Systematic Review and Case Report of a Left Gonadal Vein Anastomosing Hemangioma
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- -
- Population: Patients diagnosed with anastomosing hemangioma.
- -
- Intervention/Exposure: Treatment approach, including surgical resection or conservative management.
- -
- Comparison: Not applicable.
- -
- Outcome: Histopathological diagnosis and follow-up.
- -
- Study Design: Case reports or case series.
2.2. Search Strategy
2.3. Data Extraction and Synthesis
2.4. Quality Assessment
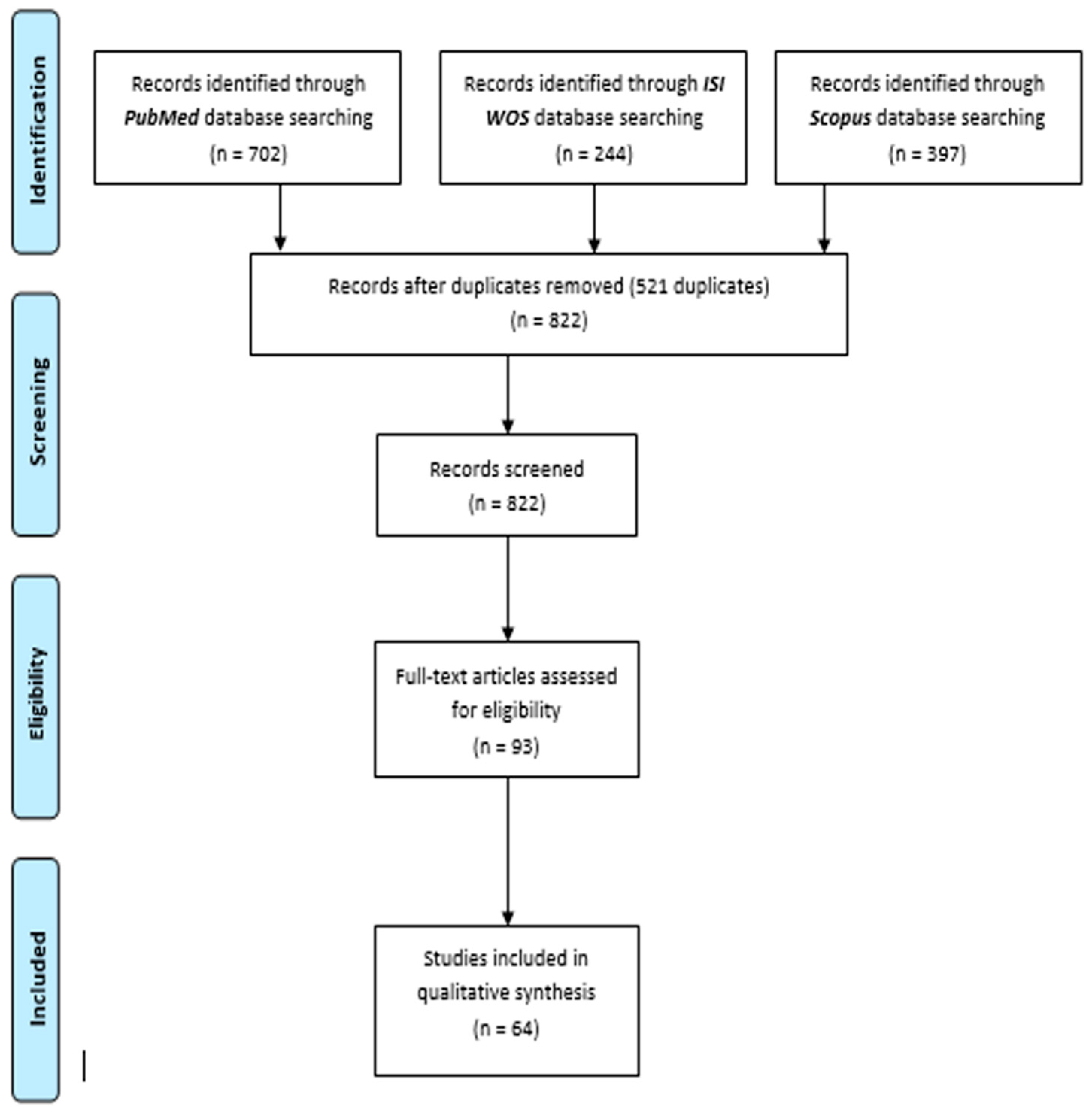
3. Results
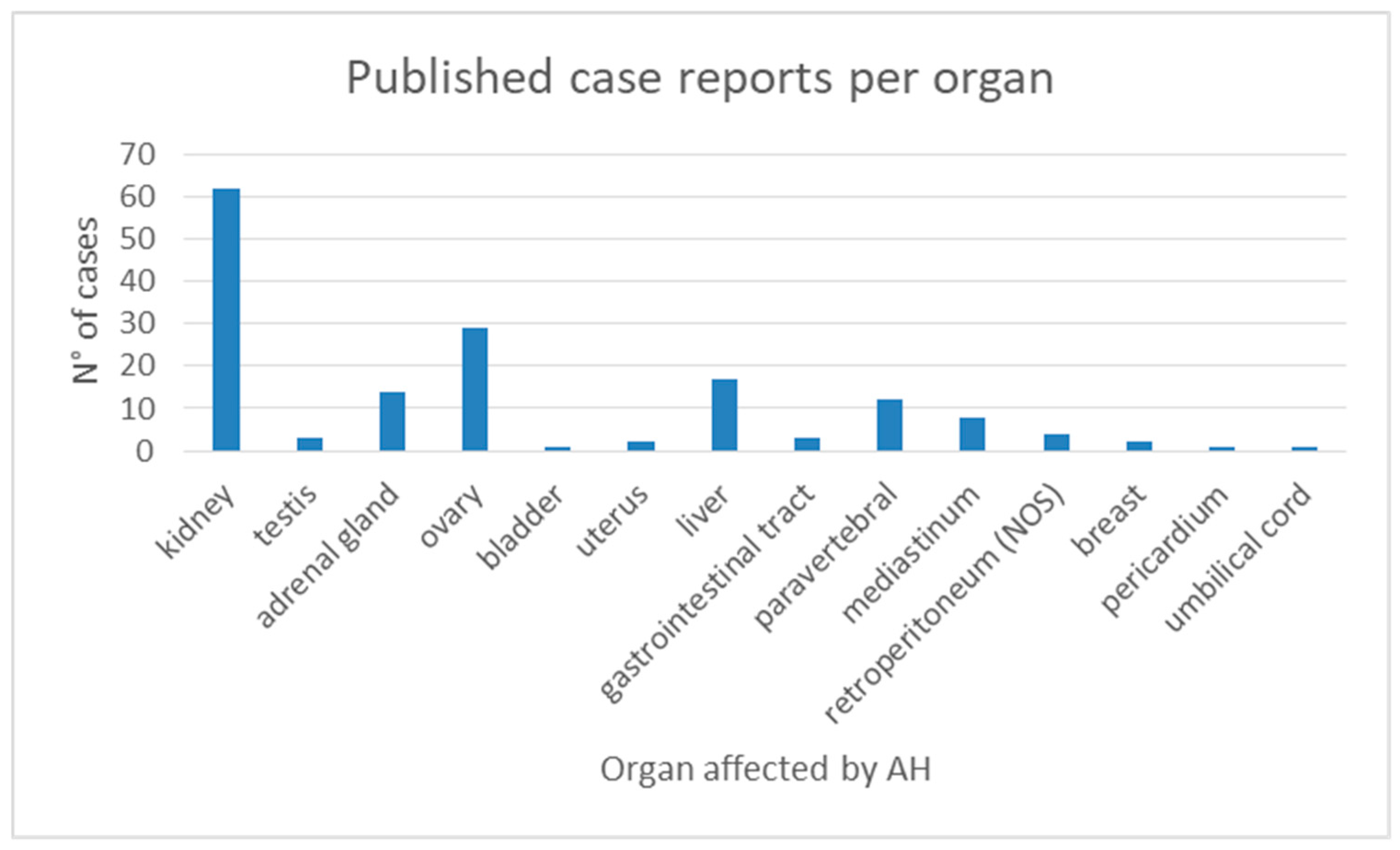
3.1. Systematic Review Results
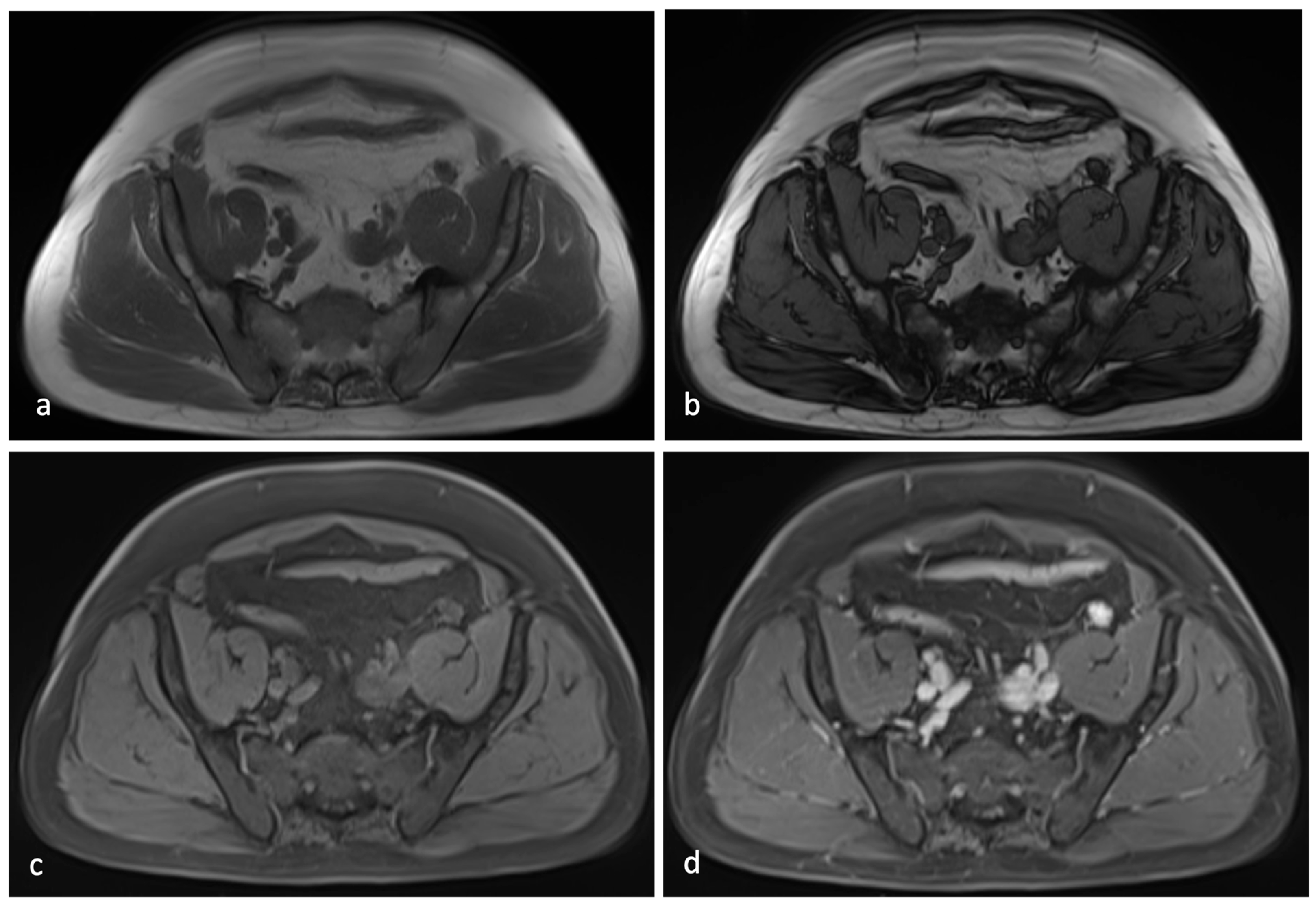
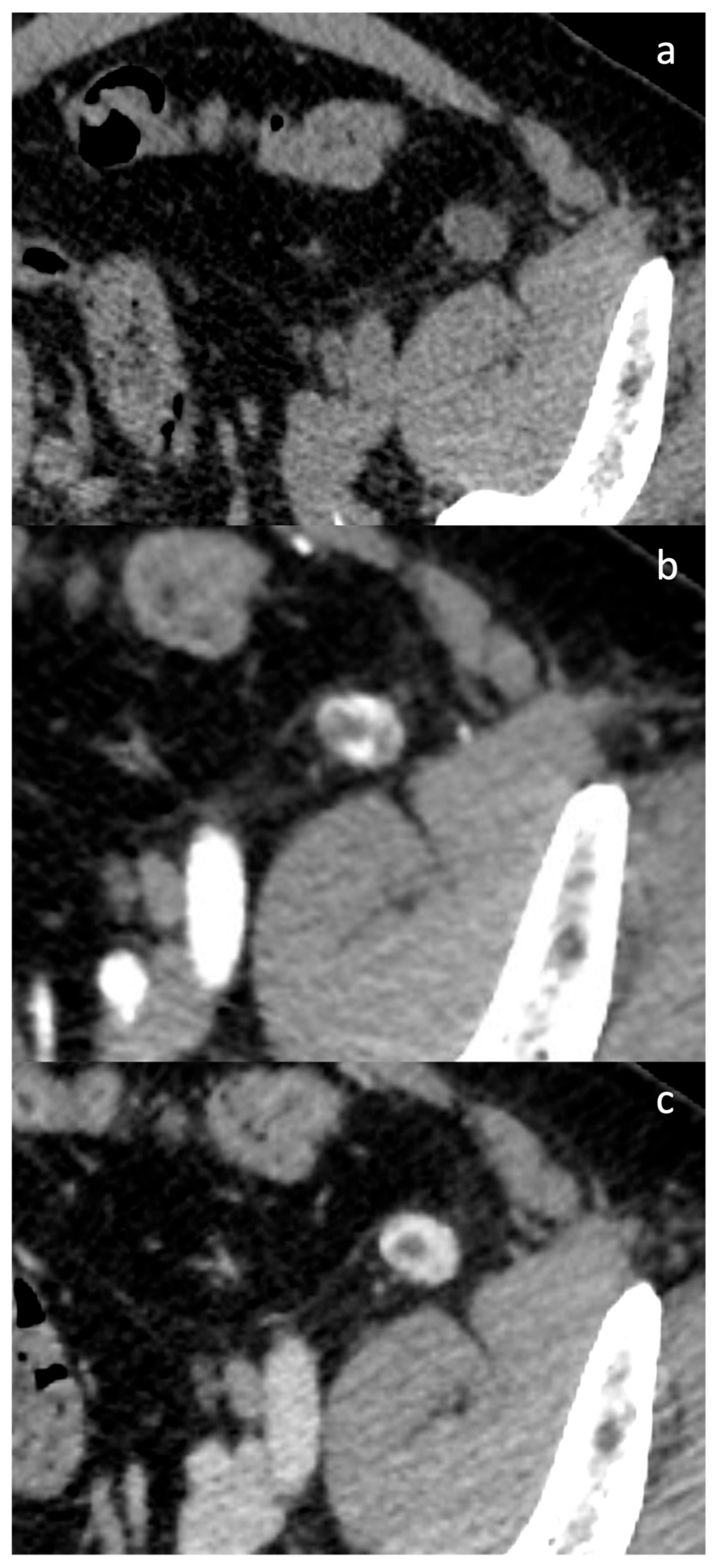
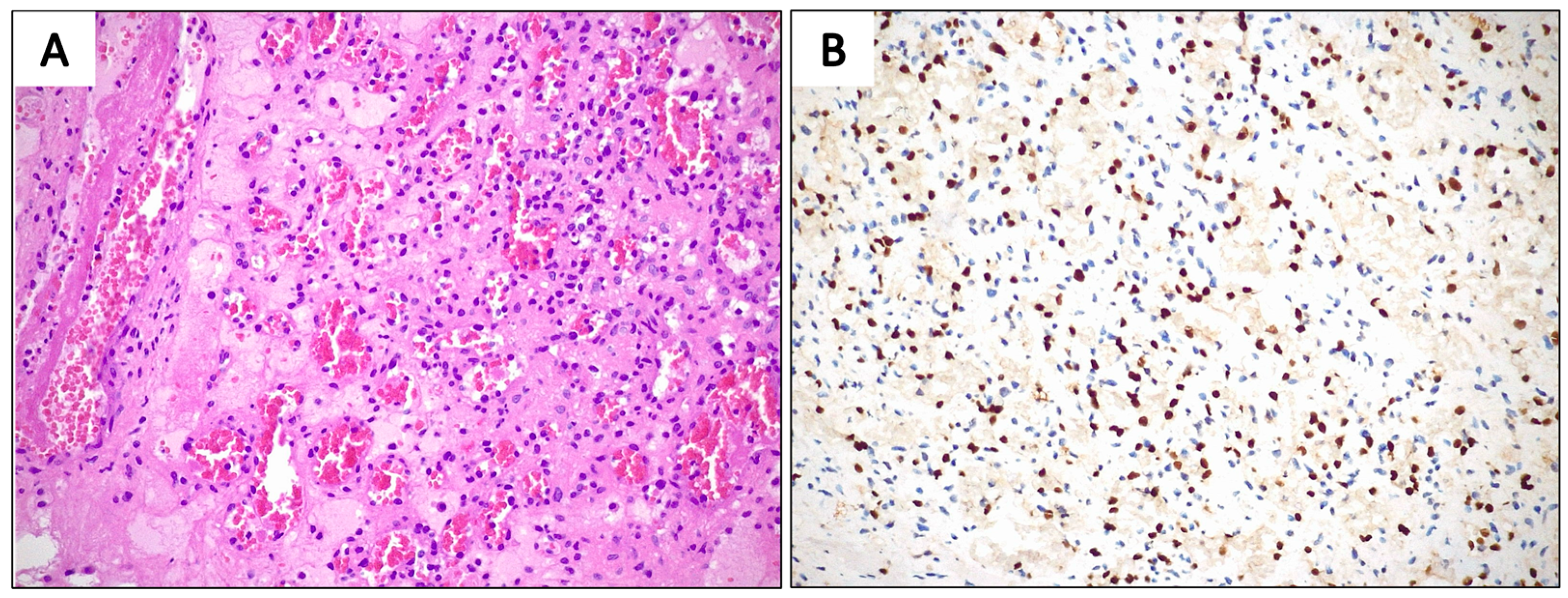
3.2. Case Presentation
4. Discussion
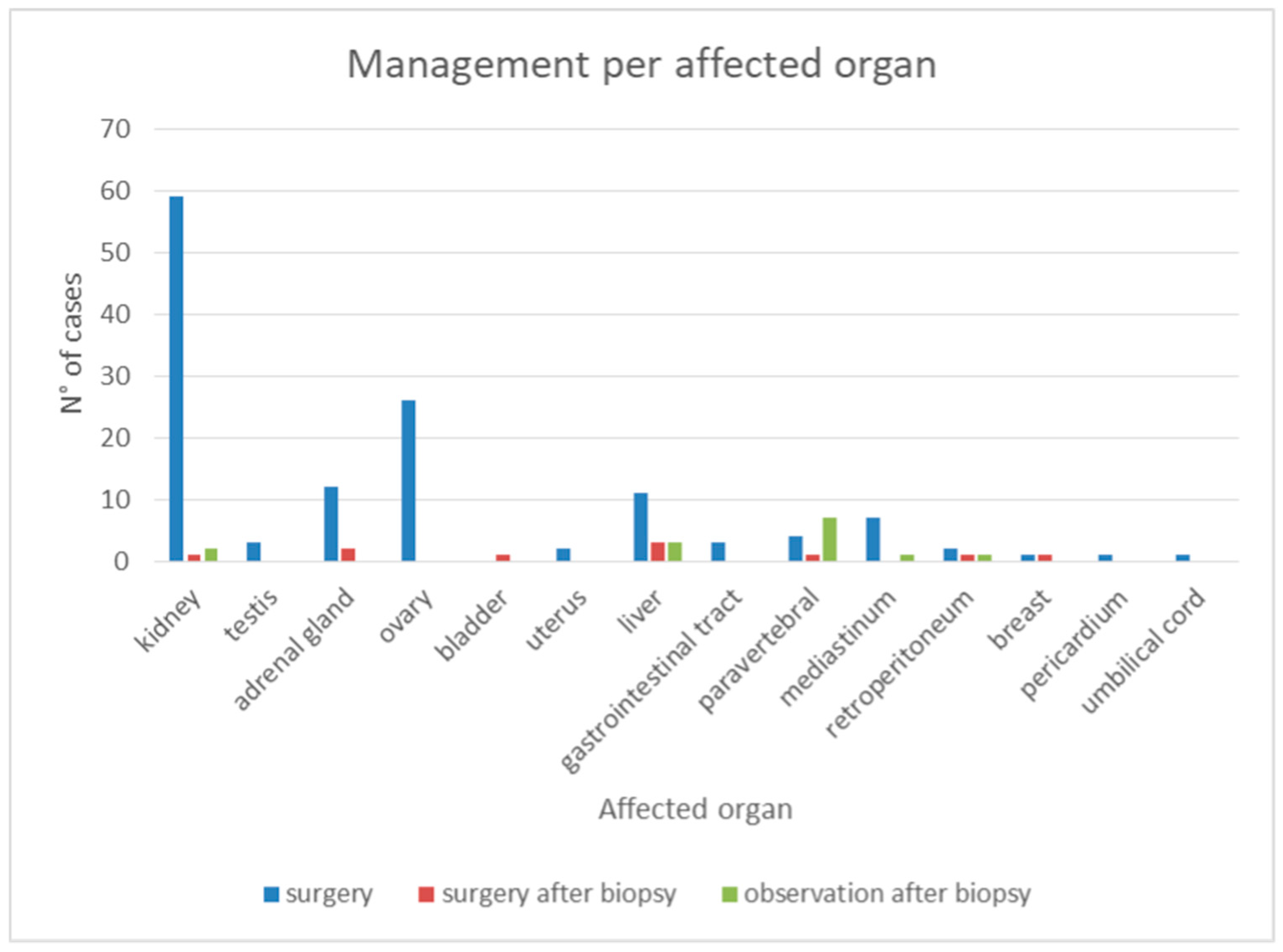
4.1. Key Findings from the Systematic Review
4.2. Demographics and Risk Factors
4.3. Diagnostic Challenges and Imaging Limitations
4.4. Histopathological Considerations and Differential Diagnosis
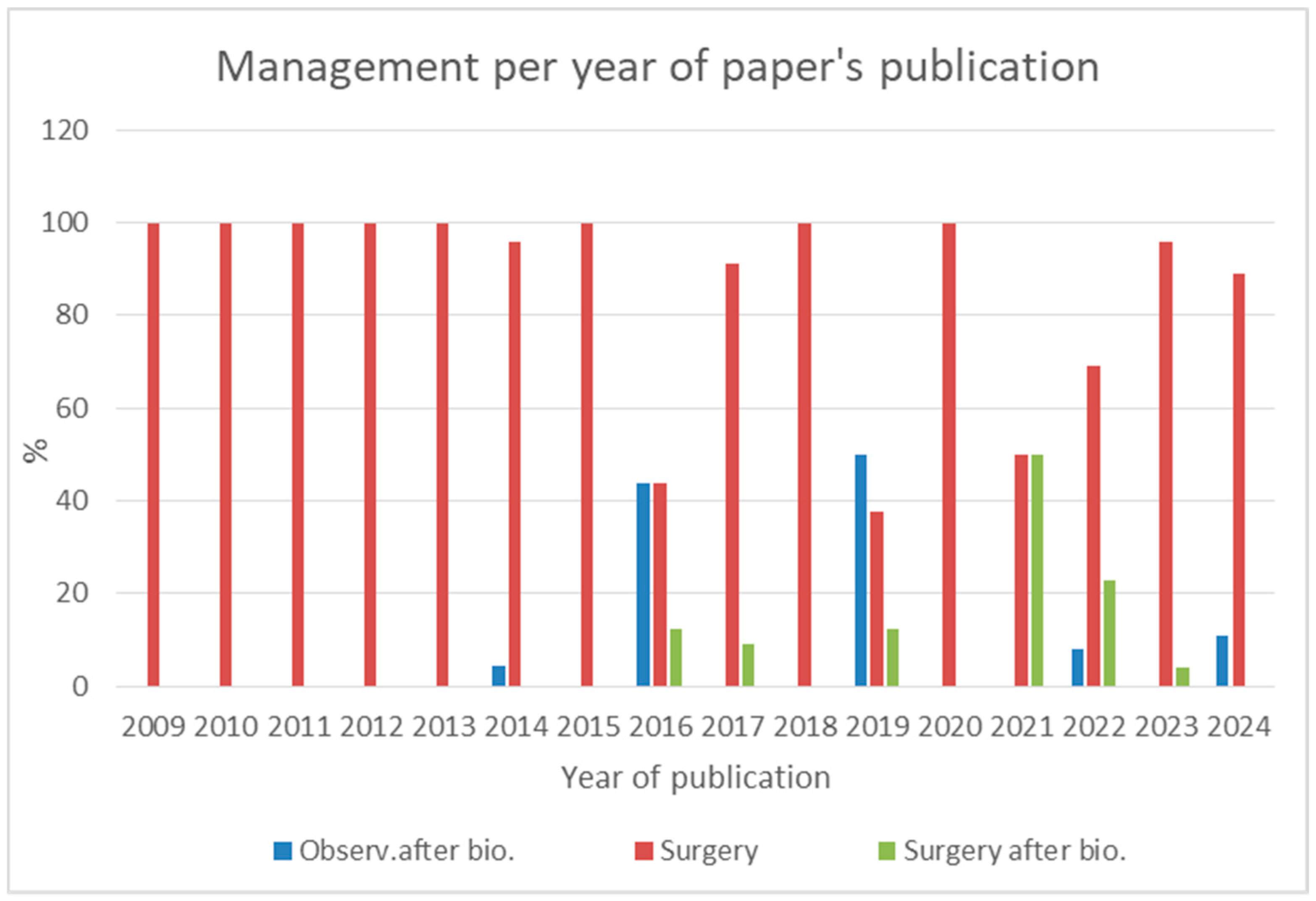
4.5. Evolution of AH Management: Shift Towards Conservative Approaches
4.6. Lessons from Our Case Report
4.7. Lessons from the Systematic Review
- -
- Middle-aged adult patient, no difference in sex;
- -
- A solid homogeneous well circumscribed lesion, without calcifications, with a characteristic CT and MRI contrast enhancement, as described in many case reports in the literature;
- -
- Minimal modifications in size during time;
- -
- Incidental discovery in asymptomatic patient;
- -
- Location in the genitourinary tract mainly, but also the liver and paravertebral space;
- -
- A history of renal impairment or ESRD;
- -
- Previous or coexistent oncological disease.
4.8. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AH | Anastomosing Hemangioma |
| AS | Angiosarcoma |
| MRI | Magnetic Resonance Imaging |
| SUV | Standardized Uptake Value |
References
- Montgomery, E.; Epstein, J.I. Anastomosing Hemangioma of the Genitourinary Tract A Lesion Mimicking Angiosarcoma. Am. J. Surg. Pathol. 2009, 33, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, C.; Zheng, J.; Sun, K. Anastomosing hemangioma of the kidney: A case report of a rare subtype of hemangioma mimicking angiosarcoma and review of the literature. Int. J. Clin. Exp. Pathol. 2013, 6, 757–765. [Google Scholar] [PubMed]
- Aparicio, L.D.; Ezquerro, J.C.; Artigas, C.; López, S.P.; Serrablo, A. AnastomosingHemangioma: Potential Differential Diagnosis for Incidental Vascular Lesion. Clin. Surg. 2021, 6, 3233. [Google Scholar]
- Al-Maghrabi, H.A.; Al Rashed, A.S. Challenging Pitfalls and Mimickers in Diagnosing Anastomosing Capillary Hemangioma of the Kidney: Case Report and Literature Review. Am. J. Case Rep. 2017, 18, 255–262. Available online: https://pubmed.ncbi.nlm.nih.gov/28286335/ (accessed on 24 March 2025). [CrossRef]
- Omiyale, A.O.; Carton, J. Clinicopathological and genetic features of anastomosing haemangioma of the kidney: A narrative review. AME Med. J. 2021, 6, 30. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85115411702&doi=10.21037%2famj-20-181&partnerID=40&md5=007f8f6bdaf93130cf7122240b7c1315 (accessed on 24 March 2025). [CrossRef]
- Perdiki, M.; Datseri, G.; Liapis, G.; Chondros, N.; Anastasiou, I.; Tzardi, M.; Delladetsima, J.K.; Drakos, E. Anastomosing hemangioma: Report of two renal cases and analysis of the literature. Diagn. Pathol. 2017, 12, 14. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Craig, J.W.; Silverman, S.G.; Alencar, R.O. Anastomosing hemangiomas: Locations of occurrence, imaging features, and diagnosis with percutaneous biopsy. Abdom. Radiol. 2016, 41, 1325–1332. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84975705716&doi=10.1007%2fs00261-016-0690-2&partnerID=40&md5=2b426192a48261c6351167d12ca50cde (accessed on 24 March 2025). [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024. [Google Scholar] [CrossRef]
- Kryvenko, O.N.; Gupta, N.S.; Meier, F.A.; Lee, M.W.; Epstein, J.I. Anastomosing Hemangioma of the Genitourinary System Eight Cases in the Kidney and Ovary With Immunohistochemical and Ultrastructural Analysis. Am. J. Clin. Pathol. 2011, 136, 450–457. [Google Scholar] [CrossRef]
- Tran, T.A.; Pernicone, P. Anastomosing hemangioma with fatty changes of the genitourinary tract: A lesion mimicking angiomyolipoma. Cent. Eur. J. Urol. 2012, 65, 40–42. [Google Scholar] [CrossRef]
- Ross, M.; Polcari, A.; Picken, M.; Sankary, H.; Milner, J. Anastomosing hemangioma arising from the adrenal gland. Urology 2012, 80, e27–e28. Available online: https://pubmed.ncbi.nlm.nih.gov/22840861/ (accessed on 24 March 2025). [CrossRef] [PubMed]
- Mehta, V.; Ananthanarayanan, V.; Antic, T.; Krausz, T.; Milner, J.; Venkataraman, G.; Picken, M.M. Primary benign vascular tumors and tumorlike lesions of the kidney: A clinicopathologic analysis of 15 cases. Virchows Arch. 2012, 461, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bigge, J.; Ulbright, T.M.; Montgomery, E. Anastomosing Hemangioma of the Liver and Gastrointestinal Tract An Unusual Variant Histologically Mimicking Angiosarcoma. Am. J. Surg. Pathol. 2013, 37, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, I.; Pichler, R.; Schäfer, G.; Zelger, B.; Zelger, B.; Aigner, F.; Bektic, J.; Horninger, W. Long-term follow up of renal anastomosing hemangioma mimicking renal angiosarcoma. Int. J. Urol. 2014, 21, 836–838. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84905051057&doi=10.1111%2fiju.12433&partnerID=40&md5=da95dbbfa7432a3a703113078b025701 (accessed on 24 March 2025). [CrossRef]
- Tao, L.L.; Dai, Y.; Yin, W.; Chen, J. A case report of a renal anastomosing hemangioma and a literature review: An unusual variant histologically mimicking angiosarcoma. Diagn. Pathol. 2014, 9, 159. [Google Scholar] [CrossRef]
- Chou, S.; Subramanian, V.; Lau, H.M.H.; Achan, A. Renal anastomosing hemangiomas with a diverse morphologic spectrum: Report of two cases and review of literature. Int. J. Surg. Pathol. 2014, 22, 369–373. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84904119131&doi=10.1177%2f1066896913492850&partnerID=40&md5=4301f035b7ae6b4cbfcdbb0a90a18597 (accessed on 24 March 2025). [CrossRef]
- Tahir, M.; Folwell, A. Anastomosing haemangioma of kidney: A rare subtype of vascular tumour of the kidney mimicking angiosarcoma. ANZ J. Surg. 2014, 86, 838–839. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85028273803&doi=10.1111%2fans.12779&partnerID=40&md5=0b6c616281dbde75787b38eb29504e1a (accessed on 24 March 2025). [CrossRef]
- Downes, M.R.; Dickson, B.C.; Cheung, C.C. Anastomosing haemangioma of kidney: Morphologic features and diagnostic considerations of an unusual vasoformative tumour. Diagn. Histopathol. 2014, 20, 208–212. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84899981223&doi=10.1016%2fj.mpdhp.2014.03.002&partnerID=40&md5=5f79d84787a6fc63bbe73b102175e42b (accessed on 24 March 2025). [CrossRef]
- Kryvenko, O.N.; Haley, S.L.; Smith, S.C.; Shen, S.S.; Paluru, S.; Gupta, N.S.; Jorda, M.; I Epstein, J.; Amin, M.B.; Truong, L.D. Haemangiomas in kidneys with end-stage renal disease: A novel clinicopathological association. Histopathology 2014, 65, 309–318. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Q.; Liu, Y.-L.; Yu, W.-J.; Liu, Y.; Zhao, H.; Zhuang, J.; Jiang, Y.-X.; Li, Y.-J. Anastomosing hemangioma arising from the kidney: A case of slow progression in four years and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 2208–2213. [Google Scholar]
- Hara, K.; Fukumura, Y.; Saito, T.; Arakawa, A.; Okabe, H.; Takeda, S.; Yao, T. A giant cord hemangioma with extramedullary hematopoiesis and elevated maternal serum human chorionic gonadotropin: A case report and review of the literature. Diagn. Pathol. 2015, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- John, I.; Folpe, A.L. Anastomosing Hemangiomas Arising in Unusual Locations: A Clinicopathologic Study of 17 Soft Tissue Cases Showing a Predilection for the Paraspinal Region. Am. J. Surg. Pathol. 2016, 40, 1084–1089. Available online: https://pubmed.ncbi.nlm.nih.gov/26945338/ (accessed on 24 March 2025). [CrossRef] [PubMed]
- Dundr, P.; Němejcová, K.; Laco, J.; Skálová, H.; Bauerová, L.; Matěj, R.; Fischerová, D. Anastomosing Hemangioma of the Ovary: A Clinicopathological Study of Six Cases with Stromal Luteinization. Pathol. Oncol. Res. 2017, 23, 717–722. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85008192274&doi=10.1007%2fs12253-016-0186-y&partnerID=40&md5=076f2fb378bd4d3eac8deeda311b0dde (accessed on 24 March 2025). [CrossRef] [PubMed]
- Burton, K.R.; Jakate, K.; Pace, K.T.; Vlachou, P.A. A case of recurrent, multifocal anastomosing haemangiomas. BMJ Case Rep. 2017, 2017, 220076. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85021094932&doi=10.1136%2fbcr-2017-220076&partnerID=40&md5=3cab3c26d4e6468a236e37edc89a02d4 (accessed on 24 March 2025). [CrossRef]
- Sun, K.; Wei, J.F.; Zhao, M.; Teng, X.D. Anastomosing hemangioma of the liver containing eosinophilic hyaline globules. Int. J. Clin. Exp. Med. 2017, 10, 7291–7295. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85018999318&partnerID=40&md5=7b25d0a4b145c69cf99a77839d13840e (accessed on 24 March 2025).
- Rodrigues, M.A.S.; Fonseca, E.K.U.N.; Yamauchi, F.I.; Baroni, R.H. Anastomosing hemangioma simulating renal cell carcinoma. Int. Braz. J. Urol. 2017, 43, 987–989. [Google Scholar] [CrossRef]
- Abboudi, H.; Tschobotko, B.; Carr, C.; DasGupta, R. Bilateral Renal Anastomosing Hemangiomas: A Tale of Two Kidneys. J. Endourol. Case Rep. 2017, 3, 176–178. [Google Scholar] [CrossRef]
- Berker, N.K.; Bayram, A.; Tas, S.; Bakir, B.; Caliskan, Y.; Ozcan, F.; Kilicaslan, I.; Ozluk, Y. Comparison of Renal Anastomosing Hemangiomas in End-Stage and Non–End-Stage Kidneys: A Meta-Analysis With a Report of 2 Cases. Int. J. Surg. Pathol. 2017, 25, 488–496. [Google Scholar] [CrossRef]
- Cheon, P.M.; Rebello, R.; Naqvi, A.; Popovic, S.; Bonert, M.; Kapoor, A. Anastomosing hemangioma of the kidney: Radiologic and pathologic distinctions of a kidney cancer mimic. Curr. Oncol. 2018, 25, e220–e223. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85049377247&doi=10.3747%2fco.25.3927&partnerID=40&md5=97abd810d949c4aef8f1d0f77632ed50 (accessed on 24 March 2025). [CrossRef]
- Gunduz, M.; Hurdogan, O.; Onder, S.; Yavuz, E. Cystic Anastomosing Hemangioma of the Ovary: A Case Report With Immunohistochemical and Ultrastructural Analysis. Int. J. Surg. Pathol. 2019, 27, 437–440. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85059593309&doi=10.1177%2f1066896918817148&partnerID=40&md5=02ba4371badec740fef72c560babe8fe (accessed on 24 March 2025). [CrossRef]
- Patel, S.R.; Abimbola, O.; Bhamber, T.; Weida, C.; Roy, O. Incidental finding of bilateral renal and adrenal anastomosing hemangiomas: A rare case report. Urol. Case Rep. 2019, 27, 100912. [Google Scholar] [CrossRef] [PubMed]
- Lunn, B.; Yasir, S.; Lam-Himlin, D.; Menias, C.O.; Torbenson, M.S.; Venkatesh, S.K. Anastomosing hemangioma of the liver: A case series. Abdom. Imaging 2019, 44, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Merritt, B.A.; Behr, S.; Umetsu, S.E.; Roberts, J.; Kolli, K.P. Anastomosing hemangioma of liver. J. Radiol. Case Rep. 2019, 13, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chandran, N.; Kannan, M.; Veeramani, M. Renal anastomosing hemangioma: A diagnosis to ponder. Indian J. Transplant. 2019, 13, 59–61. [Google Scholar] [CrossRef]
- Zheng, L.P.; Shen, W.A.; Wang, C.H.; Hu, C.D.; Chen, X.J.; Shen, Y.Y.; Wang, J. Anastomosing hemangioma arising from the left renal vein: A case report. World J. Clin. Cases 2020, 8, 4986–4992. [Google Scholar] [CrossRef]
- Rathore, K.; Yussouf, R.; Teh, M.; Jindal, S.; Wong, D.; Newman, M. Left Atrial Anastomosing Hemangioma Causing Recurrent Pericardial Effusion. Ann. Thorac. Surg. 2019, 109, e157–e159. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85079539806&doi=10.1016%2fj.athoracsur.2019.06.082&partnerID=40&md5=2377998896c2d091e40693030d9f210e (accessed on 24 March 2025). [CrossRef]
- Rezk, A.; Richards, S.; Patricia Castillo, R.; Schlumbrecht, M. Anastomosing hemangioma of the ovary mimics metastatic ovarian cancer. Gynecol. Oncol. Rep. 2020, 34, 100647. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85091101544&doi=10.1016%2fj.gore.2020.100647&partnerID=40&md5=f71190dc85ba9356c73bf8644ab814b2 (accessed on 24 March 2025). [CrossRef]
- Lin, M.S.; Ngo, T.; Schwartz, M.R.; Mehta, R.R.; Ayala, A.G.; Ro, J.Y. Anastomosing Hemangioma of the Breast: An Unusual Case at an Unusual Site. J. Breast Cancer 2020, 23, 326–330. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.; Zhou, L.; Zhao, M.; Wang, C. Anastomosing Hemangioma Incidentally Found in Kidney or Adrenal Gland: Study of 10 Cases and Review of Literature. Urol. J. 2020, 17, 650–656. [Google Scholar]
- Stewart, C.J.R.; Salfinger, S.G. Anastomosing haemangioma of the ovary with hilus cell hyperplasia. Pathology 2020, 52, 392–394. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85082146223&doi=10.1016%2fj.pathol.2019.11.010&partnerID=40&md5=5ececa352c5c0ec2c20bf0315f03c5f0 (accessed on 24 March 2025). [CrossRef]
- Johnstone, K.J.; Strutton, G.M.; Perry-Keene, J.L.; Hazratwala, K.; Delahunt, B. Multifocal anastomosing haemangioma of the kidney with intravascular growth and sinus fat invasion: A rare benign mimic of angiosarcoma. Pathology 2020, 52, 394–396. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85082092296&doi=10.1016%2fj.pathol.2020.01.681&partnerID=40&md5=2dc5636179845488678108bf570a1616 (accessed on 24 March 2025). [CrossRef] [PubMed]
- Kim, C.S.; Na Choi, S.J.; Kim, S.-S.; Suh, S.H.; Bae, E.H.; Ma, S.K.; Kim, S.W. An anastomosing hemangioma mimicking a renal cell carcinoma in a kidney transplant recipient: A case report. BMC Nephrol. 2021, 22, 262. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Cheng, S.Y. Case Report on Anastomosing Haemangioma: An Unusual Vascular Tumor in Kidney. Case Rep. Nephrol. 2021, 2021, 8847998. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85100193954&doi=10.1155%2f2021%2f8847998&partnerID=40&md5=687839f55e0abcdca175e4153ae67ebc (accessed on 24 March 2025). [CrossRef] [PubMed]
- Rogers, T.; Shah, N.; Mauro, D.; McGinty, K.A. Anastomosing hemangioma of the liver: An unusual variant in abdominal MRI imaging. Radiol. Case Rep. 2022, 17, 4889–4892. [Google Scholar] [CrossRef]
- Jha, S.; Jain, P.; Dixit, S.; Sharma, S. Anastomosing Hemangioma of Ovary with Stromal Luteinization Masquerading as Sex Cord-Stromal Tumor on Intraoperative Consultation. J. Microsc. Ultrastruct. 2022, 10, 208–210. [Google Scholar] [CrossRef]
- Chang Chien, Y.C.; Beke, L.; Méhes, G.; Mokánszki, A. Anastomosing Haemangioma: Report of Three Cases With Molecular and Immunohistochemical Studies and Comparison With Well-Differentiated Angiosarcoma. Pathol. Oncol. Res. 2022, 28, 1610498. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85136045135&doi=10.3389%2fpore.2022.1610498&partnerID=40&md5=953fbb5fdbf55ad039daae57c6ba2725 (accessed on 24 March 2025). [CrossRef]
- Chua, W.M.; Hoe, K.M.J.; Dalan, R.; Too, C.W.; Ong, S.Y.K.; Tay, T.K.Y.; Loke, K.S.H.M. Anastomosing Hemangioma on 68Ga-DOTATATE PET/CT. Clin. Nucl. Med. 2022, 47, 321–323. [Google Scholar] [CrossRef]
- Nishikimi, T.; Mizuno, H.; Kashima, A.; Morikami, H.; Ishiguro, S.; Ohashi, T.; Yamada, H. A case of robot-assisted adrenalectomy performed for an adrenal tumor (anastomosing hemangioma) exceeding 7 cm. IJU Case Rep. 2022, 5, 469–473. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Hong, P.; Deng, S.-H.; Tang, S.-Y.; Liu, Z.; He, H.-Y.; Ma, L.-L.; Zhang, S.-D.; Tian, X.-J. Spermatic cord anastomosing hemangioma mimicking a malignant inguinal tumor: A case report and literature review. Front. Surg. 2022, 9, 930160. [Google Scholar] [CrossRef]
- Xue, X.; Song, M.; Xiao, W.; Chen, F.; Huang, Q. Imaging findings of retroperitoneal anastomosing hemangioma: A case report and literature review. BMC Urol. 2022, 22, 77. [Google Scholar]
- Sasaki, K.; Kuge, A.; Shimokawa, Y.; Yamaki, T.; Kondo, R.; Sonoda, Y. Intracranial parenchymal capillary hemangioma: A case report. Surg. Neurol. Int. 2023, 14, 401. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cao, H.; Wang, H.; Qiu, L.; Shang, K.; Wen, Y. A growing liver anastomosing hemangioma. Quant. Imaging Med. Surg. 2023, 13, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-Fontanet, E.; Maillard, M.; Fasquelle, F.; Vietti-Violi, N.; Labgaa, I.; Uldry, E. A hypervascular liver mass. Surgery 2022, 172, e5–e6. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85123247491&doi=10.1016%2fj.surg.2021.12.020&partnerID=40&md5=f140476612bcee3169849b84af0f5e90 (accessed on 24 March 2025). [CrossRef] [PubMed]
- Shaker, N.; Patel, A.; Tozbikian, G.; Parwani, A. Anastomosing hemangioma: A case report of a benign tumor often misdiagnosed as a malignant epithelioid angiosarcoma. Urol. Case Rep. 2022, 42, 102023. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J. A case report of anastomosing hemangioma of the ovary. Medicine 2023, 102, e33801. [Google Scholar] [CrossRef]
- Ismayilov, R. A rare benign tumor of the liver mimicking angiosarcoma: Anastomosing hemangioma. North. Clin. Istanb. 2023, 10, 524–526. [Google Scholar] [CrossRef]
- Alaghehbandan, R.; Remer, E.M.; Berber, E.; McKenney, J.K. Anastomosing haemangioma of the adrenal gland: A clinicopathological series of seven cases. Histopathology 2023, 83, 791–797. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85167355522&doi=10.1111%2fhis.15022&partnerID=40&md5=44ae2916f508c2cc45cd427e8e179f38 (accessed on 24 March 2025). [CrossRef]
- Yang, L.; Han, P.; Liu, X.; Zhang, Y. Easily confused with hepatic angiosarcoma: Rare hepatic giant anastomosing hemangioma. Asian J. Surg. 2022, 46, 1006–1007. [Google Scholar] [CrossRef]
- Capinha, M.D.; Carvalho-Dias, E.; Cerqueira-Alves, M.; Mota, P. Renal anastomosing haemangioma. BMJ Case Rep. 2023, 16, e254131. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85171697208&doi=10.1136%2fbcr-2022-254131&partnerID=40&md5=9308537dbe5c53ee3eb2882a07a661bc (accessed on 24 March 2025). [CrossRef]
- Slutsky, H.L.; Prieto-Granada, C.N.; McCaffrey, R.L. A Case of Anastomosing Hemangioma of the Breast During Pregnancy. Am. Surg. 2023, 89, 3528–3530. [Google Scholar] [CrossRef]
- Paparo, A.J.; Hillery, S.; Gan, E.; Chai, S.; Khor, T.S. Anastomosing haemangioma of the colon. Pathology 2023, 55, 892–894. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85166214714&doi=10.1016%2fj.pathol.2023.03.014&partnerID=40&md5=5ea37f671d93ab09ff2763a7d85d6b54 (accessed on 24 March 2025). [CrossRef] [PubMed]
- McHenry, A.; Buza, N. Anastomosing Hemangioma of the Ovary With Leydig Cell Hyperplasia: A Clinicopathologic Study of 12 Cases. Int. J. Gynecol. Pathol. 2022, 42, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Ma, T.; Lin, C. Hepatic anastomosing hemangioma: Description of a rare case and a literature analysis. Quant. Imaging Med. Surg. 2023, 13, 6355–6362. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.B.; Zhang, Q.; Wu, W.X.; Li, X.; Zhang, L.; Wang, J.Y.; Guo, Z.Q.; Hu, S.Q. Primary adrenal anastomosing haemangioma. Endokrynol. Pol. 2023, 74, 119–120. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85149053367&doi=10.5603%2fEP.a2022.0091&partnerID=40&md5=439348515f39870a6b2863b54aca70b6 (accessed on 24 March 2025). [CrossRef]
- Caldwell, N.J.; Ackman, J.B.; Chebib, I.; Mino-Kenudson, M.; Nielsen, G.P.; Hung, Y.P. Anastomosing haemangioma of the mediastinum: Clinicopathological series with radiological and genetic characterisation. Histopathology 2023, 84, 463–472. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85176139173&doi=10.1111%2fhis.15085&partnerID=40&md5=51571e909f7f4fa058efd67137c7e320 (accessed on 24 March 2025). [CrossRef]
- Wu, Q.; Luo, H. Anastomosing hemangioma of the ovary: A rare benign tumor. Arch. Gynecol. Obstet. 2024, 309, 2909–2910. [Google Scholar] [CrossRef]
- Agha, R.A.; Franchi, T.; Sohrabi, C.; Mathew, G.; Kerwan, A.; Thoma, A.; Beamish, A.J.; Noureldin, A.; Rao, A.; Vasudevan, B.; et al. The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines. Int. J. Surg. 2020, 84, 226–230. [Google Scholar] [CrossRef]
- Lappa, E.; Drakos, E. Anastomosing Hemangioma: Short Review of a Benign Mimicker of Angiosarcoma. Arch. Pathol. Lab. Med. 2019, 144, 240–244. [Google Scholar] [CrossRef]
- Faraz, M.; Rosenzweig, A.; Panizo, A.; Hajiyeva, S.; Subasi, N.B.; Alghamdi, M.A.; Lightle, A.A.; Kuthi, L.; Kelemen, D.; Sangoi, A.R.; et al. Primary intrarenal hemangioma—A series of 39 cases. Ann. Diagn. Pathol. 2025, 75, 152436. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, J.; Yang, S.O. PET/CT in Benign and Malignant Musculoskeletal Tumors and Tumor-Like Conditions. Semin. Musculoskelet. Radiol. 2014, 18, 133–148. [Google Scholar]
- Bean, G.R.; Joseph, N.M.; Gill, R.M.; Folpe, A.L.; Horvai, A.E.; Umetsu, S.E. Recurrent GNAQ mutations in anastomosing hemangiomas. Mod. Pathol. 2017, 30, 722–727. [Google Scholar] [CrossRef]
- Gestrich, C.K.; Vivero, M.P.; Konczyk, D.J.; Goss, J.A.; Labow, B.I.; Pearson, G.D.; Cottrell, C.E.; Mathew, M.T.; Prasad, V.; Kozakewich, H.P.; et al. Papillary Hemangioma Harbors Somatic GNA11 and GNAQ Mutations. Am. J. Surg. Pathol. 2023, 48, 106–111. [Google Scholar] [CrossRef]

| Source | Area | Incidental | Organ | Age, Years | Sex | Malignancy in Medical History | Renal Imp. | HTN | Diagnosis to Treatment Months | Imaging Performed | Size Max, mm | Treatment ¥ | Surgery * | Postoperative Morbility | Follow Up, Months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Montgomery E et al., 2009 [1] | US | ns | kidney | 74 | m | n | n | n | 1 | ns | 15 | 2 | c | 0 | 36 |
| Montgomery E et al., 2009 [1] | US | ns | kidney | 75 | f | n | n | n | 1 | ns | 20 | 2 | c | 0 | ns |
| Montgomery E et al., 2009 [1] | US | ns | kidney | 65 | f | n | n | n | 1 | ns | 20 | 2 | a | 0 | 8 |
| Montgomery E et al., 2009 [1] | US | ns | kidney | 49 | m | n | n | n | 1 | ns | 13 | 2 | c | 0 | 12 |
| Montgomery E et al., 2009 [1] | US | ns | testis | 54 | m | n | n | n | 1 | ns | 15 | 2 | c | 0 | 8 |
| Montgomery E et al., 2009 [1] | US | ns | testis | 49 | m | n | n | n | 1 | ns | 17 | 2 | c | 0 | 12 |
| Kryvenko O et al., 2011 [10] | US | y | ovary | 70 | f | y | n | n | 1 | CT | 2 | 2 | c | 0 | 25 |
| Kryvenko O et al., 2011 [10] | US | y | ovary | 49 | f | n | n | n | 1 | CT | 1 | 2 | c | 0 | 16 |
| Kryvenko O et al., 2011 [10] | US | y | ovary | 77 | f | n | n | n | 1 | CT | 11 | 2 | c | 0 | 32 |
| Kryvenko O et al., 2011 [10] | US | y | kidney | 51 | f | n | y | n | 1 | CT | 10 | 2 | c | 0 | 122 |
| Kryvenko O et al., 2011 [10] | US | y | kidney | 39 | m | y | y | n | 1 | CT | 50 | 2 | c | 0 | 6 |
| Kryvenko O et al., 2011 [10] | US | y | kidney | 67 | f | n | n | n | 1 | CT | 12 | 2 | c | 0 | 6 |
| Kryvenko O et al., 2011 [10] | US | y | kidney | 54 | f | n | y | n | 1 | CT | 12 | 2 | c | 0 | 3 |
| Tran AT et al., 2012 [11] | US | n | kidney | 61 | m | n | n | n | 1 | CT | 21 | 2 | c | 0 | ns |
| Ross M et al., 2012 [12] | US | y | adrenal g. | 49 | m | n | y | n | 1 | CT | 37 | 2 | d | 0 | ns |
| Metha V et al., 2012 [13] | US | ns | kidney | 49 | m | n | y | n | 1 | CT | 20 | 2 | c | 0 | 3 |
| Metha V et al., 2012 [13] | US | ns | kidney | 55 | m | n | y | n | 1 | CT | 6 | 2 | c | 0 | 3 |
| Metha V et al., 2012 [13] | US | ns | kidney | 45 | m | n | y | n | 1 | CT | 19 | 2 | c | 0 | 12 |
| Lin J et al., 2013 [14] | US | y | liver | 64 | f | n | n | n | 1 | ns | 30 | 2 | b | 0 | 67 |
| Lin J et al., 2013 [14] | US | y | liver | 62 | f | y | n | n | 1 | ns | 24 | 2 | b | 0 | 14 |
| Lin J et al., 2013 [14] | US | y | g.i. tract | 70 | f | n | n | n | 1 | ns | 2 | 2 | a | 0 | ns |
| Lin J et al., 2013 [14] | US | n | g.i. tract | 68 | m | n | n | n | 1 | ns | 48 | 2 | b | 0 | 8 |
| Lin J et al., 2013 [14] | US | y | liver | 48 | m | y | n | n | 1 | ns | 20 | 2 | b | 0 | 18 |
| Lin J et al., 2013 [14] | US | n | liver | 71 | f | n | n | n | 1 | ns | 60 | 2 | b | 0 | 96 |
| Zhao M et al., 2013 [2] | China | y | kidney | 48 | m | y | n | n | 1 | CT, MRI | 23 | 2 | b | 0 | 12 |
| Heidegger I et al., 2014 [15] | EU | n | kidney | 56 | m | n | n | n | 1 | CT | 70 | 2 | c | 0 | 120 |
| Heidegger I et al., 2014 [15] | EU | n | kidney | 56 | m | n | n | n | 1 | CT | 70 | 2 | c | 0 | 144 |
| Tao L et al., 2014 [16] | China | y | kidney | 32 | m | n | n | n | 1 | CT | 34 | 2 | c | 0 | 21 |
| Chou S et al., 2014 [17] | Oceania | y | kidney | 50 | f | y | y | n | 1 | CT | 10 | 2 | c | 0 | 14 |
| Chou S et al., 2014 [17] | Oceania | y | kidney | 60 | m | n | y | y | 1 | CT | 28 | 2 | c | 0 | 8 |
| Tahir M et al., 2014 [18] | Oceania | y | kidney | 57 | m | n | n | n | 1 | CT | 46 | 2 | c | 0 | 12 |
| Downes MR et al., 2014 [19] | Canada | y | kidney | 59 | f | y | n | n | 1 | CT | 45 | 2 | c | 0 | ns |
| Downes MR et al., 2014 [19] | Canada | y | kidney | 28 | m | y | n | n | 36 | CT | 13 | 1 | 0 | ns | |
| Kryvenko ON et al., 2014 [20] | US | n | kidney | 68 | f | n | y | n | 1 | CT | 15 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | y | kidney | 51 | f | n | y | n | 1 | CT | 10 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | n | kidney | 54 | f | y | y | n | 1 | CT | 11 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | y | kidney | 29 | m | n | y | n | 1 | CT | 13 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | y | kidney | 40 | m | y | y | n | 1 | CT | 3 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | n | kidney | 34 | m | y | y | n | 1 | CT | 13 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | y | kidney | 62 | m | y | y | n | 1 | CT | 7 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | y | kidney | 40 | m | n | y | n | 1 | CT | 28 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | y | kidney | 46 | m | n | y | n | 1 | CT | 16 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | y | kidney | 60 | m | y | y | n | 1 | CT | 12 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | n | kidney | 49 | m | n | y | n | 1 | CT | 35 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | ns | kidney | 49 | m | n | y | n | 1 | CT | 13 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | n | kidney | 66 | m | y | y | n | 1 | CT | 30 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | y | kidney | 15 | m | n | y | n | 1 | CT | 7 | 2 | c | 0 | ns |
| Kryvenko ON et al., 2014 [20] | US | n | kidney | 17 | m | y | y | n | 1 | CT | 28 | 2 | c | 0 | ns |
| Zhang W et al., 2015 [21] | China | n | kidney | 29 | f | n | n | n | 48 | ns | 20 | 2 | b | 0 | ns |
| Hara K et al., 2015 [22] | Japan | n | umbilical cord | 30 | f | n | n | n | 1 | MRI | 100 | 2 | c | 0 | ns |
| Lu J et al., 2016 [23] | China | n | bladder | 46 | m | n | n | n | 1 | CT | 14 | 3 | b | 0 | ns |
| John I et al., 2016 [23] | US | ns | paravertebral | 85 | m | n | n | n | 1 | ns | 43 | 2 | a | 0 | 9 |
| John I et al., 2016 [23] | US | ns | paravertebral | 61 | m | n | n | n | 1 | ns | ns | 1 | 0 | ns | |
| John I et al., 2016 [23] | US | ns | paravertebral | 67 | m | n | n | n | 1 | ns | 27 | 1 | 0 | 46 | |
| John I et al., 2016 [23] | US | ns | paravertebral | 76 | m | n | n | n | 1 | ns | 15 | 1 | 0 | 32 | |
| John I et al., 2016 [23] | US | ns | paravertebral | 31 | f | n | n | n | 1 | ns | ns | 3 | a | 0 | ns |
| John I et al., 2016 [23] | US | ns | paravertebral | 69 | m | n | n | n | 1 | ns | 42 | 1 | 0 | ns | |
| John I et al., 2016 [23] | US | ns | paravertebral | 79 | m | n | n | n | 1 | ns | 75 | 2 | a | 0 | 12 |
| John I et al., 2016 [23] | US | ns | paravertebral | 67 | f | n | n | n | 1 | ns | 36 | 1 | 0 | ns | |
| John I et al., 2016 [23] | US | ns | paravertebral | 67 | m | n | n | n | 1 | ns | ns | 1 | 0 | 12 | |
| John I et al., 2016 [23] | US | ns | retroperitoneal | 67 | m | n | n | n | 1 | ns | ns | 1 | 0 | 12 | |
| John I et al., 2016 [23] | US | ns | mediastinum | 70 | f | n | n | n | 1 | ns | 41 | 2 | a | 0 | 1 |
| John I et al., 2016 [23] | US | ns | uterus | 74 | f | n | n | n | 1 | ns | 67 | 2 | a | 0 | ns |
| John I et al., 2016 [23] | US | ns | paravertebral | 62 | m | n | n | n | 1 | ns | 24 | 2 | a | 0 | 1 |
| John I et al., 2016 [23] | US | ns | uterus | 36 | f | n | n | n | 1 | ns | 35 | 2 | a | 0 | ns |
| John I et al., 2016 [23] | US | ns | paravertebral | 53 | m | n | n | n | 1 | ns | 40 | 2 | a | 0 | 1 |
| Dundr P et al., 2017 [24] | EU | y | ovary | 66 | f | n | n | n | 1 | ns | 5 | 2 | c | 0 | 25 |
| Dundr P et al., 2017 [24] | EU | y | ovary | 43 | f | n | n | n | 1 | ns | 13 | 2 | c | 0 | 4 |
| Dundr P et al., 2017 [24] | EU | y | ovary | 69 | f | n | n | n | 1 | ns | 15 | 2 | c | 0 | 52 |
| Dundr P et al., 2017 [24] | EU | y | ovary | 81 | f | n | n | n | 1 | ns | 35 | 2 | c | 0 | ns |
| Dundr P et al., 2017 [24] | EU | n | ovary | 68 | f | y | n | n | 1 | CT | 35 | 2 | c | 0 | ns |
| Dundr P et al., 2017 [24] | EU | y | ovary | 69 | f | y | n | n | 1 | ns | 12 | 2 | c | 0 | 13 |
| Burton KR et al., 2017 [25] | Canada | y | adrenal g. | 68 | m | y | y | n | 120 | CT | 37 | 3 | c | 0 | 24 |
| Sun k et al., 2017 [26] | China | y | liver | 51 | m | n | n | n | 1 | CT | 50 | 2 | b | 0 | 36 |
| Rodrigues MAS et al., 2017 [27] | Brazil | y | kidney | 53 | m | n | n | n | 1 | CT, MRI | 25 | 2 | a | 0 | ns |
| Abboudi H et al., 2017 [28] | EU | n | kidney | 59 | f | n | y | n | 1 | CT | 27 | 2 | c | 0 | 36 |
| Al Maghrabi HA et al., 2017 [4] | Saudi Arabia | n | kidney | 55 | f | n | y | y | 1 | CT | 20 | 2 | b | 0 | 12 |
| Perdiki M et al., 2017 [6] | EU | y | kidney | 47 | m | n | y | n | 1 | CT | 25 | 2 | c | 0 | 14 |
| Perdiki M et al., 2017 [6] | EU | n | kidney | 64 | f | n | n | n | 1 | CT | 10 | 2 | b | 0 | 25 |
| Berker NK et al., 2017 [29] | EU | y | kidney | 24 | f | n | y | n | 1 | MRI | 30 | 2 | b | 0 | 10 |
| Berker NK et al., 2017 [29] | EU | y | kidney | 57 | f | n | y | n | 1 | CT | 26 | 2 | c | 0 | 4 |
| Cheon PM et al., 2018 [30] | Canada | n | kidney | 40 | m | n | n | n | 1 | CT, MRI | 46 | 2 | c | 0 | 12 |
| Gunduz M et al., 2018 [31] | EU | y | ovary | 62 | f | n | n | n | 1 | CT | 90 | 2 | d | 0 | ns |
| Sagar R et al., 2019 [32] | US | y | kidney | 39 | f | n | y | y | 1 | CT | 15 | 1 | 0 | 24 | |
| Lunn B et al., 2019 [33] | US | n | liver | 33 | f | n | n | n | 1 | CT, MRI | 51 | 2 | b | 0 | ns |
| Lunn B et al., 2019 [33] | US | n | liver | 67 | f | n | n | n | 1 | MRI | 17 | 1 | 0 | 36 | |
| Lunn B et al., 2019 [33] | US | y | liver | 77 | m | n | n | n | 1 | CT, MRI | 21 | 1 | 0 | ns | |
| Lunn B et al., 2019 [33] | US | y | liver | 22 | m | n | n | n | 1 | MRI | 19 | 2 | b | 0 | ns |
| Lunn B et al., 2019 [33] | US | y | liver | 48 | m | y | n | n | 1 | CT, MRI | 17 | 3 | b | 0 | ns |
| Merritt B et al., 2019 [34] | US | y | liver | 56 | m | y | n | n | 1 | CT, MRI | 35 | 1 | 0 | ns | |
| Chandran N et al., 2019 [35] | India | y | kidney | 36 | m | n | y | n | 1 | CT | 17 | 2 | c | 0 | ns |
| Zheng LP et al., 2020 [36] | China | y | kidney | 74 | m | n | n | y | 1 | CT, MRI | 26 | 2 | b | 0 | 2 |
| Rathore K et al., 2020 [37] | Oceania | n | pericardium | 64 | m | n | n | y | 1 | CT, MRI | 40 | 2 | a | 0 | ns |
| Rezk A et al., 2020 [38] | US | y | ovary | 60 | f | n | n | n | 12 | CT | 65 | 2 | c | 0 | ns |
| Lin MS et al., 2020 [39] | US | y | breast | 49 | f | n | n | n | 1 | CT | 10 | 2 | a | 0 | 12 |
| Zhou J et al., 2020 [40] | China | n | kidney | 28 | m | n | n | n | 1 | CT | 3,9 | 2 | c | 0 | 46 |
| Zhou J et al., 2020 [40] | China | y | kidney | 40 | m | n | n | n | 1 | CT | 0,8 | 2 | c | 0 | 10 |
| Zhou J et al., 2020 [40] | China | y | kidney | 45 | f | n | n | n | 1 | CT | 1,5 | 2 | c | 0 | 25 |
| Zhou J et al., 2020 [40] | China | y | kidney | 48 | f | n | n | n | 1 | CT | 1,8 | 2 | c | 0 | 30 |
| Zhou J et al., 2020 [40] | China | y | kidney | 52 | m | n | n | n | 1 | CT | 2 | 2 | c | 0 | 32 |
| Zhou J et al., 2020 [40] | China | y | kidney | 55 | f | n | n | n | 1 | CT | 2 | 2 | c | 0 | 33 |
| Zhou J et al., 2020 [40] | China | y | kidney | 60 | m | n | n | n | 1 | CT | 2,2 | 2 | c | 0 | 34 |
| Zhou J et al., 2020 [40] | China | y | kidney | 63 | f | n | n | n | 1 | CT | 2,5 | 2 | c | 0 | 38 |
| Zhou J et al., 2020 [40] | China | y | adrenal g. | 67 | m | y | n | n | 1 | CT | 2,8 | 2 | c | 0 | 40 |
| Zhou J et al., 2020 [40] | China | y | adrenal g. | 71 | f | n | n | n | 1 | CT | 30 | 2 | c | 0 | 42 |
| Stewart CJR et al., 2020 [41] | Oceania | y | ovary | 48 | f | n | n | n | 1 | ns | 8 | 2 | c | 0 | ns |
| Johnstone KJ et al., 2020 [42] | Oceania | y | kidney | 70 | m | n | n | n | 1 | CT | 35 | 2 | c | 0 | ns |
| Kim et al., 2021 [43] | South Korea | y | kidney | 35 | m | n | y | y | 1 | CT, MRI | 17 | 3 | c | 0 | ns |
| Lo C et al., 2021 [44] | China | n | kidney | 84 | m | n | y | y | 1 | CT | 55 | 2 | c | 2 | 1 |
| Rogers T et al., 2022 [45] | US | y | liver | 52 | f | n | n | n | 18 | MRI | 16 | 3 | a | 0 | 24 |
| Shivani J et al., 2022 [46] | India | n | ovary | 35 | f | n | n | n | 1 | CT | 110 | 2 | c | 0 | ns |
| Chang Chien Y et al., 2022 [47] | EU | ns | ovary | 68 | f | n | n | n | 1 | ns | 18 | 2 | c | 0 | ns |
| Chang Chien Y et al., 2022 [47] | EU | ns | ovary | 76 | f | y | n | n | 1 | ns | 35 | 2 | c | 0 | ns |
| Chang Chien Y et al., 2022 [47] | EU | ns | kidney | 52 | f | n | n | n | 1 | ns | 12 | 2 | a | 0 | ns |
| Wei Ming C et al., 2022 [48] | Singapore | y | paravertebral | 32 | f | n | y | n | 1 | CT, RM, GaPET | 18 | 1 | 0 | ns | |
| Nishikimi T et al., 2022 [49] | Japan | n | adrenal g. | 49 | m | n | n | n | 4 | CT | 72 | 2 | c | 0 | ns |
| Zhang Z et al., 2022 [50] | China | n | testis | 84 | m | n | n | n | 1 | CT, MRI | 23 | 2 | c | 0 | ns |
| Xue X et al., 2022 [51] | China | y | retroperit. | 64 | f | n | n | y | 1 | CT, MRI | 108 | 2 | a | 0 | 24 |
| Sasaki Y et al., 2023 [52] | Japan | y | kidney | 65 | m | n | y | n | 36 | CT, MRI | 22 | 2 | b | 0 | 3 |
| Ma Y et al., 2022 [53] | China | y | liver | 29 | m | n | n | n | 72 | CT, MRI | 53 | 2 | a | 0 | ns |
| Lazaro-Fontanet E et al., 2022 [54] | EU | y | liver | 49 | f | y | n | n | 1 | MRI | 100 | 3 | b | 0 | ns |
| Shaker N et al., 2022 [55] | US | n | retroperit. | 66 | m | n | n | n | 1 | CT | 24 | 3 | a | 0 | 24 |
| Wang Z et al., 2023 [56] | China | y | ovary | 28 | f | n | n | n | 4 | MRI | 41 | 2 | a | 0 | 7 |
| Ismayilov R et al., 2023 [57] | EU | y | liver | 53 | f | n | n | n | 1 | CT, MRI | 25 | 2 | a | 0 | 6 |
| Alaghehbandan R et al., 2023 [58] | US | y | adrenal g. | 66 | f | n | n | n | 1 | ns | 8 | 2 | c | 0 | 5 |
| Alaghehbandan R et al., 2023 [58] | US | y | adrenal g. | 60 | m | n | n | n | 1 | ns | 15 | 2 | c | 0 | 140 |
| Alaghehbandan R et al., 2023 [58] | US | n | adrenal g. | 70 | f | n | n | n | 1 | ns | 17 | 2 | c | 0 | 12 |
| Alaghehbandan R et al., 2023 [58] | US | n | adrenal g. | 75 | f | n | n | n | 1 | ns | 7 | 2 | c | 0 | 156 |
| Alaghehbandan R et al., 2023 [58] | US | y | adrenal g. | 60 | m | n | n | n | 1 | ns | 64 | 2 | c | 0 | 95 |
| Alaghehbandan R et al., 2023 [58] | US | y | adrenal g. | 59 | m | n | n | n | 1 | ns | 10 | 2 | c | 0 | ns |
| Alaghehbandan R et al., 2023 [58] | US | ns | adrenal g. | 37 | m | n | n | n | 1 | ns | 27 | 2 | c | 0 | ns |
| Yang L et al., 2023 [59] | China | n | liver | 59 | f | n | n | n | 1 | CT, MRI | 100 | 2 | a | 0 | 12 |
| Capinha MD et al., 2023 [60] | EU | y | kidney | 70 | m | n | y | y | 3 | CT, MRI | 24 | 2 | c | 0 | 22 |
| Slutsky HL et al., 2023 [61] | US | n | breast | 37 | f | n | n | n | 1 | US | 10 | 3 | a | 0 | 36 |
| Paparo AJ et al., 2023 [62] | Oceania | y | g.i. tract | 55 | m | n | n | n | 1 | ns | 3 | 2 | a | 0 | ns |
| McHenry A et al., 2023 [63] | US | n | ovary | 55 | f | n | n | n | 1 | CT | 12 | 2 | c | 0 | ns |
| McHenry A et al., 2023 [63] | US | n | ovary | 62 | f | n | n | n | 1 | CT | 10 | 2 | d | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 67 | f | y | n | n | 1 | CT | 5 | 2 | c | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 76 | f | y | n | n | 1 | CT | 7 | 2 | d | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 58 | f | n | n | n | 1 | CT | 8 | 2 | c | 0 | ns |
| McHenry A et al., 2023 [63] | US | n | ovary | 53 | f | y | n | n | 1 | CT | 6 | 2 | c | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 73 | f | y | n | n | 1 | CT | 4 | 2 | d | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 65 | f | n | n | n | 1 | CT | 10 | 2 | d | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 50 | f | y | n | n | 1 | CT | 9 | 2 | c | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 69 | f | y | n | n | 1 | CT | 6 | 2 | d | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 63 | f | n | n | n | 1 | CT | 3 | 2 | d | 0 | ns |
| McHenry A et al., 2023 [63] | US | y | ovary | 55 | f | y | n | n | 1 | CT | 2 | 2 | d | 0 | ns |
| Fang C et al., 2023 [64] | China | y | liver | 50 | f | n | n | n | 94 | CT, MRI | 50 | 2 | b | 0 | 6 |
| Zuo ZB et al., 2023 [65] | China | y | adrenal g. | 46 | m | n | n | y | 1 | CT | 20 | 2 | c | 0 | 19 |
| Caldwell NJ et al., 2024 [66] | US | n | mediastinum | 59 | f | n | n | n | 1 | CT, MRI, FDGPET | 38 | 2 | a | 0 | 13 |
| Caldwell NJ et al., 2024 [66] | US | y | mediastinum | 55 | f | n | n | n | 29 | CT, MRI, FDGPET | 26 | 2 | a | 0 | 58 |
| Caldwell NJ et al., 2024 [66] | US | n | mediastinum | 72 | f | n | n | n | 1 | CT, MRI, FDGPET | 51 | 2 | a | 0 | 3 |
| Caldwell NJ et al., 2024 [66] | US | ns | mediastinum | 75 | m | n | n | n | 1 | CT, MRI | ns | 2 | a | 0 | ns |
| Caldwell NJ et al., 2024 [66] | US | n | mediastinum | 63 | m | n | n | n | 1 | CT | 56 | 2 | a | 0 | ns |
| Caldwell NJ et al., 2024 [66] | US | n | mediastinum | 77 | f | n | n | n | 11 | CT | 20 | 2 | a | 0 | 6 |
| Caldwell NJ et al., 2024 [66] | US | n | mediastinum | 72 | f | n | n | n | 1 | CT | 43 | 1 | 0 | 9 | |
| Wu Q et al., 2024 [67] | China | ns | ovary | 26 | f | n | n | n | 5 | CT | 43 | 2 | c | 0 | 21 |
| Present study | EU | y | retroperit. | 68 | m | n | n | y | 1 | CT, MRI, FDGPET | 15 | 2 | a | 0 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoxhaj, I.; Piccino, M.; Grossi, U.; Maffeis, V.; Beleù, A.; Baciorri, F.; Morana, G.; Zanatta, P.; Zanus, G. Systematic Review and Case Report of a Left Gonadal Vein Anastomosing Hemangioma. J. Clin. Med. 2025, 14, 3108. https://doi.org/10.3390/jcm14093108
Hoxhaj I, Piccino M, Grossi U, Maffeis V, Beleù A, Baciorri F, Morana G, Zanatta P, Zanus G. Systematic Review and Case Report of a Left Gonadal Vein Anastomosing Hemangioma. Journal of Clinical Medicine. 2025; 14(9):3108. https://doi.org/10.3390/jcm14093108
Chicago/Turabian StyleHoxhaj, Ilda, Marco Piccino, Ugo Grossi, Valeria Maffeis, Alessandro Beleù, Francesca Baciorri, Giovanni Morana, Paolo Zanatta, and Giacomo Zanus. 2025. "Systematic Review and Case Report of a Left Gonadal Vein Anastomosing Hemangioma" Journal of Clinical Medicine 14, no. 9: 3108. https://doi.org/10.3390/jcm14093108
APA StyleHoxhaj, I., Piccino, M., Grossi, U., Maffeis, V., Beleù, A., Baciorri, F., Morana, G., Zanatta, P., & Zanus, G. (2025). Systematic Review and Case Report of a Left Gonadal Vein Anastomosing Hemangioma. Journal of Clinical Medicine, 14(9), 3108. https://doi.org/10.3390/jcm14093108






