasio, E. COVID-Pain: Acute and late-onset painful clinical manifestations in COVID-19: Molecular mechanisms and research perspectives. J. Pain Res. 2021, 14, 2403–2412. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Rodríguez-Jiménez, J.; Fuensalida-Novo, S.; Palacios-Ceña, M.; Gómez-Mayordomo, V.; Florencio, L.L.; Hernández-Barrera, V.; Arendt-Nielsen, L. Myalgia as a symptom at hospital admission by severe acute respiratory syndrome coronavirus 2 infection is associated with persistent musculoskeletal pain as long-term post-COVID sequelae: A case-control study. Pain 2021, 162, 2832–2840. [Google Scholar] [CrossRef]
- Bergmans, R.S.; Clauw, D.J.; Flint, C.; Harris, H.; Lederman, S.; Schrepf, A. Chronic overlapping pain conditions increase the risk of long COVID features, regardless of acute COVID status. Pain 2024, 165, 1112–1120. [Google Scholar] [CrossRef]
- FDA Approves First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 10 January 2025).
- First COVID-19 Treatment Recommended for EU Authorization. Available online: https://www.ema.europa.eu/en/news/first-covid-19-treatment-recommended-eu-authorisation (accessed on 10 January 2025).
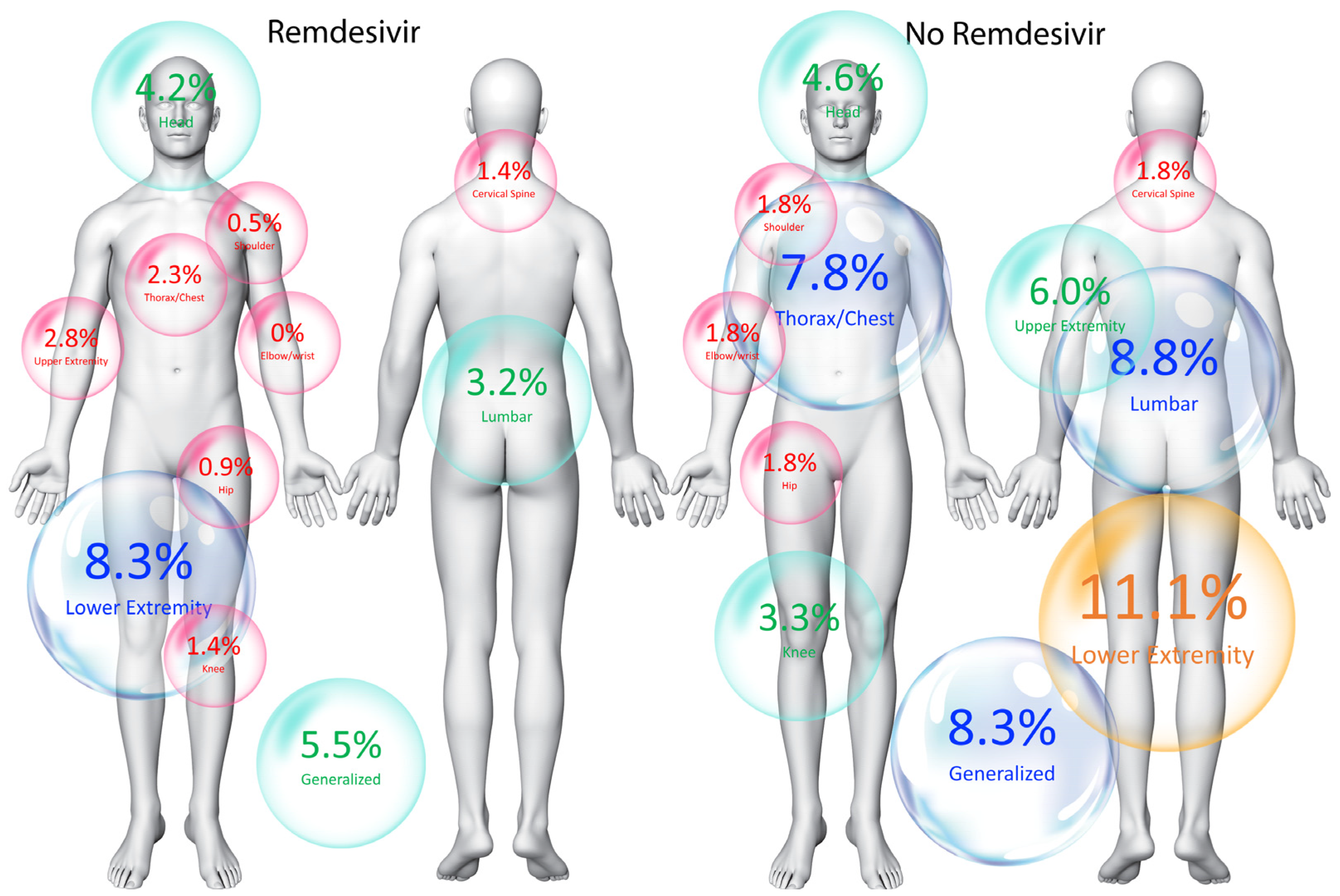
| No Remdesivir (n = 216) | Remdesivir (n = 216) | p-Value | |
|---|---|---|---|
| Female n (%) | 94 (43.5%) | 94 (43.5%) | — |
| Age (years) | 55.6 ± 12.7 | 55.4 ± 12.6 | 0.882 |
| Weight (kg) | 81.9 ± 15.2 | 80.8 ± 14.0 | 0.441 |
| Height (cm) | 167.4 ± 10.9 | 166.6 ± 9.2 | 0.409 |
| Days at hospital (mean ± SD) | 12.9 ± 12.6 | 16.6 ± 12.4 | 0.365 |
| ICU admission n (%) | 28 (12.9%) | 29 (13.4%) | 0.816 |
| Pre-existing chronic pain conditions before infection | |||
| Migraine | 5 (2.3%) | 1 (0.5%) | 0.686 |
| Headache | 10 (4.6%) | 10 (4.6%) | 0.999 |
| Arthritis | 5 (2.3%) | 2 (1.0%) | 0.528 |
| Arthrosis | 23 (10.6%) | 18 (8.3%) | 0.434 |
| Fibromyalgia | 1 (0.5%) | 1 (0.5%) | 0.999 |
| Localized musculoskeletal pain | 42 (19.4%) | 47 (21.8%) | 0.596 |
| Pre-existing medical conditions before infection | |||
| Obesity (pre-existing) | 20 (9.25%) | 28 (13.0%) | 0.248 |
| Hypertension (pre-existing) | 64 (29.6%) | 51 (23.6%) | 0.225 |
| Diabetes (pre-existing) | 25 (11.6%) | 9 (4.2%) | 0.242 |
| Asthma (pre-existing) | 10 (4.6%) | 11 (5.1%) | 0.827 |
| COPD (pre-existing) | 7 (3.25%) | 6 (2.8%) | 0.781 |
| Cardiac diseases (pre-existing) | 23 (10.65) | 18 (8.3%) | 0.435 |
| Rheumatological diseases (pre-existing) | 1 (0.45%) | 0 (0.0%) | 0.318 |
| Location of post-COVID-19 pain symptomatology | |||
| Generalized | 18 (8.3%) | 12 (5.5%) | 0.273 |
| Head | 10 (4.6%) | 9 (4.2%) | 0.818 |
| Cervical spine | 4 (1.8%) | 3 (1.4%) | 0.705 |
| Shoulder | 4 (1.8%) | 1 (0.5%) | 0.178 |
| Elbow–wrist | 4 (1.8%) | 1 (0.5%) | 0.178 |
| Hip | 4 (1.8%) | 2 (0.9%) | 0.414 |
| Knee | 7 (3.3%) | 3 (1.4%) | 0.206 |
| Thorax/chest | 17 (7.8%) | 5 (2.3%) | 0.02 * |
| Lumbar | 19 (8.8%) | 7 (3.2%) | 0.02 * |
| Lower extremity | 24 (11.1%) | 18 (8.3%) | 0.354 |
| Upper extremity | 13 (6.0%) | 6 (2.8%) | 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-de-las-Peñas, C.; Franco-Moreno, A.; Ruiz-Ruigómez, M.; Arrieta-Ortubay, E.; Ryan-Murua, P.; Lumbreras-Bermejo, C.; del-Valle-Loarte, P.; Pellicer-Valero, O.J.; Torres-Macho, J.; Giordano, R.; et al. Intravenous Administration of Remdesivir at the Acute Phase of SARS-CoV-2 Infection Is Associated with a Lower Prevalence of Post-COVID-19 Pain. J. Clin. Med. 2025, 14, 3156. https://doi.org/10.3390/jcm14093156
Fernández-de-las-Peñas C, Franco-Moreno A, Ruiz-Ruigómez M, Arrieta-Ortubay E, Ryan-Murua P, Lumbreras-Bermejo C, del-Valle-Loarte P, Pellicer-Valero OJ, Torres-Macho J, Giordano R, et al. Intravenous Administration of Remdesivir at the Acute Phase of SARS-CoV-2 Infection Is Associated with a Lower Prevalence of Post-COVID-19 Pain. Journal of Clinical Medicine. 2025; 14(9):3156. https://doi.org/10.3390/jcm14093156
Chicago/Turabian StyleFernández-de-las-Peñas, César, Anabel Franco-Moreno, María Ruiz-Ruigómez, Estibaliz Arrieta-Ortubay, Pablo Ryan-Murua, Carlos Lumbreras-Bermejo, Pablo del-Valle-Loarte, Oscar J. Pellicer-Valero, Juan Torres-Macho, Rocco Giordano, and et al. 2025. "Intravenous Administration of Remdesivir at the Acute Phase of SARS-CoV-2 Infection Is Associated with a Lower Prevalence of Post-COVID-19 Pain" Journal of Clinical Medicine 14, no. 9: 3156. https://doi.org/10.3390/jcm14093156
APA StyleFernández-de-las-Peñas, C., Franco-Moreno, A., Ruiz-Ruigómez, M., Arrieta-Ortubay, E., Ryan-Murua, P., Lumbreras-Bermejo, C., del-Valle-Loarte, P., Pellicer-Valero, O. J., Torres-Macho, J., Giordano, R., & Arendt-Nielsen, L. (2025). Intravenous Administration of Remdesivir at the Acute Phase of SARS-CoV-2 Infection Is Associated with a Lower Prevalence of Post-COVID-19 Pain. Journal of Clinical Medicine, 14(9), 3156. https://doi.org/10.3390/jcm14093156






