Pantera Lux Drug-Coated Balloon for the Treatment of Coronary Artery Lesions in Routine Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Study Device
2.3. Endpoint Definition
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Lesion and Procedural Characteristics
3.3. Clinical Endpoints
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCB | Drug-coated balloon |
| PCB | Paclitaxel-coated balloon |
| ISR | In-stent restenosis |
| DES | Drug-eluting stent |
| BMS | Bare-metal stent |
| TLR | Target-lesion revascularization |
| TV-MI | Target-vessel myocardial infarction |
References
- Scheller, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; Haghi, D.; Dietz, U.; Böhm, M.; Speck, U. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N. Engl. J. Med. 2006, 355, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [PubMed]
- Byrne, A.R.; Neumann, F.-J.; Mehilli, J.; Pinieck, S.; Wolff, B.; Tiroch, K.; Schulz, S.; Fusaro, M.; Ott, I.; Ibrahim, T.; et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): A randomised, open-label trial. Lancet 2013, 381, 461–467. [Google Scholar] [CrossRef]
- Yeh, R.W.; Shlofmitz, R.; Moses, J.; Bachinsky, W.; Dohad, S.; Rudick, S.; Stoler, R.; Jefferson, B.K.; Nicholson, W.; Altman, J.; et al. Paclitaxel-Coated Balloon vs Uncoated Balloon for Coronary In-Stent Restenosis: The AGENT IDE Randomized Clinical Trial. JAMA 2024, 331, 1015–1024. [Google Scholar] [CrossRef]
- Cortese, B.; Kalkat, H.; Bathia, G.; Basavarajaiah, S. The evolution and revolution of drug coated balloons in coronary angioplasty: An up-to-date review of literature data. Catheter. Cardiovasc. Interv. 2023, 102, 1069–1077. [Google Scholar] [CrossRef]
- Speck, U.; Cremers, B.; Kelsch, B.; Biedermann, M.; Clever, Y.P.; Schaffner, S.; Mahnkopf, D.; Hanisch, U.; Böhm, M.; Scheller, B. Do pharmacokinetics explain persistent restenosis inhibition by a single dose of paclitaxel? Circ. Cardiovasc. Interv. 2012, 5, 392–400. [Google Scholar] [CrossRef]
- Tanaka, A.; Latib, A.; Jabbour, R.J.; Kawamoto, H.; Giannini, F.; Ancona, M.; Regazzoli, D.; Mangieri, A.; Mattioli, R.; Chieffo, A.; et al. Impact of Angiographic Result After Predilatation on Outcome After Drug-Coated Balloon Treatment of In-Stent Coronary Restenosis. Am. J. Cardiol. 2016, 118, 1460–1465. [Google Scholar] [CrossRef]
- Jeger, R.V.; Eccleshall, S.; Wan Ahmad, W.A.; Ge, J.; Poerner, T.C.; Shin, E.S.; Alfonso, F.; Latib, A.; Ong, P.J.; Rissanen, T.T.; et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc. Interv. 2020, 13, 1391–1402. [Google Scholar] [CrossRef]
- Cortese, B.; Di Palma, G.; Guimaraes, M.G.; Piraino, D.; Orrego, P.S.; Buccheri, D.; Rivero, F.; Perotto, A.; Zambelli, G.; Alfonso, F. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 2840–2849. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.-A.; van Es, G.-A.; Zuckerman, B.; et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Eur. Heart J. 2018, 39, 2192–2207. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T.; Kozuma, K.; Tanabe, K.; Morino, Y.; Ako, J.; Nakamura, S.; Yamaji, K.; Kohsaka, S.; Amano, T.; Kobayashi, Y.; et al. Clinical expert consensus document on drug-coated balloon for coronary artery disease from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc. Interv. Ther. 2023, 38, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Wessely, R.; Blaich, B.; Belaiba, R.S.; Merl, S.; Görlach, A.; Kastrati, A.; Schömig, A. Comparative characterization of cellular and molecular anti-restenotic profiles of paclitaxel and sirolimus. Implications for local drug delivery. Thromb. Haemost. 2007, 97, 1003–1012. [Google Scholar] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2023, 82, 833–955. [Google Scholar]
- Giacoppo, D.; Alvarez-Covarrubias, H.; Koch, T.; Cassese, S.; Xhepa, E.; Kessler, T.; Wiebe, J.; Joner, M.; Hochholzer, W.; Laugwitz, K.-L.; et al. Coronary artery restenosis treatment with plain balloon, drug-coated balloon, or drug-eluting stent: 10-year outcomes of the ISAR-DESIRE 3 trial. Eur. Heart J. 2023, 44, 1343–1357. [Google Scholar] [CrossRef]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.E.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.-Y.; Degenhardt, R.; Pleva, L.; et al. Drug-coated balloon angioplasty versus drug-eluting stent implantation in patients with coronary stent restenosis. J. Am. Coll. Cardiol. 2020, 75, 2664–2678. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mahmoud, A.N.; Elgendy, A.Y.; Mojadidi, M.K.; Elbadawi, A.; Eshtehardi, P.; Pérez-Vizcayno, M.J.; Wayangankar, S.A.; Jneid, H.; Anderson, R.D.; et al. Drug-eluting balloons versus everolimus-eluting stents for in-stent restenosis: A meta-analysis of randomized trials. Cardiovasc. Revasc Med. 2019, 20, 612–618. [Google Scholar] [CrossRef]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef]
- Gao, C.; He, X.; Ouyang, F.; Zhang, Z.; Shen, G.; Wu, M.; Yang, P.; Ma, L.; Yang, F.; Ji, Z.; et al. Drug-coated balloon angioplasty with rescue stenting versus intended stenting for the treatment of patients with de novo coronary artery lesions (REC-CAGEFREE I): An open-label, randomised, non-inferiority trial. Lancet 2024, 404, 1040–1050. [Google Scholar] [CrossRef]
- Fezzi, S.; Giacoppo, D.; Fahrni, G.; Latib, A.; Alfonso, F.; Colombo, A.; Mahfoud, F.; Scheller, B.; Jeger, R.; Cortese, B. Individual patient data meta-analysis of paclitaxel-coated balloons vs. drug-eluting stents for small-vessel coronary artery disease: The ANDROMEDA study. Eur. Heart J. 2025, ehaf002. [Google Scholar] [CrossRef]
- Gao, X.; Tian, N.; Kan, J.; Li, P.; Wang, M.; Sheiban, I.; Figini, F.; Deng, J.; Chen, X.; Santoso, T.; et al. Drug-Coated Balloon Angioplasty of the Side Branch During Provisional Stenting: The Multicenter Randomized DCB-BIF Trial. J. Am. Coll. Cardiol. 2025, 85, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.; Krackhardt, F.; Bogaerts, K.; Urban, P.; Meis, S.; Morice, M.-C.; Eccleshall, S. Comparing a strategy of sirolimus-eluting balloon treatment to drug-eluting stent implantation in de novo coronary lesions in all-comers: Design and rationale of the Selution DeNovo Trial. Am. Heart J. 2023, 258, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.J.; Richardt, G.; Tölg, R.; Erglis, A.; Skurk, C.; Jung, W.; Neumann, F.J.; Stangl, K.; Brachmann, J.; Fischer, D.; et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: The BIOLUX randomised controlled trial. EuroIntervention 2018, 14, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Pérez-Vizcayno, M.J.; Cuesta, J.; García Del Blanco, B.; García-Touchard, A.; López-Mínguez, J.R.; Masotti, M.; Zueco, J.; Cequier, A.; Velázquez, M.; et al. 3-Year Clinical Follow-Up of the RIBS IV Clinical Trial: A Prospective Randomized Study of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients with in-Stent Restenosis in Coronary Arteries Previously Treated with Drug-Eluting Stents. JACC Cardiovasc. Interv. 2018, 11, 981–991. [Google Scholar] [CrossRef]
- Unverdorben, M.; Vallbracht, C.; Cremers, B.; Heuer, H.; Hengstenberg, C.; Maikowski, C.; Werner, G.S.; Antoni, D.; Kleber, F.X.; Bocksch, W.; et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation 2009, 119, 2986–2994. [Google Scholar] [CrossRef]
- Alfonso, F.; Byrne, R.A.; Rivero, F.; Kastrati, A. Current treatment of in-stent restenosis. J. Am. Coll. Cardiol. 2014, 63, 2659–2673. [Google Scholar] [CrossRef]
- Qian, J.; Wu, Y.; Li, C.; Yin, J.; Fu, G.; Wang, J.; He, Y.; Ma, G.; Chen, Y.; Xia, Y.; et al. Drug-coated balloon for the treatment of small vessel disease: 9 months of angiographic results and 12 months of clinical outcomes of the PEPCAD China SVD study. Catheter. Cardiovasc. Interv. 2023, 101, 33–43. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef]
- Sedhom, R.; Hamed, M.; Elbadawi, A.; Mohsen, A.; Swamy, P.; Athar, A.; Bharadwaj, A.S.; Prasad, V.; Elgendy, I.Y.; Alfonso, F.; et al. Outcomes with Limus- vs. Paclitaxel-Coated Balloons for Percutaneous Coronary Intervention: Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc. Interv. 2024, 17, 1533–1543. [Google Scholar] [CrossRef]
| Total Cohort (n = 386) | DES-ISR (n = 191) | BMS-ISR (n = 127) | De Novo (n = 68) | p-Value | |
|---|---|---|---|---|---|
| Age | 71.3 ± 9.7 | 71.5 ± 9.7 | 70.9 ± 9.8 | 71.5 ± 9.7 | 0.7 |
| Female | 96/386 (24.9%) | 42/191 (22.0%) | 33/127 (26.0%) | 21/68 (30.9%) | 0.3 |
| BMI | 28.0 ± 4.4 | 28.3 ± 4.8 | 28.0 ± 3.8 | 27.2 ± 4.6 | 0.4 |
| Hyperlipidemia | 244/386 (63.2%) | 127/191 (66.5%) | 85/127 (66.9%) | 32/68 (47.1%) | 0.010 |
| Hypertension | 365/386 (94.6%) | 182/191 (95.3%) | 121/127 (95.3%) | 62/68 (91.2%) | 0.4 |
| Diabetes mellitus | 123/386 (31.9%) | 69/191 (36.1%) | 31/127 (24.4%) | 23/68 (33.8%) | 0.4 |
| Smoking history | 162/386 (42.0) | 98/191 (50.8%) | 40/127 (31.5%) | 25/68 (36.8%) | 0.01 |
| LVEF [%] | 52.3 ± 11.4 | 51.0 ± 11.9 | 54.1 ± 10.8 | 52.5 ± 11.0 | 0.092 |
| Previous PCI | 363/386 (94.0%) | 191/191 (100.0%) | 127/127 (100.0%) | 45/68 (66.2%) | <0.001 |
| Peripheral artery disease | 63/386 (16.3%) | 38/191 (19.9%) | 16/127 (12.6%) | 9/68 (13.2%) | 0.2 |
| Carotid artery disease | 84/386 (21.8%) | 45/191 (23.6%) | 28/127 (22.0%) | 11/68 (16.2%) | 0.4 |
| Impaired renal function | 67/386 (17.4%) | 39/191 (20.4%) | 20/127 (15.7%) | 8/68 (11.8%) | 0.4 |
| Clinical presentation | 0.854 | ||||
| STEMI | 6/386 (1.6%) | 3/191 (1.6%) | 2/127 (1.6%) | 1/68 (1.5%) | |
| NSTEMI | 34/386 (8.8%) | 17/191 (8.9%) | 10/127 (7.9%) | 7/68 (10.3%) | |
| Unstable angina | 26/386 (6.7%) | 18/191 (9.4%) | 5/127 (3.9%) | 3/68 (4.4%) | |
| Chronic coronary syndrome | 320/386 (82.9%) | 153/191 (80.1%) | 110/127 (86.6%) | 57/68 (83.8%) |
| Total Cohort % (n = 386) | DES-ISR (n = 191) | BMS-ISR (n = 127) | De Novo (n = 68) | p-Value | |
|---|---|---|---|---|---|
| LESION CHARACTERISTICS | |||||
| No. of lesions | 407 | 212 | 127 | 68 | |
| Target Vessel | <0.001 | ||||
| LM | 8/407 (2.0%) | 8/212 (3.8%) | 0/127 (0.0%) | 0/68 (0.0%) | |
| LAD | 145/407 (35.6%) | 68/212 (32.1%) | 38/127 (29.9%) | 39/68 (57.4%) | |
| LCX | 100/407 (24.6%) | 48/212 (22.6%) | 31/127 (24.4%) | 21/68 (30.9%) | |
| RCA | 134/407 (32.9%) | 74/212 (34.9%) | 53/127 (41.7%) | 7/68 (10.3%) | |
| Venous CABG | 20/407 (4.9%) | 14/212 (6.6%) | 5/127 (3.9%) | 1/68 (1.5%) | |
| ISR Mehran classification | 0.6 | ||||
| Class I | 103/212 (48.6%) | 70/127 (55.1%) | - | ||
| Class II | 68/212 (32.1%) | 34/127 (26.8%) | - | ||
| Class III | 20/212 (9.4%) | 9/127 (7.1%) | - | ||
| Class IV | 21/212 (9.9%) | 14/127 (11.0%) | - | ||
| Lesion type AHA/ACC class | <0.001 | ||||
| Type A | 37/407 (9.1%) | 4/212 (1.9%) | 17/127 (13.4%) | 16/68 (23.5%) | |
| Type B1 | 212/407 (52.1%) | 104/212 (49.1%) | 70/127 (55.1%) | 38/68 (55.9%) | |
| Type B2 | 110/407 (27.0%) | 71/212 (33.5%) | 30/127 (23.6%) | 9/68 (13.2%) | |
| Type C | 48/407 (11.8%) | 33/212 (15.6%) | 10/127 (7.9%) | 5/68 (7.4%) | |
| Bifurcation | 97/407 (23.8%) | 59/212 (27.8%) | 15/127 (11.8%) | 23/68 (33.8%) | <0.001 |
| Calcification | <0.001 | ||||
| Moderate | 146/407 (35.9%) | 86/212 (40.6%) | 54/127 (42.5%) | 6/68 (8.8%) | |
| Severe | 44/407 (10.8%) | 39/212 (18.4%) | 3/127 (2.4%) | 2/68 (2.9%) | |
| Reference vessel diameter [mm] | 2.9 ± 0.6 | 3.1 ± 0.5 | 3.0 ± 0.4 | 2.1 ± 0.3 | <0.001 |
| Lesion length [mm] | 13.3 ± 9.6 | 14.6 ± 11.7 | 11.4 ± 6.3 | 12.6 ± 6.2 | 0.01 |
| Diameter stenosis [%] | 83.2 ± 11.3 | 83.3 ± 10.4 | 80.4 ± 12.8 | 88.3 ± 9.5 | <0.001 |
| PROCEDURAL CHARACTERISTICS | |||||
| Lesion preparation technique | |||||
| POBA | 329/407 (80.8%) | 164/212 (77.4%) | 102/127 (80.3%) | 63/68 (92.6%) | <0.001 |
| Cutting | 113/407 (27.8%) | 87/212 (41.0%) | 25/127 (19.7%) | 1/68 (1.5%) | <0.001 |
| Scoring | 57/407 (14.0%) | 28/212 (13.2%) | 26/127 (20.5%) | 3/68 (4.4%) | <0.001 |
| Rotational atherectomy | 2/407 (0.5%) | 2/212 (0.9%) | 0/127 (0.0%) | 0/68 (0.0%) | 0.006 |
| Intravascular lithotripsy | 1/407 (0.2%) | 1/212 (0.5%) | 0/127 (0.0%) | 0/68 (0.0%) | 0.005 |
| Thrombus aspiration | 1/407 (0.2%) | 1/212 (0.5%) | 0/127 (0.0%) | 0/68 (0.0%) | 0.005 |
| Pantera Lux Balloon | |||||
| No. of balloons used | <0.001 | ||||
| 1 | 335/386 (86.8%) | 147/191 (77.0%) | 123/127 (96.9%) | 65/68 (95.6%) | |
| 2 | 39/386 (10.1%) | 32/191 (16.8%) | 4/127 (3.1%) | 3/68 (4.4%) | |
| 3 | 10/386 (2.6%) | 10/191 (5.2%) | 0/127 (0.0%) | 0/68 (0.0%) | |
| 4 | 2/386 (0.5%) | 2/191 (1.0%) | 0/127 (0.0%) | 0/68 (0.0%) | |
| Diameter [mm] | 2.9 (0.5) | 3.1 (0.5) | 3.0 (0.4) | 2.1 (0.3) | <0.001 |
| Length [mm] | 19.7 (6.2) | 20.6 (6.3) | 18.7 (5.9) | 18.2 (5.6) | 0.003 |
| Pressure [atm] | 14.8 (3.3) | 15.3 (3.3) | 15.0 (3.0) | 12.8 (2.9) | <0.001 |
| Inflation time [s] | 41.0 (8.9) | 41.3 (8.8) | 39.1 (7.8) | 43.3 (10.6) | 0.016 |
| Dissection requiring stenting | 10/407 (2.5%) | 4/212 (1.9%) | 2/127 (1.6%) | 4/68 (5.9%) | 0.14 |
| Residual stenosis requiring stent | 9/407 (2.2%) | 5/212 (2.4%) | 2/127 (1.6%) | 2/68 (2.9%) | 0.7 |
| Residual stenosis post-DCB | 6.3 ± 16.4 | 7.6 ± 17.6 | 3.3 ± 13.4 | 7.3 ± 16.8 | 0.002 |
| Residual stenosis > 20% | 38/451 (8.4%) | 27/249 (10.8%) | 6/131 (4.6%) | 5/71 (7.0%) | 0.102 |
| Perforation | 1/386 (0.3%) | 1/191 (0.5%) | 0/127 (0.0%) | 0/68 (0.0%) | 0.600 |
| Vascular occlusion | 1/386 (0.3%) | 1/191 (0.5%) | 0/127 (0.0%) | 0/68 (0.0%) | 0.600 |
| Periprocedural myocardial infarction | 5/386 (1.3%) | 3/191 (1.6%) | 0/127 (0.0%) | 2/68 (2.9%) | 0.200 |
| Total Cohort (n = 386) | DES-ISR (n = 191) | BMS-ISR (n = 127) | De Novo (n = 68) | p-Value | |
|---|---|---|---|---|---|
| ENDPOINTS | |||||
| Device success | 446/451 (98.9%) | 246/249 (98.8%) | 129/131 (98.5%) | 71/71 (100.0%) | 0.8 |
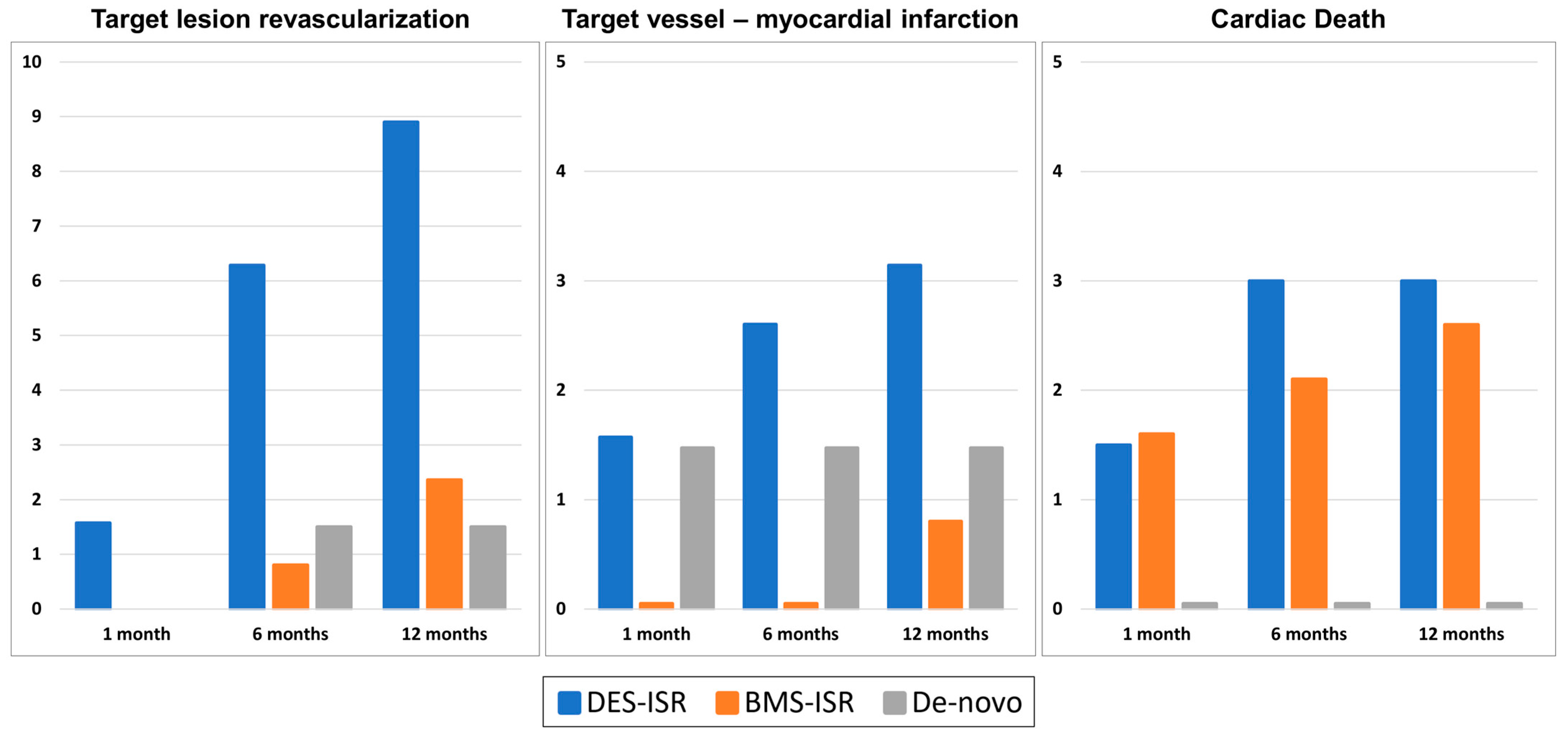
| TLR at 12 months | 21/386 (5.4%) | 17/191 (8.9%) | 3/127 (2.4%) | 1/68 (1.5%) | 0.013 |
| TVMI at 12 months | 8/386 (2.1%) | 6/191 (3.2%) | 1/127 (0.8%) | 1/68 (1.5%) | 0.5 |
| Cardiac death at 12 months | 7/386 (1.8%) | 5/191 (2.6%) | 0/127 (0.0%) | 2/68 (2.9%) | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemetsberger, R.; Mankerious, N.; Hamzaraj, K.; Alali, A.; Richardt, G.; Tölg, R. Pantera Lux Drug-Coated Balloon for the Treatment of Coronary Artery Lesions in Routine Practice. J. Clin. Med. 2025, 14, 3133. https://doi.org/10.3390/jcm14093133
Hemetsberger R, Mankerious N, Hamzaraj K, Alali A, Richardt G, Tölg R. Pantera Lux Drug-Coated Balloon for the Treatment of Coronary Artery Lesions in Routine Practice. Journal of Clinical Medicine. 2025; 14(9):3133. https://doi.org/10.3390/jcm14093133
Chicago/Turabian StyleHemetsberger, Rayyan, Nader Mankerious, Kevin Hamzaraj, Ahmed Alali, Gert Richardt, and Ralph Tölg. 2025. "Pantera Lux Drug-Coated Balloon for the Treatment of Coronary Artery Lesions in Routine Practice" Journal of Clinical Medicine 14, no. 9: 3133. https://doi.org/10.3390/jcm14093133
APA StyleHemetsberger, R., Mankerious, N., Hamzaraj, K., Alali, A., Richardt, G., & Tölg, R. (2025). Pantera Lux Drug-Coated Balloon for the Treatment of Coronary Artery Lesions in Routine Practice. Journal of Clinical Medicine, 14(9), 3133. https://doi.org/10.3390/jcm14093133






