Preclinical Volume Retention of Fat Grafts Processed with REVOLVE™ Technology or Decantation Methods in Irradiated and Nonirradiated Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Adipose Tissue Harvest
2.2. Adipose Tissue Processing
2.2.1. Decantation
2.2.2. REVOLVE™ Technology Processing
2.2.3. Quantification of Graft Components Through Centrifugation
2.3. Preparation of Adipose Grafts
2.3.1. Animal Model of Adipose Grafting
2.3.2. Dermal Radiation
2.3.3. Surgical Implantation of Adipose Grafts
2.4. MRI Imaging
2.5. Volumetric Analysis of MRI Scans
2.6. Harvest of Adipose Tissue Grafts
2.7. Histology and Immunohistochemistry
2.8. Statistical Analysis
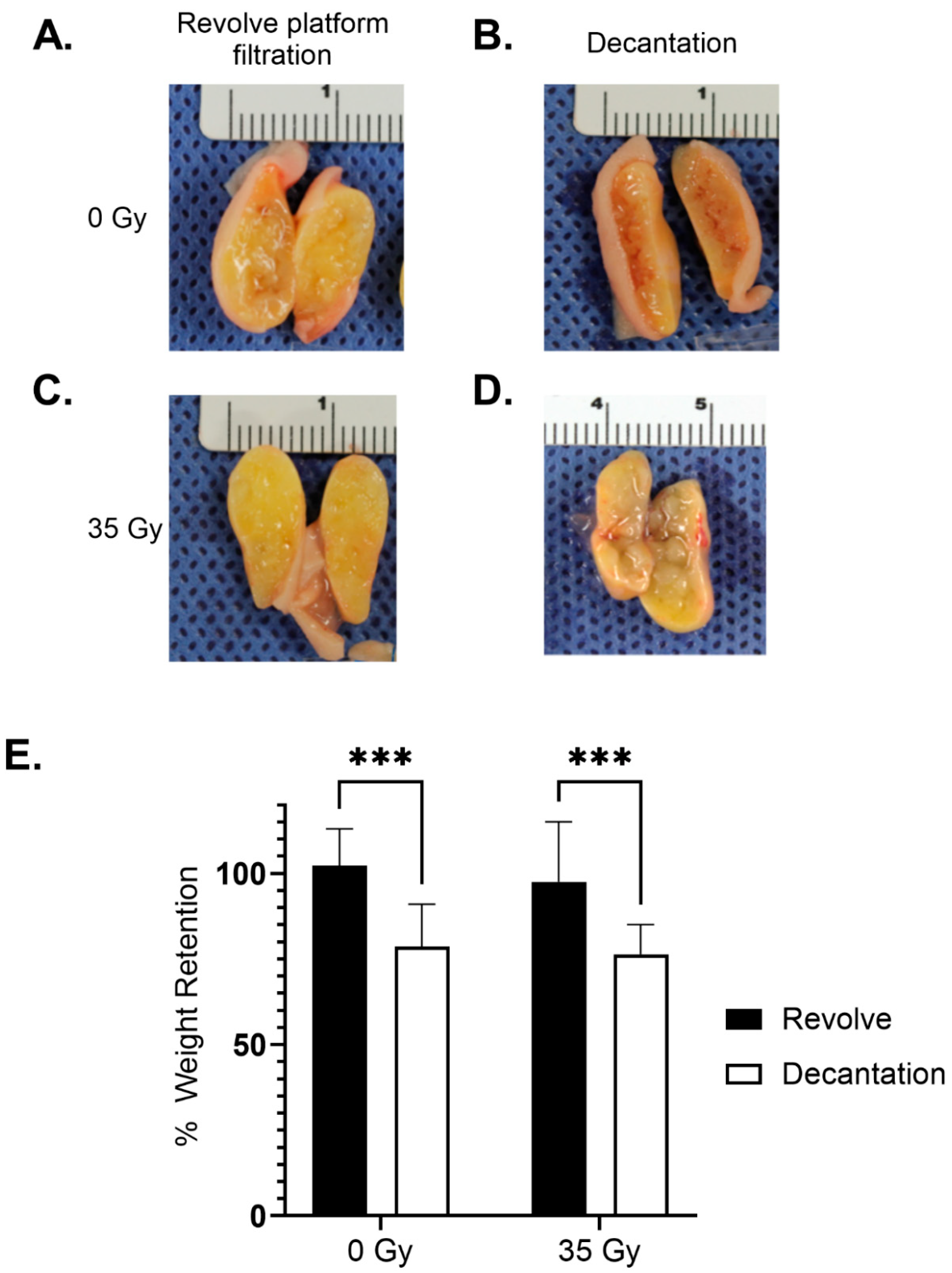
3. Results
3.1. Adipose Tissue Processing
3.2. Preparation of Adipose Grafts
Surgical Implantation of Adipose Grafts
3.3. Long-Term Graft Characteristics
3.4. Histology and Immunohistochemistry
3.4.1. Fibrosis
3.4.2. Adipocyte Necrosis
3.4.3. Oil Vacuoles
3.4.4. Inflammation
3.4.5. Adipocyte Size and Shape
3.4.6. Vascularization (CD31)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pazmiño, P.; Del Vecchio, D. Static Injection, Migration, and Equalization (SIME): A New Paradigm for Safe Ultrasound-Guided Brazilian Butt Lift: Safer, Faster, Better. Aesthet. Surg. J. 2023, 43, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Maruyama, N.; Onodera, T. What Patient-related Factors Most Strongly Influence Autologous Fat Grafting Volume Retention in Breast Augmentation? Plast. Reconstr. Surg. Glob. Open 2024, 12, e6194. [Google Scholar] [CrossRef] [PubMed]
- Erdim, M.; Tezel, E.; Numanoglu, A.; Sav, A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: An experimental study. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1210–1214. [Google Scholar] [CrossRef]
- Berkane, Y.; Oubari, H.; van Dieren, L.; Charlès, L.; Lupon, E.; McCarthy, M.; Cetrulo, C.L., Jr.; Bertheuil, N.; Uygun, B.E.; Smadja, D.M.; et al. Tissue engineering strategies for breast reconstruction: A literature review of current advances and future directions. Ann. Transl. Med. 2024, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Anjos, S.D.; Matas-Palau, A.; Mercader, J.; Katz, A.J.; Llull, R. Reproducible volume restoration and efficient long-term volume retention after point-of-care standardized cell-enhanced fat grafting in breast surgery. Plast. Reconstr. Surg. Glob. Open 2015, 3, e547. [Google Scholar] [CrossRef]
- Poglio, S.; Galvani, S.; Bour, S.; André, M.; Prunet-Marcassus, B.; Pénicaud, L.; Casteilla, L.; Cousin, B. Adipose Tissue Sensitivity to Radiation Exposure. Am. J. Pathol. 2009, 174, 44. [Google Scholar] [CrossRef]
- Tsimponis, A.; Dionyssiou, D.; Papamitsou, T.; Demiri, E. The effect of host tissue and radiation on fat-graft survival: A comparative experimental study. JPRAS Open 2023, 38, 134–146. [Google Scholar] [CrossRef]
- Gabriel, A.; Kabaria, N.; Fang, C.H.; Lombardi, J.A.; Stec, E.; Huang, L.T.; Li, H.; Sandor, M. In Vitro Characterization of Fat Grafts Processed Using the REVOLVE ENVI System versus Decantation. Plast. Reconstr. Surg. Glob. Open 2024, 12, E5615. [Google Scholar] [CrossRef]
- Fang, C.; Patel, P.; Li, H.; Huang, L.T.; Wan, H.; Collins, S.; Connell, T.L.; Xu, H. Physical, Biochemical, and Biologic Properties of Fat Graft Processed via Different Methods. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3010. [Google Scholar] [CrossRef]
- Haubner, F.; Ohmann, E.; Pohl, F.; Strutz, J.; Gassner, H.G. Wound healing after radiation therapy: Review of the literature. Radiat. Oncol. 2012, 7, 162. [Google Scholar] [CrossRef]
- Phulpin, B.; Gangloff, P.; Tran, N.; Bravetti, P.; Merlin, J.L.; Dolivet, G. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast. Reconstr. Surg. 2009, 123, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Facial Recontouring with Lipostructure. Clin. Plast. Surg. 1997, 24, 347–367. [Google Scholar] [CrossRef]
- Coleman, S.R. Hand Rejuvenation with Structural Fat Grafting. Plast. Reconstr. Surg. 2002, 110, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Structural Fat Grafts: The Ideal Filler? Clin. Plast. Surg. 2001, 28, 111–119. [Google Scholar] [CrossRef]
- Pu, L.L.Q.; Coleman, S.R.; Cui, X.; Ferguson, R.E.H.; Vasconez, H.C. Autologous fat grafts harvested and refined by the coleman technique: A comparative study. Plast. Reconstr. Surg. 2008, 122, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Cottler, P.S.; Sun, N.; Thuman, J.M.; Bielak, K.M.H.; Salopek, L.S.; Pineros-Fernandez, A.; Hu, S.; Campbell, C.A. The Biointegration of a Porcine Acellular Dermal Matrix in a Novel Radiated Breast Reconstruction Model. Ann. Plast. Surg. 2020, 84, S417–S423. [Google Scholar] [CrossRef]
- Rodriguez, J.J.; Kung, T.; Wang, Y.; Nelson, N.S.; Polyatskaya, Y.; Deshpande, S.S.; Zheutlin, A.R.; Donneys, A.; Buchman, S.R.; Momoh, A.O. Changes in skin vascularity in a murine model for postmastectomy radiation. Ann. Plast. Surg. 2016, 76, 494–498. [Google Scholar] [CrossRef]
- Arnautovic, A.; Karinja, S.; Olafsson, S.; Carty, M.J.; Erdmann-Sager, J.; Caterson, S.A.; Broyles, J.M. Optimal Timing of Delayed Microvascular Breast Reconstruction after Radiation Therapy. J. Reconstr. Microsurg. 2023, 39, 165–170. [Google Scholar] [CrossRef]
- Lins, C.F.; Salmon, C.E.G.; Nogueira-Barbosa, M.H. Applications of the dixon technique in the evaluation of the musculoskeletal system. Radiol. Bras. 2021, 54, 33–42. [Google Scholar] [CrossRef]
- Shoshani, O.; Livne, E.; Armoni, M.; Shupak, A.; Berger, J.; Ramon, Y.; Fodor, L.; Gilhar, A.; Peled, I.J.; Ullmann, Y. The effect of interleukin-8 on the viability of injected adipose tissue in nude mice. Plast. Reconstr. Surg. 2005, 115, 853–859. [Google Scholar] [CrossRef]
- Garza, R.M.; Paik, K.J.; Chung, M.T.; Duscher, D.; Gurtner, G.C.; Longaker, M.T.; Wan, D.C. Studies in fat grafting: Part III. Fat grafting irradiated tissue—Improved skin quality and decreased fat graft retention. Plast. Reconstr. Surg. 2014, 134, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Atik, B.; Öztürk, G.; Erdoǧan, E.; Tan, Ö. Comparison of techniques for long-term storage of fat grafts: An experimental study. Plast. Reconstr. Surg. 2006, 118, 1533–1537. [Google Scholar] [CrossRef]
- Lee, J.H.; Kirkham, J.C.; McCormack, M.C.; Nicholls, A.M.; Randolph, M.A.; Austen, W.G. The effect of pressure and shear on autologous fat grafting. Plast. Reconstr. Surg. 2013, 131, 1125–1136. [Google Scholar] [CrossRef]
- Ramon, Y.; Shoshani, O.; Peled, I.J.; Gilhar, A.; Carmi, N.; Fodor, L.; Risin, Y.; Ullmann, Y. Enhancing the take of injected adipose tissue by a simple method for concentrating fat cells. Plast. Reconstr. Surg. 2005, 115, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Pan, Y.; Zhen, Y.; Zhang, L.; Zhang, X.; Shu, M.; Han, Y.; Guo, S. Enhancement of viability of fat grafts in nude mice by endothelial progenitor cells. Dermatol. Surg. 2006, 32, 1437–1443. [Google Scholar] [CrossRef]
- Nelissen, X.; Licciardi, S.; Nizet, C.; Delay, E.; Roche, R. Comparative Analysis of a New Automatic System and Four Existing Techniques for Autologous Fat Grafting. Plast. Reconstr. Surg. Glob. Open 2023, 11, E5349. [Google Scholar] [CrossRef]
- Maheta, B.; Yesantharao, P.S.; Thawanyarat, K.; Akhter, M.F.; Rowley, M.; Nazerali, R.S. Timing of autologous fat grafting in implant-based breast reconstruction: Best practices based on systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2023, 86, 273–279. [Google Scholar] [CrossRef]
- Abboud, M.H.; Dibo, S.A.; Abboud, N.M. Power-assisted liposuction and lipofilling: Techniques and experience in large-volume fat grafting. Aesthet. Surg. J. 2020, 40, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Agostini, T.; Lazzeri, D.; Pini, A.; Marino, G.; Quattrini, A.L.; Bani, D.; Dini, M. Wet and dry techniques for structural fat graft harvesting: Histomorphometric and cell viability assessments of lipoaspirated samples. Plast. Reconstr. Surg. 2012, 130, 331e–339e. [Google Scholar] [CrossRef]
- Keck, M.; Zeyda, M.; Gollinger, K.; Burjak, S.; Kamolz, L.P.; Frey, M.; Stulnig, T.M. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast. Reconstr. Surg. 2010, 126, 1500–1505. [Google Scholar] [CrossRef]
- Small, K.; Choi, M.; Petruolo, O.; Lee, C.; Karp, N. Is There an Ideal Donor Site of Fat for Secondary Breast Reconstruction? Aesthet. Surg. J. 2014, 34, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.; Kober, J.; Riedl, O.; Kitzinger, H.B.; Wolf, S.; Stulnig, T.M.; Zeyda, M.; Gugerell, A. Power assisted liposuction to obtain adipose-derived stem cells: Impact on viability and differentiation to adipocytes in comparison to manual aspiration. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, e1. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Han, X.; Li, F. The Importance of Protecting the Structure and Viability of Adipose Tissue for Fat Grafting. Plast. Reconstr. Surg. 2022, 149, 1357–1368. [Google Scholar] [CrossRef] [PubMed]


 denotes area of fibrosis (blue collagen fibers). (C) Example of an H&E stain depicting the presence of adipocyte necrosis.
denotes area of fibrosis (blue collagen fibers). (C) Example of an H&E stain depicting the presence of adipocyte necrosis.  denotes necrotic adipocyte. (D) Example image of H&E stain depicting the presence of cysts and vacuoles.
denotes necrotic adipocyte. (D) Example image of H&E stain depicting the presence of cysts and vacuoles.  denotes cyst or vacuole. (E) Example image of H&E stain depicting the presence of inflammation. Star denotes presence of inflammation. Scale bar = 100 µm.
denotes cyst or vacuole. (E) Example image of H&E stain depicting the presence of inflammation. Star denotes presence of inflammation. Scale bar = 100 µm.
 denotes area of fibrosis (blue collagen fibers). (C) Example of an H&E stain depicting the presence of adipocyte necrosis.
denotes area of fibrosis (blue collagen fibers). (C) Example of an H&E stain depicting the presence of adipocyte necrosis.  denotes necrotic adipocyte. (D) Example image of H&E stain depicting the presence of cysts and vacuoles.
denotes necrotic adipocyte. (D) Example image of H&E stain depicting the presence of cysts and vacuoles.  denotes cyst or vacuole. (E) Example image of H&E stain depicting the presence of inflammation. Star denotes presence of inflammation. Scale bar = 100 µm.
denotes cyst or vacuole. (E) Example image of H&E stain depicting the presence of inflammation. Star denotes presence of inflammation. Scale bar = 100 µm.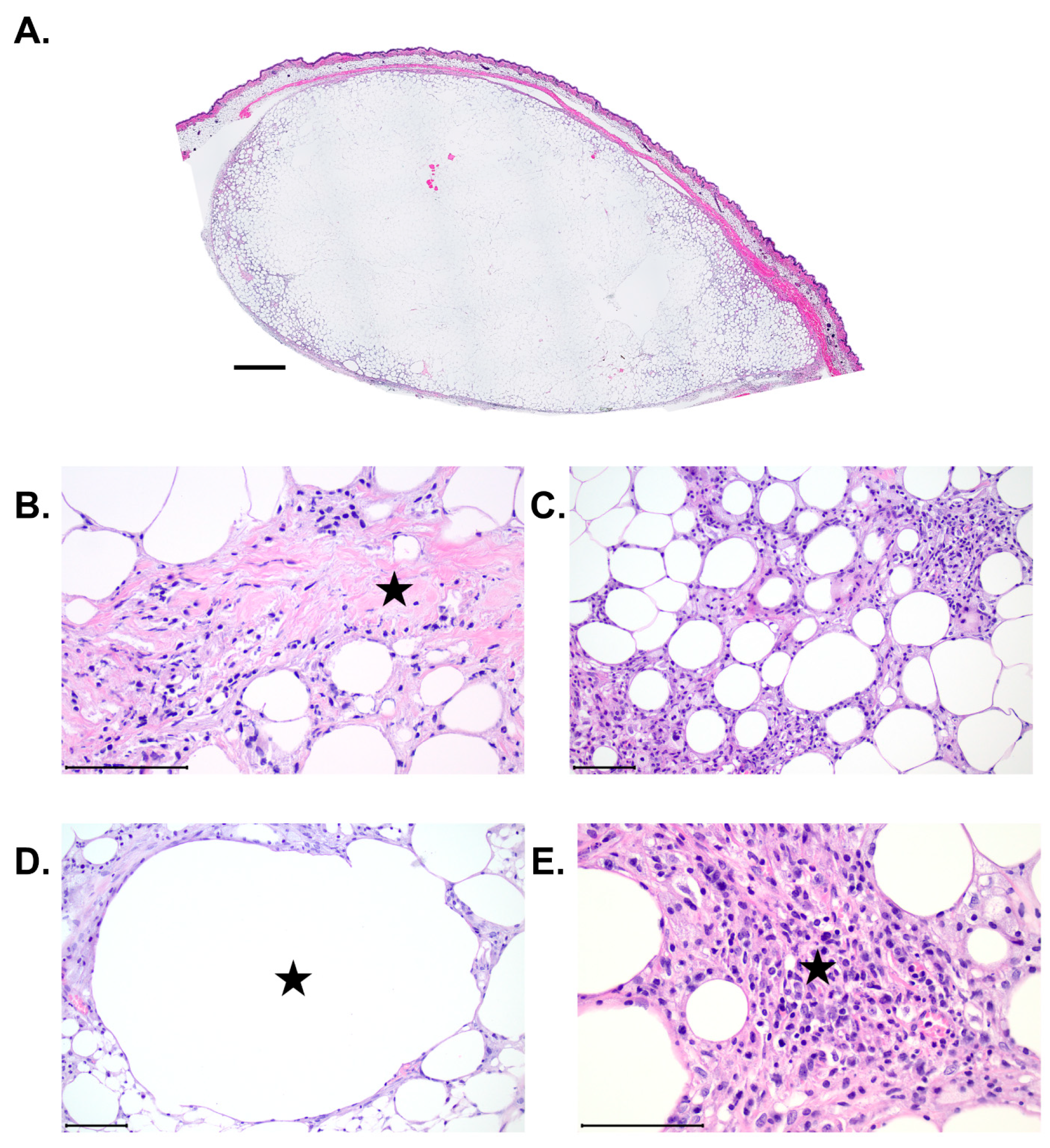


| Revolve (0 Gy) n = 18 | Revolve (35 Gy) n = 18 |
Decantation (0 Gy) n = 12 |
Decantation (35 Gy) n = 12 | p | |
|---|---|---|---|---|---|
| Mouse weight (g) | 20.1 ± 1.2 | 19.3 ± 1.5 | 20.2 ± 1.5 | 19.6 ± 1.7 | 0.05 |
| Implant weight (g) | 0.59 ± 0.16 | 0.61 ± 0.07 | 0.56 ± 0.19 | 0.61 ± 0.06 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, C.A.; Grogan, G.M.; St. Jean, S.; Kabaria, N.; Gardocki-Sandor, M.; Cottler, P.S. Preclinical Volume Retention of Fat Grafts Processed with REVOLVE™ Technology or Decantation Methods in Irradiated and Nonirradiated Wounds. J. Clin. Med. 2025, 14, 3100. https://doi.org/10.3390/jcm14093100
Campbell CA, Grogan GM, St. Jean S, Kabaria N, Gardocki-Sandor M, Cottler PS. Preclinical Volume Retention of Fat Grafts Processed with REVOLVE™ Technology or Decantation Methods in Irradiated and Nonirradiated Wounds. Journal of Clinical Medicine. 2025; 14(9):3100. https://doi.org/10.3390/jcm14093100
Chicago/Turabian StyleCampbell, Christopher A., Graham M. Grogan, Samantha St. Jean, Nimesh Kabaria, Maryellen Gardocki-Sandor, and Patrick S. Cottler. 2025. "Preclinical Volume Retention of Fat Grafts Processed with REVOLVE™ Technology or Decantation Methods in Irradiated and Nonirradiated Wounds" Journal of Clinical Medicine 14, no. 9: 3100. https://doi.org/10.3390/jcm14093100
APA StyleCampbell, C. A., Grogan, G. M., St. Jean, S., Kabaria, N., Gardocki-Sandor, M., & Cottler, P. S. (2025). Preclinical Volume Retention of Fat Grafts Processed with REVOLVE™ Technology or Decantation Methods in Irradiated and Nonirradiated Wounds. Journal of Clinical Medicine, 14(9), 3100. https://doi.org/10.3390/jcm14093100






