Effects of Age, Gender and Laterality on the sEMG of the Orbicularis Oculi in Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Electrode Placement
2.3. sEMG Measurement Protocol
2.4. Ethics Approval
2.5. Statistical Analysis
3. Results
3.1. Differences Between Males and Females
3.2. Differences Between the Values for the Left and the Right Eye
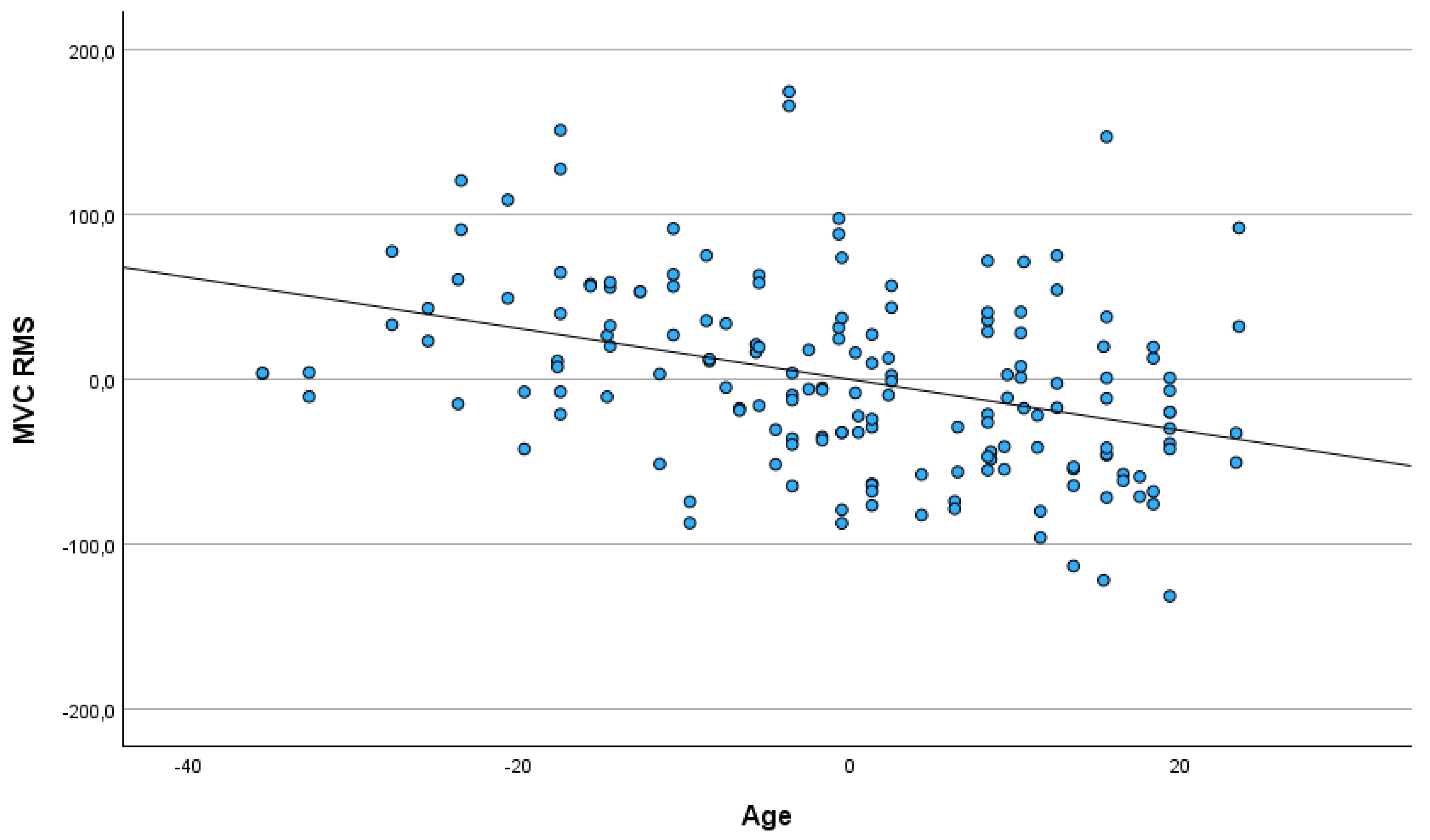
3.3. Age of Participants
3.4. The Differences Between Males and Females, Between the Left and the Right Eye and the Age of Participants as Predictors of Analyzed Variables
4. Discussion
4.1. Age-Related Decline in Neuromuscular Function
4.2. Gender Differences in Neuromuscular Function
4.3. Laterality: Left vs. Right Eye Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OOM | orbicularis oculi muscle |
| sEMG | surface electromyography |
| MVC | maximal voluntary contraction |
| GEC | gentle eyelid closure |
References
- Tong, J.; Lopez, M.J.; Fakoya, A.O.; Patel, B.C. Anatomy, Head and Neck: Orbicularis Oculi Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441907/ (accessed on 12 April 2025).
- Ali, M.J.; Zetzsche, M.; Scholz, M.; Hahn, D.; Gaffling, S.; Heichel, J.; Hammer, C.M.; Bräuer, L.; Paulsen, F. New insights into the lacrimal pump. Ocul. Surf. 2020, 18, 689–698. [Google Scholar] [CrossRef] [PubMed]
- May, P.J. The Eyelid: Anatomy, Neural Control and Pathology. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2024; p. B9780443138201000025. [Google Scholar] [CrossRef]
- Scherrer, E.; Chaloupka, K. Future treatment options for facial nerve palsy: A review on electrical stimulation devices for the orbicularis oculi muscle. Neurol. Sci. 2024, 45, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.H.; Gameiro, G.R.; Osaki, M.H.; Osaki, T.; Campos, E.D.; Belfort, R.; Marie, S.K.N. Orbicularis Oculi Muscle Immunohistochemical, Metabolic, and Morphometric Differences in Affected and Nonaffected Sides in Hemifacial Spasm vs Healthy Subjects. J. Neuro-Ophthalmol. 2023, 43, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J. Alteration of the Orbicularis Oculi Reflex by Pontine Lesions: Study in Multiple Sclerosis. Arch. Neurol. 1970, 22, 156. [Google Scholar] [CrossRef]
- Radecka, A.; Lubkowska, A. The usefulness of surface electromyography in rehabilitation and physiotherapy: Systematic review. Pomeranian J. Life Sci. 2020, 66, 49–56. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Hu, S. Sex Differences Found in Facial EMG Activity Provoked by Viewing Pleasant and Unpleasant Photographs. Percept. Mot. Skills 2009, 109, 371–381. [Google Scholar] [CrossRef]
- Olmos, A.A.; Sterczala, A.J.; Parra, M.E.; Dimmick, H.L.; Miller, J.D.; Deckert, J.A.; Sontag, S.A.; Gallagher, P.M.; Fry, A.C.; Herda, T.J.; et al. Sex-related differences in motor unit behavior are influenced by myosin heavy chain during high- but not moderate-intensity contractions. Acta Physiol. 2023, 239, e14024. [Google Scholar] [CrossRef]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-Based Differences in Skeletal Muscle Kinetics and Fiber-Type Composition. Physiology 2015, 30, 30–39. [Google Scholar] [CrossRef]
- Krajewska-Węglewicz, L.; Felczak, P.; Dorobek, M. Effects of Aging on Orbicularis Oculi Muscle Strength and Ultrastructure in Dermatochalasis: A Pilot Study. J. Clin. Med. 2024, 14, 162. [Google Scholar] [CrossRef]
- Piasecki, M.; Ireland, A.; Jones, D.A.; McPhee, J.S. Age-dependent motor unit remodelling in human limb muscles. Biogerontology 2016, 17, 485–496. [Google Scholar] [CrossRef]
- Shinohara, M. Adaptations in motor unit behavior in elderly adults. Curr. Aging Sci. 2011, 4, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Okuda, I.; Irimoto, M.; Nakajima, Y.; Sakai, S.; Hirata, K.; Shirakabe, Y. Using multidetector row computed tomography to evaluate baggy eyelid. Aesthetic Plast. Surg. 2012, 36, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Fukada, K.; Kajiya, K. Age-related structural alterations of skeletal muscles and associated capillaries. Angiogenesis 2020, 23, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Rango, M.; Galante, D.; Bresolin, N.; Ferrario, V.F. Spontaneous blinking in healthy persons: An optoelectronic study of eyelid motion. Ophthalmic Physiol. Opt. 2008, 28, 345–353. [Google Scholar] [CrossRef]
- Young, A.; Stokes, M.; Crowe, M. The size and strength of the quadriceps muscles of old and young men. Clin. Physiol. 1985, 5, 145–154. [Google Scholar] [CrossRef]
- Sepic, S.B.; Murray, M.P.; Mollinger, L.A.; Spurr, G.B.; Gardner, G.M. Strength and range of motion in the ankle in two age groups of men and women. Am. J. Phys. Med. 1986, 65, 75–84. [Google Scholar]
- Rice, C.L.; Cunningham, D.A.; Paterson, D.H.; Rechnitzer, P.A. Strength in an elderly population. Arch. Phys. Med. Rehabil. 1989, 70, 391–397. [Google Scholar]
- Bertozzi, N.; Bianchi, B.; Salvagni, L.; Raposio, E. Activity Evaluation of Facial Muscles by Surface Electromyography. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3081. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kim, K.H.; Kim, L.-H.; Kim, J.-U.; Yook, T.-H. Difference between Right and Left Facial Surface Electromyography in Healthy People. Evid.-Based Complement. Altern. Med. ECAM 2018, 2018, 4069530. [Google Scholar] [CrossRef]
- Abe, T.; Wong, V.; Spitz, R.W.; Viana, R.B.; Bell, Z.W.; Yamada, Y.; Chatakondi, R.N.; Loenneke, J.P. Influence of sex and resistance training status on orofacial muscle strength and morphology in healthy adults between the ages of 18 and 40: A cross-sectional study. Am. J. Hum. Biol. 2020, 32, e23401. [Google Scholar] [CrossRef]
- Volk, G.F.; Sauer, M.; Pohlmann, M.; Guntinas-Lichius, O. Reference values for dynamic facial muscle ultrasonography in adults. Muscle Nerve 2014, 50, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Koschmieder, A.; Pisowocki, P.; Zietz, C.; Bader, R.; Stachs, O.; Jünemann, A. Measurement of the Strength of Human Musculus Orbicularis Oculi Using Video Analysis. Klin. Monatsbl. Augenheilkd. 2017, 234, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.; Trentzsch, V.; Grassme, R.; Guntinas-Lichius, O.; Volk, G.F.; Anders, C. High-resolution surface electromyographic activities of facial muscles during mimic movements in healthy adults: A prospective observational study. Front. Hum. Neurosci. 2022, 16, 1029415. [Google Scholar] [CrossRef] [PubMed]
- Franz, L.; de Filippis, C.; Daloiso, A.; Biancoli, E.; Iannacone, F.P.; Cazzador, D.; Tealdo, G.; Marioni, G.; Nicolai, P.; Zanoletti, E. Facial surface electromyography: A systematic review on the state of the art and current perspectives. Am. J. Otolaryngol. 2024, 45, 104041. [Google Scholar] [CrossRef]
- Volk, G.F.; Karamyan, I.; Klingner, C.M.; Reichenbach, J.R.; Guntinas-Lichius, O. Quantitative magnetic resonance imaging volumetry of facial muscles in healthy patients with facial palsy. Plast. Reconstr. Surg. Glob. Open 2014, 2, e173. [Google Scholar] [CrossRef]


| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | t | df | p | |
| RMS baseline | 11.20 | 5.28 | 10.23 | 5.81 | 1.13 | 166 | 0.261 | |
| MVC | ampl. max | 1197.26 | 411.63 | 1283.51 | 449.58 | −1.30 | 166 | 0.196 |
| ampl. Mean | 349.37 | 91.03 | 369.67 | 95.44 | −1.41 | 166 | 0.160 | |
| RMS | 146.45 | 56.48 | 157.80 | 56.43 | −1.30 | 166 | 0.195 | |
| ΔRMS | 135.25 | 57.32 | 147.56 | 58.00 | −1.38 | 166 | 0.169 | |
| GEC | ampl. max | 52.15 | 23.15 | 49.26 | 19.40 | 0.87 | 166 | 0.385 |
| RMS | 8.35 | 4.86 | 7.42 | 2.94 | 1.49 | 166 | 0.138 | |
| ΔRMS | −2.84 | 6.85 | −2.81 | 5.39 | −0.03 | 166 | 0.974 | |
| Left | Right | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | t | df | p | |
| RMS baseline | 10.40 | 6.39 | 11.08 | 4.56 | −0.80 | 166 | 0.426 | |
| MVC | ampl. max | 1221.26 | 448.77 | 1255.41 | 414.44 | −0.51 | 166 | 0.609 |
| ampl. Mean | 358.38 | 96.45 | 359.69 | 90.89 | −0.09 | 166 | 0.928 | |
| RMS | 152.24 | 57.73 | 151.47 | 55.74 | 0.09 | 166 | 0.930 | |
| ΔRMS | 141.84 | 59.22 | 140.39 | 56.70 | 0.16 | 166 | 0.871 | |
| GEC | ampl. max | 52.35 | 22.16 | 49.19 | 20.70 | 0.96 | 166 | 0.340 |
| RMS | 8.29 | 5.04 | 7.53 | 2.79 | 1.21 | 166 | 0.227 | |
| ΔRMS | −2.11 | 7.47 | −3.55 | 4.47 | 1.52 | 166 | 0.130 | |
| Age | |||
|---|---|---|---|
| Variable | r | p | |
| RMS baseline | 0.073 | 0.344 | |
| MVC | ampl. max | −0.344 | 0.001 |
| ampl. Mean | −0.358 | 0.001 | |
| RMS | −0.380 | 0.001 | |
| ΔRMS | −0.379 | 0.001 | |
| GEC | ampl. max | −0.066 | 0.393 |
| RMS | −0.018 | 0.813 | |
| ΔRMS | −0.078 | 0.315 | |
| Variables | Predictors | Beta | t | p | F | df | p | R2 | |
|---|---|---|---|---|---|---|---|---|---|
| MVC | ampl. max | Males vs. females | 0.09 | 1.17 | 0.243 | 7.96 | 3164 | 0.001 | 0.127 |
| Eye left vs. right | 0.04 | 0.55 | 0.587 | ||||||
| Age | −0.34 | −4.66 | 0.001 | ||||||
| ampl. mean | Males vs. females | 0.09 | 1.29 | 0.199 | 8.68 | 3164 | 0.001 | 0.137 | |
| Eye left vs. right | 0.01 | 0.10 | 0.923 | ||||||
| Age | −0.35 | −4.88 | 0.001 | ||||||
| RMS | Males vs. females | 0.08 | 1.17 | 0.244 | 9.75 | 3164 | 0.001 | 0.151 | |
| Eye left vs. right | −0.01 | −0.10 | 0.925 | ||||||
| Age | −0.38 | −5.23 | 0.001 | ||||||
| ΔRMS | Males vs. females | 0.09 | 1.26 | 0.210 | 9.79 | 3164 | 0.001 | 0.152 | |
| Eye left vs. right | −0.01 | −0.18 | 0.861 | ||||||
| Age | −0.38 | −5.21 | 0.001 | ||||||
| GEC | ampl. max | Males vs. females | −0.07 | −0.91 | 0.365 | 0.08 | 3164 | 0.483 | 0.015 |
| Eye left vs. right | −0.07 | −0.96 | 0.341 | ||||||
| Age | −0.07 | −0.90 | 0.372 | ||||||
| RMS | Males vs. females | −0.12 | −1.50 | 0.136 | 1.26 | 3164 | 0.291 | 0.022 | |
| Eye left vs. right | −0.09 | −1.21 | 0.227 | ||||||
| Age | −0.02 | −0.30 | 0.762 | ||||||
| ΔRMS | Males vs. females | 0.00 | −0.01 | 0.991 | 1.11 | 3164 | 0.349 | 0.020 | |
| Eye left vs. right | −0.12 | −1.52 | 0.131 | ||||||
| Age | −0.08 | −1.01 | 0.315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska-Węglewicz, L.; Dorobek, M. Effects of Age, Gender and Laterality on the sEMG of the Orbicularis Oculi in Healthy Adults. J. Clin. Med. 2025, 14, 3119. https://doi.org/10.3390/jcm14093119
Krajewska-Węglewicz L, Dorobek M. Effects of Age, Gender and Laterality on the sEMG of the Orbicularis Oculi in Healthy Adults. Journal of Clinical Medicine. 2025; 14(9):3119. https://doi.org/10.3390/jcm14093119
Chicago/Turabian StyleKrajewska-Węglewicz, Larysa, and Małgorzata Dorobek. 2025. "Effects of Age, Gender and Laterality on the sEMG of the Orbicularis Oculi in Healthy Adults" Journal of Clinical Medicine 14, no. 9: 3119. https://doi.org/10.3390/jcm14093119
APA StyleKrajewska-Węglewicz, L., & Dorobek, M. (2025). Effects of Age, Gender and Laterality on the sEMG of the Orbicularis Oculi in Healthy Adults. Journal of Clinical Medicine, 14(9), 3119. https://doi.org/10.3390/jcm14093119




