How New Technologies Will Transform Total Knee Arthroplasty from a Singular Surgical Procedure to a Holistic Standardized Process
Abstract
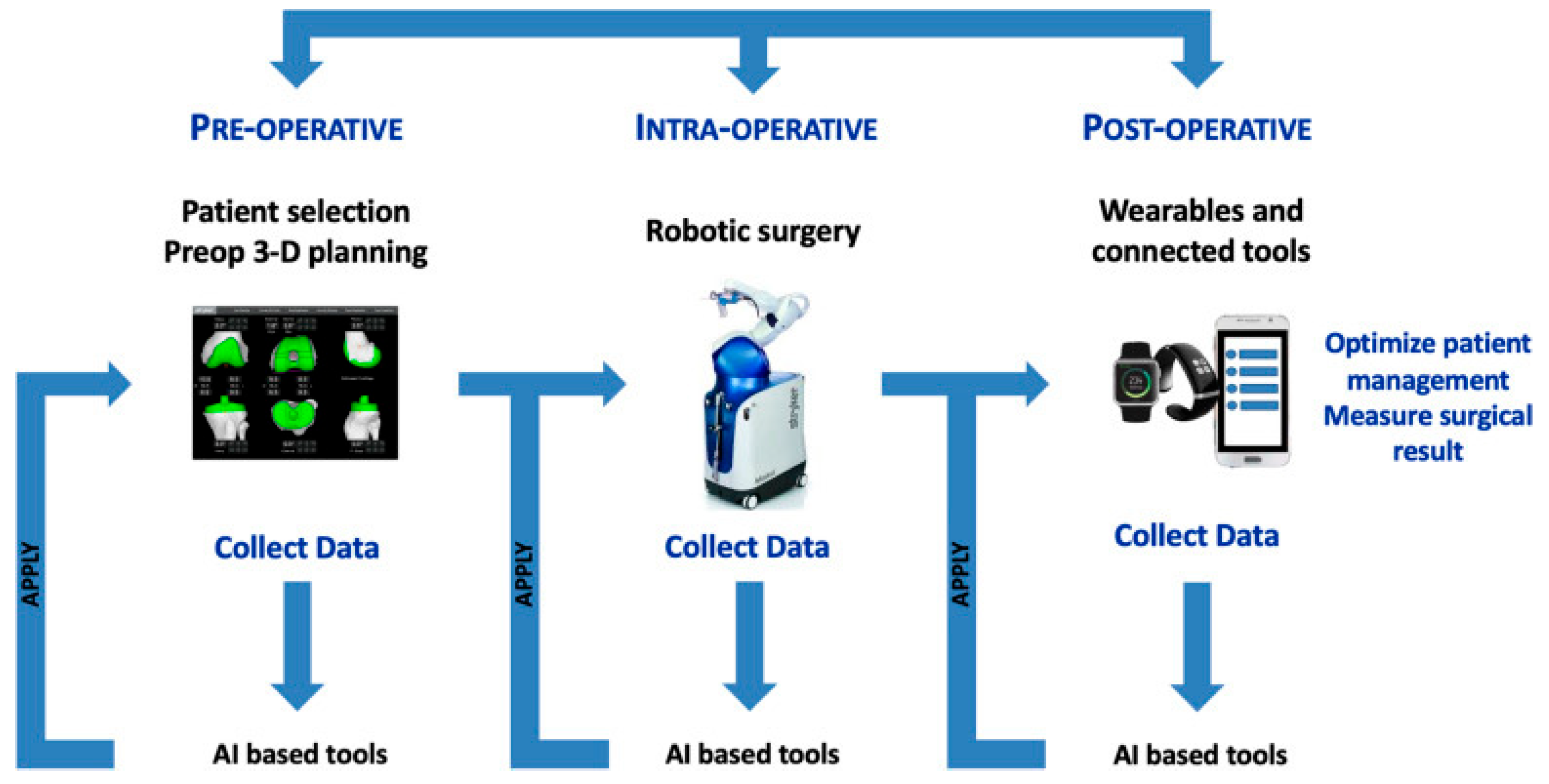
1. Introduction
2. Artificial Intelligence
3. Robotic-Assisted Surgery
4. Custom Implant and Personalized Alignment
- Ensuring an optimal fit between the bone and implant to prevent either overhang or inadequate coverage;
- Enhancing ligament balance by avoiding laxity caused by asymmetric resection;
- Improving stability and joint movement in the middle range of flexion by restoring the knee’s native curvatures;
- Enhancing the tracking of the patella in relation to the femur by replicating natural femoral torsion and creating a customized trochlea;
- Facilitating the restoration of the limb’s original, pre-arthritic alignment.
5. Discussion
- (i)
- (ii)
- (iii)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.C.; Conditt, M.A.; Cook, K.F.; Mathis, K.B. The John Insall Award: Patient Expectations Affect Satisfaction with Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2006, 452, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Batailler, C.; Swan, J.; Sappey Marinier, E.; Servien, E.; Lustig, S. New Technologies in Knee Arthroplasty: Current Concepts. J. Clin. Med. 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Abdel, M.P.; Parratte, S.; Blanc, G.; Ollivier, M.; Pomero, V.; Viehweger, E.; Argenson, J.-N.A. No Benefit of Patient-Specific Instrumentation in TKA on Functional and Gait Outcomes: A Randomized Clinical Trial. Clin. Orthop. Relat. Res. 2014, 472, 2468–2476. [Google Scholar] [CrossRef]
- Abane, L.; Anract, P.; Boisgard, S.; Descamps, S.; Courpied, J.P.; Hamadouche, M. A Comparison of Patient-Specific and Conventional Instrumentation for Total Knee Arthroplasty: A Multicentre Randomised Controlled Trial. Bone Jt. J. 2015, 97-B, 56–63. [Google Scholar] [CrossRef]
- Vundelinckx, B.J.; Bruckers, L.; De Mulder, K.; De Schepper, J.; Van Esbroeck, G. Functional and Radiographic Short-Term Outcome Evaluation of the Visionaire System, a Patient-Matched Instrumentation System for Total Knee Arthroplasty. J. Arthroplast. 2013, 28, 964–970. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chin, P.L.; Tay, D.K.J.; Chia, S.-L.; Lo, N.N.; Yeo, S.J. Functional Outcome and Quality of Life after Patient-Specific Instrumentation in Total Knee Arthroplasty. J. Arthroplast. 2015, 30, 1724–1728. [Google Scholar] [CrossRef]
- Gustke, K.A.; Golladay, G.J.; Roche, M.W.; Elson, L.C.; Anderson, C.R. A New Method for Defining Balance: Promising Short-Term Clinical Outcomes of Sensor-Guided TKA. J. Arthroplast. 2014, 29, 955–960. [Google Scholar] [CrossRef]
- Jones, C.W.; Jerabek, S.A. Current Role of Computer Navigation in Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 1989–1993. [Google Scholar] [CrossRef]
- Li, J.-T.; Gao, X.; Li, X. Comparison of iASSIST Navigation System with Conventional Techniques in Total Knee Arthroplasty: A Systematic Review and Meta-Analysis of Radiographic and Clinical Outcomes. Orthop. Surg. 2019, 11, 985–993. [Google Scholar] [CrossRef]
- Bellman, R. An Introduction to Artificial Intelligence: Can Computers Think? Course Technology: San Francisco, CA, USA, 1978. [Google Scholar]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Overview of Deep Learning in Medical Imaging. Radiol. Phys. Technol. 2017, 10, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Soffer, S.; Ben-Cohen, A.; Shimon, O.; Amitai, M.M.; Greenspan, H.; Klang, E. Convolutional Neural Networks for Radiologic Images: A Radiologist’s Guide. Radiology 2019, 290, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Do, S.; Song, K.D.; Chung, J.W. Basics of Deep Learning: A Radiologist’s Guide to Understanding Published Radiology Articles on Deep Learning. Korean J. Radiol. 2020, 21, 33–41. [Google Scholar] [CrossRef]
- Laur, O.; Wang, B. Musculoskeletal Trauma and Artificial Intelligence: Current Trends and Projections. Skelet. Radiol. 2022, 51, 257–269. [Google Scholar] [CrossRef]
- Batailler, C.; Shatrov, J.; Sappey-Marinier, E.; Servien, E.; Parratte, S.; Lustig, S. Artificial Intelligence in Knee Arthroplasty: Current Concept of the Available Clinical Applications. Arthroplasty 2022, 4, 17. [Google Scholar] [CrossRef]
- Navarro, S.M.; Wang, E.Y.; Haeberle, H.S.; Mont, M.A.; Krebs, V.E.; Patterson, B.M.; Ramkumar, P.N. Machine Learning and Primary Total Knee Arthroplasty: Patient Forecasting for a Patient-Specific Payment Model. J. Arthroplast. 2018, 33, 3617–3623. [Google Scholar] [CrossRef]
- Gould, D.J.; Bailey, J.A.; Spelman, T.; Bunzli, S.; Dowsey, M.M.; Choong, P.F.M. Predicting 30-Day Readmission Following Total Knee Arthroplasty Using Machine Learning and Clinical Expertise Applied to Clinical Administrative and Research Registry Data in an Australian Cohort. Arthroplasty 2023, 5, 30. [Google Scholar] [CrossRef]
- Kunze, K.N.; Polce, E.M.; Sadauskas, A.J.; Levine, B.R. Development of Machine Learning Algorithms to Predict Patient Dissatisfaction After Primary Total Knee Arthroplasty. J. Arthroplast. 2020, 35, 3117–3122. [Google Scholar] [CrossRef]
- Jo, C.; Ko, S.; Shin, W.C.; Han, H.-S.; Lee, M.C.; Ko, T.; Ro, D.H. Transfusion after Total Knee Arthroplasty Can Be Predicted Using the Machine Learning Algorithm. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1757–1764. [Google Scholar] [CrossRef]
- Kunze, K.N.; Polce, E.M.; Patel, A.; Courtney, P.M.; Levine, B.R. Validation and Performance of a Machine-Learning Derived Prediction Guide for Total Knee Arthroplasty Component Sizing. Arch. Orthop. Trauma. Surg. 2021, 141, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Farooq, H.; Deckard, E.R.; Arnold, N.R.; Meneghini, R.M. Machine Learning Algorithms Identify Optimal Sagittal Component Position in Total Knee Arthroplasty. J. Arthroplast. 2021, 36, S242–S249. [Google Scholar] [CrossRef]
- Hinterwimmer, F.; Lazic, I.; Suren, C.; Hirschmann, M.T.; Pohlig, F.; Rueckert, D.; Burgkart, R.; von Eisenhart-Rothe, R. Machine Learning in Knee Arthroplasty: Specific Data Are Key-a Systematic Review. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, W.; Raghupathi, V. Big Data Analytics in Healthcare: Promise and Potential. Health Inf. Sci. Syst. 2014, 2, 3. [Google Scholar] [CrossRef]
- Baker, P.N.; van der Meulen, J.H.; Lewsey, J.; Gregg, P.J.; National Joint Registry for England and Wales. The Role of Pain and Function in Determining Patient Satisfaction after Total Knee Replacement. Data from the National Joint Registry for England and Wales. J. Bone Jt. Surg. Br. 2007, 89, 893–900. [Google Scholar] [CrossRef]
- Baker, P.N.; Deehan, D.J.; Lees, D.; Jameson, S.; Avery, P.J.; Gregg, P.J.; Reed, M.R. The Effect of Surgical Factors on Early Patient-Reported Outcome Measures (PROMS) Following Total Knee Replacement. J. Bone Jt. Surg. Br. 2012, 94, 1058–1066. [Google Scholar] [CrossRef]
- Judge, A.; Arden, N.K.; Cooper, C.; Kassim Javaid, M.; Carr, A.J.; Field, R.E.; Dieppe, P.A. Predictors of Outcomes of Total Knee Replacement Surgery. Rheumatology 2012, 51, 1804–1813. [Google Scholar] [CrossRef]
- Brander, V.A.; Stulberg, S.D.; Adams, A.D.; Harden, R.N.; Bruehl, S.; Stanos, S.P.; Houle, T. Predicting Total Knee Replacement Pain: A Prospective, Observational Study. Clin. Orthop. Relat. Res. 2003, 416, 27–36. [Google Scholar] [CrossRef]
- Wylde, V.; Rooker, J.; Halliday, L.; Blom, A. Acute Postoperative Pain at Rest after Hip and Knee Arthroplasty: Severity, Sensory Qualities and Impact on Sleep. Orthop. Traumatol. Surg. Res. 2011, 97, 139–144. [Google Scholar] [CrossRef]
- Tolk, J.J.; Waarsing, J.E.H.; Janssen, R.P.A.; van Steenbergen, L.N.; Bierma-Zeinstra, S.M.A.; Reijman, M. Development of Preoperative Prediction Models for Pain and Functional Outcome After Total Knee Arthroplasty Using The Dutch Arthroplasty Register Data. J. Arthroplast. 2020, 35, 690–698.e2. [Google Scholar] [CrossRef]
- Escobar, A.; Quintana, J.M.; Bilbao, A.; Ibañez, B.; Arenaza, J.C.; Gutiérrez, L.; Azkárate, J.; Güenaga, J.I.; Vidaurreta, I. Development of Explicit Criteria for Prioritization of Hip and Knee Replacement. J. Eval. Clin. Pract. 2007, 13, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Perera, R.A.; Jiranek, W.A.; Dumenci, L. Using Surgical Appropriateness Criteria to Examine Outcomes of Total Knee Arthroplasty in a United States Sample. Arthritis Care Res. 2015, 67, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, P.N.; Haeberle, H.S.; Ramanathan, D.; Cantrell, W.A.; Navarro, S.M.; Mont, M.A.; Bloomfield, M.; Patterson, B.M. Remote Patient Monitoring Using Mobile Health for Total Knee Arthroplasty: Validation of a Wearable and Machine Learning-Based Surveillance Platform. J. Arthroplast. 2019, 34, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.H.; Wei, J.; Kim, T.K.; Sair, H.I.; Hui, F.K.; Hager, G.D.; Fritz, J.; Oni, J.K. Automated Detection & Classification of Knee Arthroplasty Using Deep Learning. Knee 2020, 27, 535–542. [Google Scholar] [CrossRef]
- Karnuta, J.M.; Luu, B.C.; Roth, A.L.; Haeberle, H.S.; Chen, A.F.; Iorio, R.; Schaffer, J.L.; Mont, M.A.; Patterson, B.M.; Krebs, V.E.; et al. Artificial Intelligence to Identify Arthroplasty Implants From Radiographs of the Knee. J. Arthroplast. 2021, 36, 935–940. [Google Scholar] [CrossRef]
- Ramkumar, P.N.; Karnuta, J.M.; Navarro, S.M.; Haeberle, H.S.; Scuderi, G.R.; Mont, M.A.; Krebs, V.E.; Patterson, B.M. Deep Learning Preoperatively Predicts Value Metrics for Primary Total Knee Arthroplasty: Development and Validation of an Artificial Neural Network Model. J. Arthroplast. 2019, 34, 2220–2227.e1. [Google Scholar] [CrossRef]
- Wei, C.; Quan, T.; Wang, K.Y.; Gu, A.; Fassihi, S.C.; Kahlenberg, C.A.; Malahias, M.-A.; Liu, J.; Thakkar, S.; Gonzalez Della Valle, A.; et al. Artificial Neural Network Prediction of Same-Day Discharge Following Primary Total Knee Arthroplasty Based on Preoperative and Intraoperative Variables. Bone Jt. J. 2021, 103-B, 1358–1366. [Google Scholar] [CrossRef]
- Shah, R.F.; Bini, S.A.; Martinez, A.M.; Pedoia, V.; Vail, T.P. Incremental Inputs Improve the Automated Detection of Implant Loosening Using Machine-Learning Algorithms. Bone Jt. J. 2020, 102-B, 101–106. [Google Scholar] [CrossRef]
- Bonnin, M.; Müller-Fouarge, F.; Estienne, T.; Bekadar, S.; Pouchy, C.; Ait Si Selmi, T. Artificial Intelligence Radiographic Analysis Tool for Total Knee Arthroplasty. J. Arthroplast. 2023, 38, S199–S207.e2. [Google Scholar] [CrossRef]
- Kazarian, G.S.; Lawrie, C.M.; Barrack, T.N.; Donaldson, M.J.; Miller, G.M.; Haddad, F.S.; Barrack, R.L. The Impact of Surgeon Volume and Training Status on Implant Alignment in Total Knee Arthroplasty. J. Bone Jt. Surg. Am. 2019, 101, 1713–1723. [Google Scholar] [CrossRef]
- Batailler, C.; Fernandez, A.; Swan, J.; Servien, E.; Haddad, F.S.; Catani, F.; Lustig, S. MAKO CT-Based Robotic Arm-Assisted System Is a Reliable Procedure for Total Knee Arthroplasty: A Systematic Review. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 3585–3598. [Google Scholar] [CrossRef] [PubMed]
- van der List, J.P.; Chawla, H.; Joskowicz, L.; Pearle, A.D. Current State of Computer Navigation and Robotics in Unicompartmental and Total Knee Arthroplasty: A Systematic Review with Meta-Analysis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3482–3495. [Google Scholar] [CrossRef] [PubMed]
- Kayani, B.; Konan, S.; Pietrzak, J.R.T.; Haddad, F.S. Iatrogenic Bone and Soft Tissue Trauma in Robotic-Arm Assisted Total Knee Arthroplasty Compared With Conventional Jig-Based Total Knee Arthroplasty: A Prospective Cohort Study and Validation of a New Classification System. J. Arthroplast. 2018, 33, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Sires, J.D.; Wilson, C.J. CT Validation of Intraoperative Implant Position and Knee Alignment as Determined by the MAKO Total Knee Arthroplasty System. J. Knee Surg. 2021, 34, 1133–1137. [Google Scholar] [CrossRef]
- Kayani, B.; Konan, S.; Huq, S.S.; Tahmassebi, J.; Haddad, F.S. Robotic-Arm Assisted Total Knee Arthroplasty Has a Learning Curve of Seven Cases for Integration into the Surgical Workflow but No Learning Curve Effect for Accuracy of Implant Positioning. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1132–1141. [Google Scholar] [CrossRef]
- Sultan, A.A.; Samuel, L.T.; Khlopas, A.; Sodhi, N.; Bhowmik-Stoker, M.; Chen, A.; Orozco, F.; Kolisek, F.; Mahoney, O.; Smith, L.; et al. Robotic-Arm Assisted Total Knee Arthroplasty More Accurately Restored the Posterior Condylar Offset Ratio and the Insall-Salvati Index Compared to the Manual Technique; A Cohort-Matched Study. Surg. Technol. Int. 2019, 34, 409–413. [Google Scholar]
- Gilmour, A.; MacLean, A.D.; Rowe, P.J.; Banger, M.S.; Donnelly, I.; Jones, B.G.; Blyth, M.J.G. Robotic-Arm-Assisted vs. Conventional Unicompartmental Knee Arthroplasty. The 2-Year Clinical Outcomes of a Randomized Controlled Trial. J. Arthroplast. 2018, 33, S109–S115. [Google Scholar] [CrossRef]
- Bhimani, S.J.; Bhimani, R.; Smith, A.; Eccles, C.; Smith, L.; Malkani, A. Robotic-Assisted Total Knee Arthroplasty Demonstrates Decreased Postoperative Pain and Opioid Usage Compared to Conventional Total Knee Arthroplasty. Bone Jt. Open 2020, 1, 8–12. [Google Scholar] [CrossRef]
- Naziri, Q.; Cusson, B.C.; Chaudhri, M.; Shah, N.V.; Sastry, A. Making the Transition from Traditional to Robotic-Arm Assisted TKA: What to Expect? A Single-Surgeon Comparative-Analysis of the First-40 Consecutive Cases. J. Orthop. 2019, 16, 364–368. [Google Scholar] [CrossRef]
- Blyth, M.J.G.; Anthony, I.; Rowe, P.; Banger, M.S.; MacLean, A.; Jones, B. Robotic Arm-Assisted versus Conventional Unicompartmental Knee Arthroplasty: Exploratory Secondary Analysis of a Randomised Controlled Trial. Bone Jt. Res. 2017, 6, 631–639. [Google Scholar] [CrossRef]
- Hansen, D.C.; Kusuma, S.K.; Palmer, R.M.; Harris, K.B. Robotic Guidance Does Not Improve Component Position or Short-Term Outcome in Medial Unicompartmental Knee Arthroplasty. J. Arthroplast. 2014, 29, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, O.M.; Kinsey, T. Overhang of the Femoral Component in Total Knee Arthroplasty: Risk Factors and Clinical Consequences. J. Bone Jt. Surg. Am. 2010, 92, 1115–1121. [Google Scholar] [CrossRef]
- Beckers, L.; Müller, J.H.; Daxhelet, J.; Saffarini, M.; Aït-Si-Selmi, T.; Bonnin, M.P. Sexual Dimorphism and Racial Diversity Render Bone-Implant Mismatch Inevitable after off-the-Shelf Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Lustig, S.; Sappey-Marinier, E.; Fary, C.; Servien, E.; Parratte, S.; Batailler, C. Personalized Alignment in Total Knee Arthroplasty: Current Concepts. SICOT-J 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, M.P.; Beckers, L.; Leon, A.; Chauveau, J.; Müller, J.H.; Tibesku, C.O.; Aït-Si-Selmi, T. Custom Total Knee Arthroplasty Facilitates Restoration of Constitutional Coronal Alignment. Knee Surg. Sports Traumatol. Arthrosc. 2020, 30, 464–475. [Google Scholar] [CrossRef]
- Gousopoulos, L.; Dobbelaere, A.; Ratano, S.; Bondoux, L.; ReSurg; Tibesku, C.O.; Aït-Si-Selmi, T.; Bonnin, M.P. Custom Total Knee Arthroplasty Combined with Personalised Alignment Grants 94% Patient Satisfaction at Minimum Follow-up of 2 Years. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1276–1283. [Google Scholar] [CrossRef]
- Ratano, S.; Müller, J.H.; Daxhelet, J.; Beckers, L.; Bondoux, L.; Tibesku, C.O.; Aït-Si-Selmi, T.; Bonnin, M.P. Custom TKA Combined with Personalised Coronal Alignment Yield Improvements That Exceed KSS Substantial Clinical Benefits. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2958–2965. [Google Scholar] [CrossRef]
- Daxhelet, J.; Aït-Si-Selmi, T.; Müller, J.H.; Saffarini, M.; Ratano, S.; Bondoux, L.; Mihov, K.; Bonnin, M.P. Custom TKA Enables Adequate Realignment with Minimal Ligament Release and Grants Satisfactory Outcomes in Knees That Had Prior Osteotomies or Extra-Articular Fracture Sequelae. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1212–1219. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sappey-Marinier, E.; Dutra Vieira, T.; Schmidt, A.; Aït Si Selmi, T.; Bonnin, M. How New Technologies Will Transform Total Knee Arthroplasty from a Singular Surgical Procedure to a Holistic Standardized Process. J. Clin. Med. 2025, 14, 3102. https://doi.org/10.3390/jcm14093102
Sappey-Marinier E, Dutra Vieira T, Schmidt A, Aït Si Selmi T, Bonnin M. How New Technologies Will Transform Total Knee Arthroplasty from a Singular Surgical Procedure to a Holistic Standardized Process. Journal of Clinical Medicine. 2025; 14(9):3102. https://doi.org/10.3390/jcm14093102
Chicago/Turabian StyleSappey-Marinier, Elliot, Thais Dutra Vieira, Axel Schmidt, Tarik Aït Si Selmi, and Michel Bonnin. 2025. "How New Technologies Will Transform Total Knee Arthroplasty from a Singular Surgical Procedure to a Holistic Standardized Process" Journal of Clinical Medicine 14, no. 9: 3102. https://doi.org/10.3390/jcm14093102
APA StyleSappey-Marinier, E., Dutra Vieira, T., Schmidt, A., Aït Si Selmi, T., & Bonnin, M. (2025). How New Technologies Will Transform Total Knee Arthroplasty from a Singular Surgical Procedure to a Holistic Standardized Process. Journal of Clinical Medicine, 14(9), 3102. https://doi.org/10.3390/jcm14093102






