Physical Activity and Sedentary Behaviour in People with Long COVID: A Follow-Up from 12 to 18 Months After Discharge
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
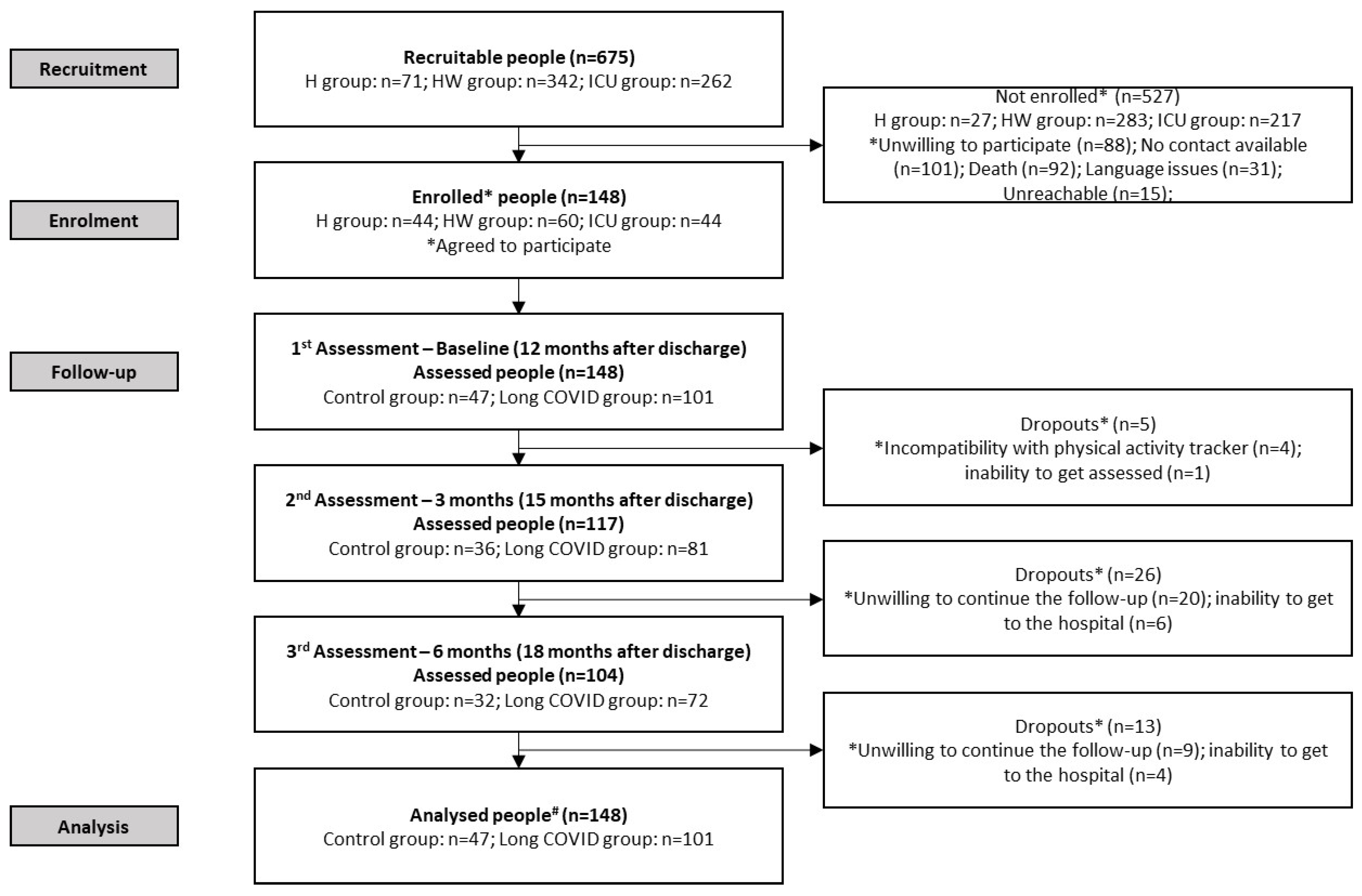
2.2. Participants
2.3. Procedures
2.4. Physical Activity and Sedentary Behaviour
2.5. Secondary Outcomes
2.6. Statistical Analyses
2.6.1. Physical Activity and Sedentary Behaviour in People with and Without Long COVID
2.6.2. Potential Contributing Factors for PA and Sedentary Behaviour Change
3. Results
3.1. Participants’ Baseline Characteristics
3.2. Physical Activity and Sedentary Behaviour in People with and Without Long COVID
3.3. Potential Contributing Factors for Physical Activity and Sedentary Behaviour Change
3.4. Secondary Outcomes
3.5. Exploratory Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1minSTS | 1-min sit-to-stand test |
| 6MWD | 6-min walk test |
| COVID-19 | Coronavirus Disease 2019 |
| EQ-5D-5L | European quality of life—5 Dimensions—5 Levels |
| FACIT-FS | Functional Assessment of Chronic Illness Therapy—Fatigue |
| HADS | Hospital Anxiety and Depression Scale |
| HRQoL | Health-related quality of life |
| mMRC | Modified Medical Research Council |
| PA | Physical activity |
| QMVC | Quadriceps muscle voluntary contraction |
References
- The Lancet Respiratory Medicine. Long COVID: Confronting a Growing Public Health Crisis. Lancet Respir. Med. 2023, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Hou, Y.; Gu, T.; Ni, Z.; Shi, X.; Ranney, M.L.; Mukherjee, B. Global Prevalence of Long COVID, Its Subtypes and Risk Factors: An Updated Systematic Review and Meta-Analysis. medRxiv 2025. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Baldwin, M.M.; Daynes, E.; Evans, R.A.; Greening, N.J.; Jenkins, R.G.; Lone, N.I.; McAuley, H.; Mehta, P.; Newman, J.; et al. Respiratory Sequelae of COVID-19: Pulmonary and Extrapulmonary Origins, and Approaches to Clinical Care and Rehabilitation. Lancet Respir. Med. 2023, 11, 709–725. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and Mechanism of Long COVID: A Comprehensive Review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Shah, D.P.; Thaweethai, T.; Karlson, E.W.; Bonilla, H.; Horne, B.D.; Mullington, J.M.; Wisnivesky, J.P.; Hornig, M.; Shinnick, D.J.; Klein, J.D.; et al. Sex Differences in Long COVID. JAMA Netw. Open 2025, 8, e2455430. [Google Scholar] [CrossRef]
- Mansell, V.; Hall Dykgraaf, S.; Kidd, M.; Goodyear-Smith, F. Long COVID and Older People. Lancet Healthy Longev. 2022, 3, e849–e854. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More Than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Fernandez-de-las-Peñas, C.; Palacios-Cena, D.; Gomez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of Post-COVID-19 Symptoms in Hospitalized and Non-Hospitalized COVID-19 Survivors: A Systematic Review and Meta-Analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, Predictors and Clinical Correlates of Post-COVID Syndrome (PCS) in Germany: A Prospective, Multi-Centre, Population-Based Cohort Study. EClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Serra-Sutton, V.; Soriano, J.B.; Ferrer, M.; Trejo, A.; Benavides, F.G.; Lumbreras, B.; Pérez-Gómez, B.; Pijoan, J.I.; Monguet, J.M.; et al. Consensus on Post COVID in the Spanish National Health System: Results of the CIBERPOSTCOVID EDelphi Study. J. Infect. Public Health 2023, 16, 1784–1792. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Sanchis-Gomar, F. Physical Activity, Long-COVID, and Inactivity: A Detrimental Endless Loop. J. Phys. Act. Health 2024, 21, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, A.; Savci, S.; Kahraman, B.O.; Ozpelit, E. Extrapulmonary Features of Post-COVID-19 Patients: Muscle Function, Physical Activity, Mood, and Sleep Quality. Ir. J. Med. Sci. 2022, 191, 969–975. [Google Scholar] [CrossRef]
- Delbressine, J.M.; Machado, F.V.C.; Goërtz, Y.M.J.; Van Herck, M.; Meys, R.; Houben-Wilke, S.; Burtin, C.; Franssen, F.M.E.; Spies, Y.; Vijlbrief, H.; et al. The Impact of Post-COVID-19 Syndrome on Self-Reported Physical Activity. Int. J. Environ. Res. Public Health 2021, 18, 6017. [Google Scholar] [CrossRef]
- Plekhanova, T.; Rowlands, A.V.; Evans, R.A.; Edwardson, C.L.; Bishop, N.C.; Bolton, C.E.; Chalmers, J.D.; Davies, M.J.; Daynes, E.; Dempsey, P.C.; et al. Device-Assessed Sleep and Physical Activity in Individuals Recovering from a Hospital Admission for COVID-19: A Multicentre Study. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Ballouz, T.; Menges, D.; Anagnostopoulos, A.; Domenghino, A.; Aschmann, H.E.; Frei, A.; Fehr, J.S.; Puhan, M.A. Recovery and Symptom Trajectories up to Two Years After SARS-CoV-2 Infection: Population Based, Longitudinal Cohort Study. BMJ 2023, 381, e074425. [Google Scholar] [CrossRef]
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Impact of Physical Inactivity on the World’s Major Non-Communicable Diseases. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef]
- Demeyer, H.; Mohan, D.; Burtin, C.; Vaes, A.W.; Heasley, M.; Bowler, R.P.; Casaburi, R.; Cooper, C.B.; Corriol-Rohou, S.; Frei, A.; et al. Objectively Measured Physical Activity in Patients with COPD: Recommendations from an International Task Force on Physical Activity. Chronic Obstr. Pulm. Dis. 2021, 8, 528–550. [Google Scholar] [CrossRef] [PubMed]
- Yarowsky, D. Unsupervised Word Sense Disambiguation Rivaling Supervised Methods. In Proceedings of the Annual Meeting of the Association for Computational Linguistics, Cambridge, MA, USA, 26–30 June 1995; pp. 189–196. [Google Scholar]
- Schölkopf, B.; Smola, A.J. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond; The MIT Press: Cambridge, MA, USA, 2018; ISBN 9780262256933. [Google Scholar]
- Muhsen, H.; Al-Amaydeh, O.; Al-Hamlan, R. Algorithm Design for Accurate Steps Counting Based on Smartphone Sensors for Indoor Applications. Adv. Sci. Technol. Eng. Syst. 2020, 5, 811–816. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Lluva-Plaza, S.; Jiménez-Martín, A.; Gualda-Gómez, D.; Villadangos-Carrizo, J.M.; García-Domínguez, J.J. Multisensory System for Long-Term Activity Monitoring to Facilitate Aging-in-Place. Sensors 2023, 23, 8646. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An Official European Respiratory Society/American Thoracic Society Technical Standard: Field Walking Tests in Chronic Respiratory Disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Crook, S.; Büsching, G.; Schultz, K.; Lehbert, N.; Jelusic, D.; Keusch, S.; Wittmann, M.; Schuler, M.; Radtke, T.; Frey, M.; et al. A Multicentre Validation of the 1-Min Sit-to-Stand Test in Patients with COPD. Eur. Respir. J. 2017, 49, 1601871. [Google Scholar] [CrossRef]
- Lesnak, J.; Anderson, D.; Farmer, B.; Katsavelis, D.; Grindstaff, T.L. Validity of Hand-Held Dynamometry in Measuring Quadriceps Strength and Rate of Torque Development. Int. J. Sports Phys. Ther. 2019, 14, 180–187. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Kindig, J.; Sabo, G.; Duni, A.E.; Cram, P. Isometric Knee Extension Force Measured Using a Handheld Dynamometer with and Without Belt-Stabilization. Physiother. Theory Pract. 2012, 28, 562–568. [Google Scholar] [CrossRef]
- Gimeno-Santos, E.; Arbillaga-Etxarri, A.; Vilaró, J.; Balañá, A.; Barberán-Garcia, A.; Del Corral-Núñez, T.; Fernández, J.C.; Jiménez, B.; López, A.; López, D.; et al. Reference Equations for 6-Minute Walk Test in Spanish Population. Eur. Respir. J. 2015, 46, PA1543. [Google Scholar] [CrossRef]
- Vilarinho, R.; Montes, A.M.; Noites, A.; Silva, F.; Melo, C. Reference Values for the 1-Minute Sit-to-Stand and 5 Times Sit-to-Stand Tests to Assess Functional Capacity: A Cross-Sectional Study. Physiotherapy 2024, 124, 85–92. [Google Scholar] [CrossRef]
- Tanguay, S.; Saey, D.; Marklund, S.; Nyberg, A.; Gephine, S.; Frykholm, E.; De Brandt, J.; Burtin, C.; Maltais, F. Reference Equations for Quadriceps Strength, Endurance and Power: A Multicentre Study. ERJ Open Res. 2023, 9, 00313-02023. [Google Scholar] [CrossRef] [PubMed]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) Dyspnoea Scale as a Measure of Disability in Patients with Chronic Obstructive Pulmonary Disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, S.; Dures, E.; Almeida, C. Measures of Fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for Severity, Effect, and Coping, Chalder Fatigue Questionnaire (CFQ), Checklist. Arthritis Care Res. 2011, 63, S263–S286. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The Validity of the Hospital Anxiety and Depression Scale. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Hernandez, G.; Garin, O.; Pardo, Y.; Vilagut, G.; Pont, À.; Suárez, M.; Neira, M.; Rajmil, L.; Gorostiza, I.; Ramallo-Fariña, Y.; et al. Validity of the EQ–5D–5L and Reference Norms for the Spanish Population. Qual. Life Res. 2018, 27, 2337–2348. [Google Scholar] [CrossRef]
- Garcia-Gordillo, M.A.; Adsuar, J.C.; Olivares, P.R. Normative Values of EQ-5D-5L: In a Spanish Representative Population Sample from Spanish Health Survey, 2011. Qual. Life Res. 2016, 25, 1313–1321. [Google Scholar] [CrossRef]
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: An International Classification of Diseases for the Twenty-First Century. BMC Med. Inform. Decis. Mak. 2021, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- WHO. Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC Classification and DDD Assignment 2024; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2023. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; Miller, M.R.; et al. Standardization of Spirometry 2019 Update an Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, E70–E88. [Google Scholar] [CrossRef]
- Collins, G.S.; Dhiman, P.; Ma, J.; Schlussel, M.M.; Archer, L.; Van Calster, B.; Harrell, F.E.; Martin, G.P.; Moons, K.G.M.; van Smeden, M.; et al. Evaluation of Clinical Prediction Models (Part 1): From Development to External Validation. BMJ 2024, 384, e074819. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, H.; Burtin, C.; Hornikx, M.; Camillo, C.A.; Van Remoortel, H.; Langer, D.; Janssens, W.; Troosters, T. The Minimal Important Difference in Physical Activity in Patients with COPD. PLoS ONE 2016, 11, e0154587. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Souza, F.J.; Freire, Y.A.; Galliano, L.M.; Dalton-Alves, F.; de Lima Pinto, J.C.B.; Godtsfriedt, C.E.S.; Delevatti, R.S.; Gerage, A.M.; Rech, C.R.; Ritti-Dias, R.M.; et al. Association of Physical Symptoms with Accelerometer-Measured Movement Behaviors and Functional Capacity in Individuals with Long COVID. Sci. Rep. 2024, 14, 20652. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle Abnormalities Worsen after Post-Exertional Malaise in Long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.P.C.F.; Lira, F.S.; Morano, A.E.v.A.; Pereira, T.; Coelho-E-Silva, M.-J.; Caseiro, A.; Christofaro, D.G.D.; Júnior, O.M.; Dorneles, G.P.; Minuzzi, L.G.; et al. Role of Body Mass and Physical Activity in Autonomic Function Modulation on Post-COVID-19 Condition: An Observational Subanalysis of Fit-COVID Study. Int. J. Environ. Res. Public Health 2022, 19, 2457. [Google Scholar] [CrossRef]
- Daines, L.; Zheng, B.; Elneima, O.; Harrison, E.; Lone, N.I.; Hurst, J.R.; Brown, J.S.; Sapey, E.; Chalmers, J.D.; Quint, J.K.; et al. Characteristics and Risk Factors for Post-COVID-19 Breathlessness after Hospitalisation for COVID-19. ERJ Open Res. 2023, 9, 00274-02022. [Google Scholar] [CrossRef]
- Hanania, N.A.; O’Donnell, D.E. Activity-Related Dyspnea in Chronic Obstructive Pulmonary Disease: Physical and Psychological Consequences, Unmet Needs, and Future Directions. Int. J. Chron. Obs. Pulmon Dis. 2019, 14, 1127–1138. [Google Scholar] [CrossRef]
- Smith, M.P.; Sharpe, H.; Damant, R.W.; Ferrara, G.; Lim, R.K.; Stickland, M.K.; Lam, G.Y. Factors Associated with Phenotypes of Dyspnea in Post-COVID-19 Condition: A Cross-Sectional Study. Sci. Rep. 2024, 14, 13387. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Hoffman, M.; Jones, A.W.; Holland, A.E.; Borghi-Silva, A. Effect of Pulmonary Rehabilitation on Exercise Capacity, Dyspnea, Fatigue, and Peripheral Muscle Strength in Patients with Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2024, 105, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ortigosa, L.; Gálvez-Álvarez, P.; Viñolo-Gil, M.J.; Rodriguez-Huguet, M.; Góngora-Rodríguez, J.; Martín-Valero, R. Effectiveness of Pulmonary Rehabilitation Programmes and/or Respiratory Muscle Training in Patients with Post-COVID Conditions: A Systematic Review. Respir. Res. 2024, 25, 248. [Google Scholar] [CrossRef] [PubMed]
- Poppele, I.; Ottiger, M.; Stegbauer, M.; Schlesinger, T.; Müller, K. Device-Assessed Physical Activity and Sleep Quality of Post-COVID Patients Undergoing a Rehabilitation Program. BMC Sports Sci. Med. Rehabil. 2024, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.; Sails, J.; Stavropoulos-Kalinoglou, A.; Birch, R.J.; McKenna, J.; Clifton, I.J.; Peckham, D.; Birch, K.M.; Price, O.J. Physical Activity Promotion Interventions in Chronic Airways Disease: A Systematic Review and Meta-Analysis. Eur. Respir. Rev. 2023, 32, 220109. [Google Scholar] [CrossRef] [PubMed]
- Megaritis, D.; Hume, E.; Chynkiamis, N.; Buckley, C.; Polhemus, A.M.; Watz, H.; Troosters, T.; Vogiatzis, I. Effects of Pharmacological and Non-Pharmacological Interventions on Physical Activity Outcomes in COPD: A Systematic Review and Meta-Analysis. ERJ Open Res. 2023, 9, 00409-02023. [Google Scholar] [CrossRef]
- Sanal-Hayes, N.E.M.; Mclaughlin, M.; Hayes, L.D.; Mair, J.L.; Ormerod, J.; Carless, D.; Hilliard, N.; Meach, R.; Ingram, J.; Sculthorpe, N.F. A Scoping Review of ‘Pacing’ for Management of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Lessons Learned for the Long COVID Pandemic. J. Transl. Med. 2023, 21, 720. [Google Scholar] [CrossRef]
- N3C—Home. Available online: https://covid.cd2h.org/ (accessed on 9 May 2025).
- Home | RECOVER COVID Initiative. Available online: https://recovercovid.org/ (accessed on 9 May 2025).
- REICOP | Red Española de Investigación en COVID Persistente. Available online: https://reicop.org/ (accessed on 9 May 2025).
- Ewing, A.G.; Joffe, D.; Blitshteyn, S.; Brooks, A.E.S.; Wist, J.; Bar-Yam, Y.; Bilodeau, S.; Curtin, J.; Duncan, R.; Faghy, M.; et al. Long COVID Clinical Evaluation, Research and Impact on Society: A Global Expert Consensus. Ann. Clin. Microbiol. Antimicrob. 2025, 24, 27. [Google Scholar] [CrossRef]
- Keller, B.; Receno, C.N.; Franconi, C.J.; Harenberg, S.; Stevens, J.; Mao, X.; Stevens, S.R.; Moore, G.; Levine, S.; Chia, J.; et al. Cardiopulmonary and Metabolic Responses During a 2-Day CPET in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Translating Reduced Oxygen Consumption to Impairment Status to Treatment Considerations. J. Transl. Med. 2024, 22, 627. [Google Scholar] [CrossRef]

| All | Control Group | Long COVID Group | Sig. | |
|---|---|---|---|---|
| Participants n | 148 | 47 | 101 | |
| Demographic and anthropometric data | ||||
| Age (years) mean ± SD | 58 ± 15 | 54 ± 17 | 60 ± 14 | p = 0.04 * |
| Male | 80 (54) | 31 (66) | 49 (49) | p = 0.05 |
| BMI (kg·m−2) mean ± SE | 27.9 ± 0.4 | 27.0 ± 0.7 | 28.8 ± 0.5 | p = 0.30 |
| Lung function | ||||
| FEV1%predicted mean ± SE | 90 ± 1 | 92 ± 2 | 87 ± 2 | p = 0.76 |
| FVC%predicted mean ± SE | 86 ± 1 | 88 ± 2 | 84 ± 2 | p = 0.65 |
| Medical records | ||||
| Comorbidities median (Q1; Q3) | 2 (1; 3) | 1 (0; 2) | 2 (1; 3) | p < 0.01 * |
| Endocrine or metabolic diseases ¶ n (%) | 60 (41) | 11 (23) | 49 (49) | p < 0.01 * |
| Circularity system diseases ¶ n (%) | 59 (40) | 18 (38) | 41 (41) | p = 0.86 |
| Musculoskeletal system diseases ¶ n (%) | 30 (20) | 6 (13) | 24 (24) | p = 0.13 |
| Respiratory system diseases ¶ n (%) | 22 (15) | 4 (9) | 18 (18) | p = 0.21 |
| Immune system diseases ¶ n (%) | 17 (12) | 4 (9) | 13 (13) | p = 0.58 |
| Nervous system diseases ¶ n (%) | 12 (8) | 2 (4) | 10 (10) | p = 0.34 |
| Others ¶ n (%) | 33 (22) | 9 (19) | 24 (24) | p = 0.67 |
| Charlson comorbidity index median (Q1; Q3) | 2 (0; 3) | 2 (0; 3) | 2 (1; 3) | p = 0.09 |
| Charlson comorbidity categories (mild/moderate/severe) | 53 (36)/48 (32)/8 (5) | 16 (34)/15 (32)/0 (0) | 37 (37)/33 (33)/8 (8) | p = 0.14 |
| Medications median (Q1; Q3) | 1 (0; 2) | 1 (0; 1) | 1 (1; 2) | p < 0.01 * |
| Cardiovascular system # n (%) | 65 (44) | 19 (40) | 46 (46) | p = 0.60 |
| Alimentary track and metabolism # n (%) | 25 (17) | 3 (6) | 22 (22) | p = 0.02 * |
| Respiratory system # n (%) | 20 (14) | 1 (2) | 14 (14) | p = 0.30 |
| Systemic hormonal preparations, and insulins # n (%) | 19 (13) | 1 (2) | 18 (18) | p < 0.01 * |
| Nervous system # n (%) | 15 (10) | 1 (2) | 3 (3) | p = 0.04 * |
| Others # | 13 (9) | 3 (6) | 10 (10) | p = 0.76 |
| All # | Control Group # | Long COVID Group # | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Twelve Months | Fifteen Months | Eighteen Months | Twelve Months | Fifteen Months | Eighteen Months | Twelve Months | Fifteen Months | Eighteen Months | Sig. | |
| Participants n | 148 | 117 | 104 | 47 | 36 | 32 | 101 | 81 | 72 | |
| Primary outcomes | ||||||||||
| LPA (min·day−1) | 272 ± 13 | 260 ± 12 | 277 ± 12 | 290 ± 22 | 268 ± 20 | 275 ± 20 | 253 ± 16 | 251 ± 13 | 279 ± 14 † | p = 0.29 |
| MVPA (min·day−1) | 11 ± 1 | 8 ± 1 † | 7 ± 1 † | 11 ± 2 | 8 ± 1 | 7 ± 1 | 12 ± 1 | 8 ± 1 † | 7 ± 1 † | p = 0.46 |
| Steps·day−1 | 5306 ± 301 | 4541 ± 252 † | 4513 ± 270 † | 5927 ± 486 | 5062 ± 412 | 5179 ± 446 | 4684 ± 356 | 4021 ± 285 | 3847 ± 306 | p = 0.81 |
| Steps·day−1 ≤ 5000 n (%) | 50 (34) | 62 (53) | 59 (57) † | 14 (30) | 18 (50) | 15 (47) | 36 (36) | 44 (54) | 44 (61) † | p = 0.61 |
| Sedentary time (min·day−1) | 425 ± 15 | 387 ± 12 † | 378 ± 14 † | 416 ± 24 | 386 ± 19 | 366 ± 22 | 434 ± 18 | 387 ± 13 † | 390 ± 15 | p = 0.61 |
| Secondary outcomes | ||||||||||
| 6MWD (m) | 578 ± 12 | 597 ± 12 † | 601 ± 13 † | 632 ± 19 | 653 ± 20 † | 663 ± 22 † | 523 ± 13 | 542 ± 13 † | 538 ± 15 | p = 0.44 |
| 6MWD ≤ 70% predicted n (%) | 30 (20) | 19 (16) | 12 (11) | 3 (6) | 1 (3) | 0 (0) | 27 (27) | 18 (22) † | 12(17) † | p = 0.48 |
| 1 min STS (reps) | 26 ± 1 | 28 ± 1 † | 29 ± 1 † | 30 ± 1 | 31 ± 1 † | 33 ± 2 † | 23 ± 1 | 25 ± 1 † | 25 ± 1 † | p = 0.11 |
| 1minSTS ≤ 70% predicted n (%) | 44 (30) | 29 (25) | 28 (27) | 7 (15) | 4 (11) | 4 (13) | 37 (37) | 25 (31) | 24 (33) † | p = 0.11 |
| QMVC (kgf) | 18 ± 1 | 18 ± 1 | 18 ± 1 | 20 ± 1 | 20 ± 1 | 21 ± 1 | 15 ± 1 | 15 ± 1 | 16 ± 1 | p = 0.37 |
| QMVC ≤ 70% predicted n (%) | 26 (18) | 19 (16) | 20 (19) | 2 (4) | 1 (3) | 2 (6) | 24 (24) | 18 (22) | 18 (25) | p = 0.95 |
| Dyspnoea mMRC | 1 (0; 1) | 1 (0; 1) | 1 (0; 1) | 0 (0; 1) | 0 (0; 1) | 0 (0; 1) | 1 (0; 1) | 1 (1; 1) | 1 (0; 1) | p = 0.94 |
| mMRC ≥ 2 n (%) | 18 (12) | 14 (12) | 17 (16) | 1 (2) | 0 (0) † | 0 (0) † | 18 (18) | 14 (17) | 17 (24) | p = 0.42 |
| Fatigue FACIT-FS | 42 (33; 49) | 43 (34; 48) | 44 (35; 50) † | 50 (48; 51) | 50 (47; 51) | 50 (48; 52) | 37 (30; 42) | 38 (30; 45) | 38 (31; 46) | p = 0.24 |
| FACIT-FS ≤ 43 n (%) | 84 (57) | 60 (51) † | 52 (50) † | 0 (0) | 4 (11) | 4 (13) | 84 (83) | 56 (69) † | 48 (67) † | p < 0.01 * |
| Anxiety HADS-A | 4 (2; 7) | 5 (2; 7) | 4 (2; 8) | 2 (1; 4) | 2 (0; 4) | 2 (1; 4) | 6 (3; 9) | 6 (3; 8) | 6 (3; 9) | p = 0.84 |
| HADS-A ≥ 8 n (%) | 41 (28) | 37 (32) † | 37 (36) † | 0 (0) | 3 (8) | 3 (9) | 41 (41) | 34 (42) | 34 (47) | p < 0.01 * |
| Depression HADS-D | 4 (3; 6) | 3 (1; 6) † | 3 (1; 6) † | 3 (1; 4) | 1 (0; 2) † | 1 (0; 2) † | 5 (3; 7) | 4 (3; 7) | 4 (3; 7) | p = 0.09 |
| HADS-D ≥ 8 n (%) | 29 (20) | 23 (20) | 19 (18) | 0 (0) | 1 (3) | 0 (0) | 29 (29) | 22 (27) | 19 (26) | p < 0.01 * |
| EQ-5D-5L | 80 ± 1 | 79 ± 1 | 80 ± 2 | 90 ± 2 | 86 ± 2 | 88 ± 3 | 70 ± 2 | 72 ± 2 | 73 ± 2 | p = 0.22 |
| EQ-5D-5L ≤ 74/female or 78/male n (%) | 60 (41) | 45 (38) | 38 (37) † | 0 (0) | 2 (5) | 2 (6) | 60 (59) | 43 (53) | 36 (50) | p < 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diciolla, N.S.; Ampuero-López, A.; Marques, A.; Jiménez-Martín, A.; García-De Villa, S.; Torres-Lacomba, M.; Yuste-Sánchez, M.J. Physical Activity and Sedentary Behaviour in People with Long COVID: A Follow-Up from 12 to 18 Months After Discharge. J. Clin. Med. 2025, 14, 3641. https://doi.org/10.3390/jcm14113641
Diciolla NS, Ampuero-López A, Marques A, Jiménez-Martín A, García-De Villa S, Torres-Lacomba M, Yuste-Sánchez MJ. Physical Activity and Sedentary Behaviour in People with Long COVID: A Follow-Up from 12 to 18 Months After Discharge. Journal of Clinical Medicine. 2025; 14(11):3641. https://doi.org/10.3390/jcm14113641
Chicago/Turabian StyleDiciolla, Nicola S., Ana Ampuero-López, Alda Marques, Ana Jiménez-Martín, Sara García-De Villa, María Torres-Lacomba, and María José Yuste-Sánchez. 2025. "Physical Activity and Sedentary Behaviour in People with Long COVID: A Follow-Up from 12 to 18 Months After Discharge" Journal of Clinical Medicine 14, no. 11: 3641. https://doi.org/10.3390/jcm14113641
APA StyleDiciolla, N. S., Ampuero-López, A., Marques, A., Jiménez-Martín, A., García-De Villa, S., Torres-Lacomba, M., & Yuste-Sánchez, M. J. (2025). Physical Activity and Sedentary Behaviour in People with Long COVID: A Follow-Up from 12 to 18 Months After Discharge. Journal of Clinical Medicine, 14(11), 3641. https://doi.org/10.3390/jcm14113641







