Anti-Inflammatory Interleukin Levels Reflect Th1/Th2 Imbalance in Spondyloarthritis Patients with Concomitant Atopy Under Biological Therapy
Abstract
1. Introduction and Objectives
Atopy and Spondyloarthritis (SpA): Intersecting Immune Pathways
2. Materials and Methods
2.1. Patients and Controls
2.2. Study Design
2.3. Serum Cytokine Measurement
2.4. Group Matching Considerations
2.5. Statistical Analysis
3. Results
3.1. Characteristics of BT and BN SpA Patients in the Main SpA Cohort
3.1.1. Epidemiologic Data for Main SpA Cohort Comparative Between Biotreated (BT) and Bio-Naïve (BN) (Table 1)
- Demographics
- Disease-Related Characteristics
- Therapeutic Characteristics
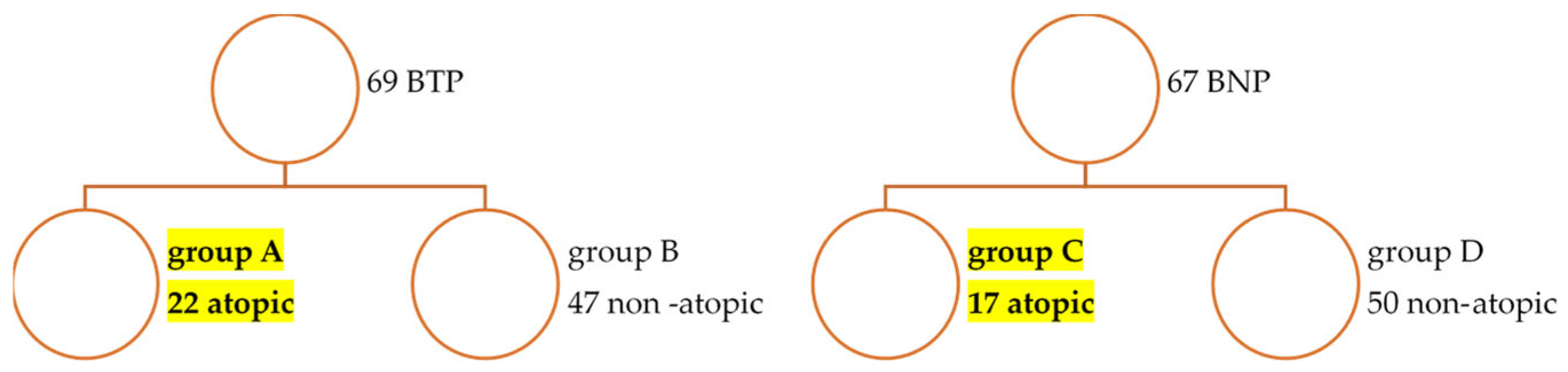
3.1.2. Characteristics of Atopic SpA Groups (Table 2)
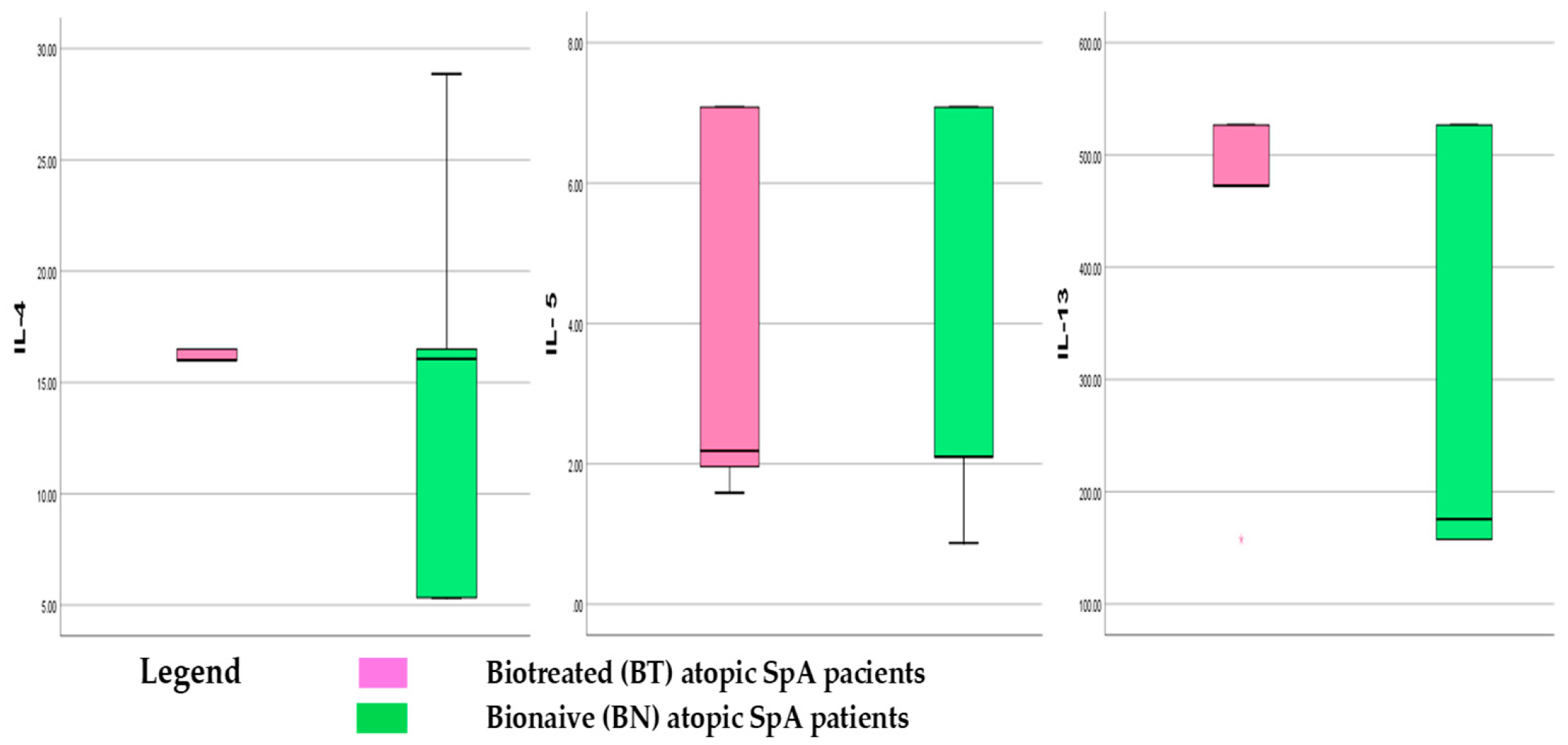
3.1.3. Th2 Cytokines Panel in Atopic SpA Patients
3.2. Th2 Cytokines in Different Atopy Subtypes Associated with SpA in Our Study
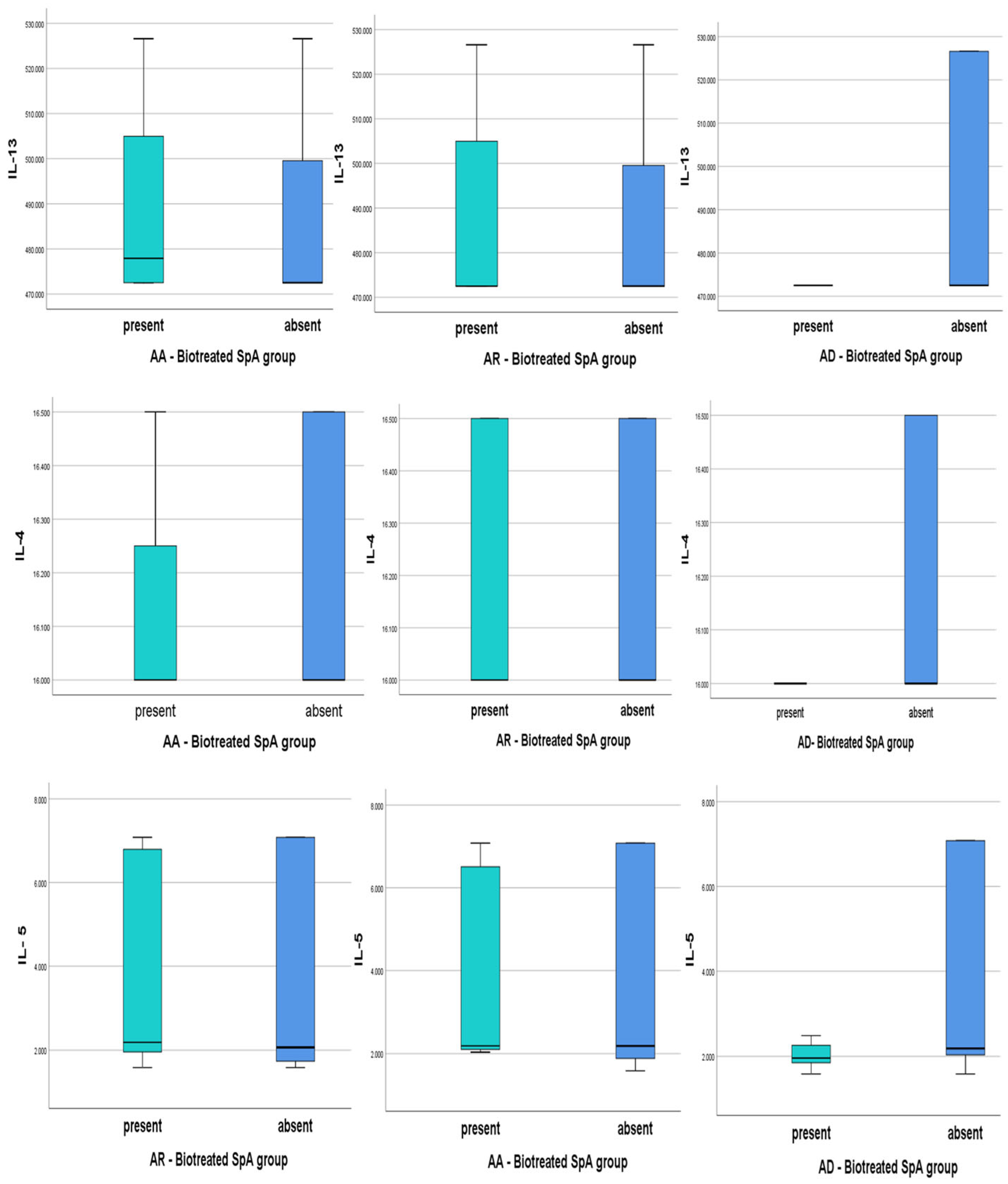
- Biologically Treated Atopic SpA and Th2 cytokines levels
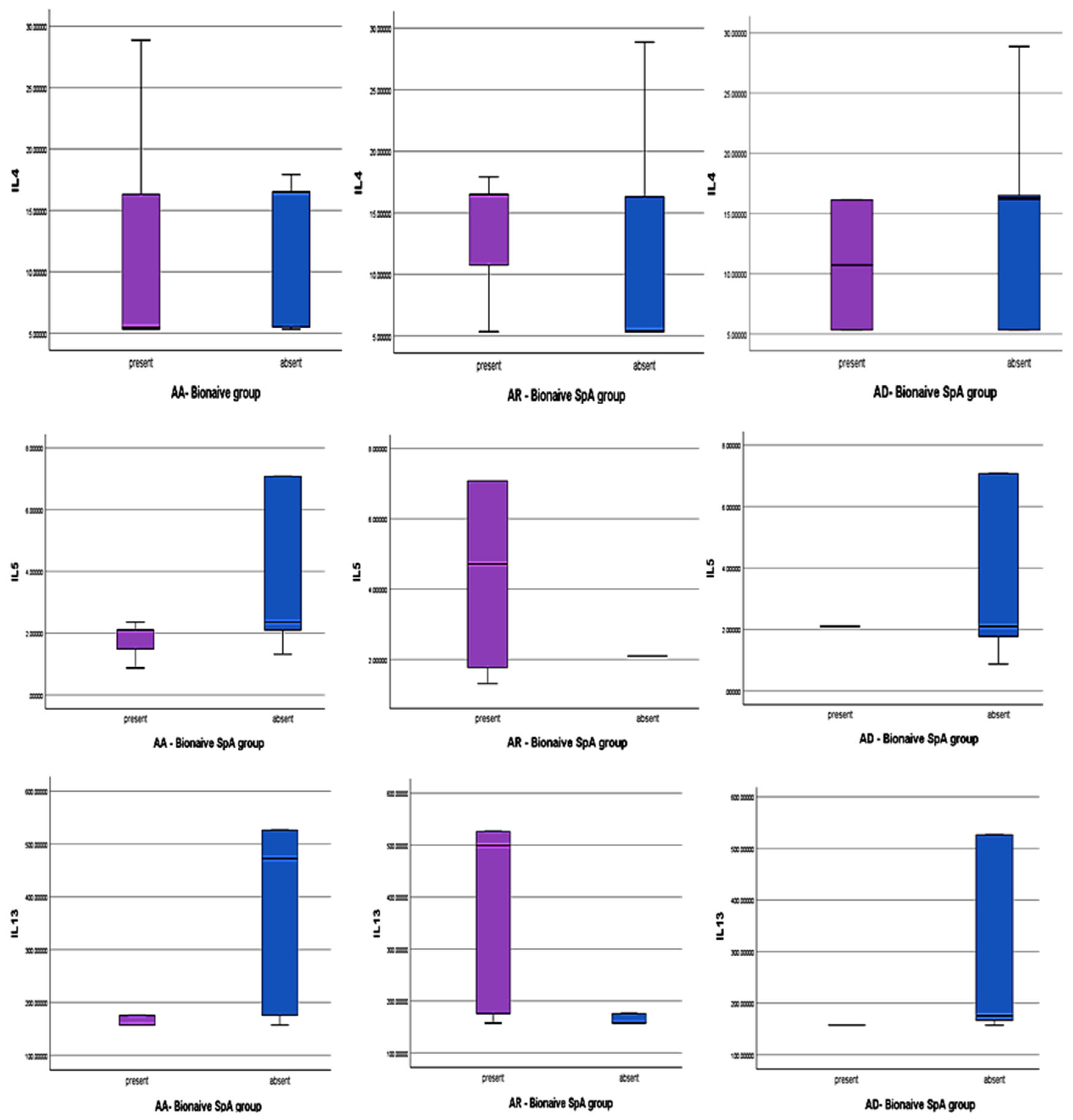
- Bio-Naïve atopic SpA and Th2 cytokines levels
3.3. Quantitative Analysis of Mean LLOQ Levels of Th2 Cytokines in BT and BN Atopic SPA
3.3.1. The Results for AR Patients Associated with SPA in Our Groups
3.3.2. The Results for AA Patients Associated with SPA in Our Groups
3.3.3. The Results for AD Patients Associated with SPA in Our Groups
4. Discussion
Perspectives and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SpA | Spondyloarthritis |
| AA | Allergic Asthma |
| AR | Atopic Rhinitis |
| AD | Atopic Dermatitis |
| BN | Bio-Naïve |
| BT | Biologically treated |
| INF | Interferon |
| IL | Interleukin |
| Th | T helper |
| TNF | Tumor Necrosis Factor |
References
- Rosine, N.; Fogel, O.; Koturan, S.; Rogge, L.; Bianchi, E.; Miceli-Richard, C. T cells in the pathogenesis of axial spondyloarthritis. Jt. Bone Spine 2023, 90, 105619. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Chang, W.-P.; Kuo, C.-N.; Kuo, L.-N.; Wang, Y.-T.; Perng, W.-T.; Kuo, H.-C.; Wei, J.C.-C. Increase risk of allergic diseases in patients with ankylosing spondylitis. Medicine 2016, 95, e5172. [Google Scholar] [CrossRef]
- van Roon, J.A.G.; Bijlsma, J.W.J. Th2 mediated regulation in RA and the spondyloarthropathies. Ann. Rheum. Dis. 2002, 61, 951–954. [Google Scholar] [CrossRef]
- Rudwaleit, M. Atopic disorders in ankylosing spondylitis and rheumatoid arthritis. Ann. Rheum. Dis. 2002, 61, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2014, 135, 3–626. [Google Scholar] [CrossRef]
- Bridgewood, C.; Newton, D.; Bragazzi, N.; Wittmann, M.; McGonagle, D. Unexpected connections of the IL-23/IL-17 and IL-4/IL-13 cytokine axes in inflammatory arthritis and enthesitis. Semin. Immunol. 2021, 58, 101520. [Google Scholar] [CrossRef] [PubMed]
- Felice, C.; Buono, A.D.; Gabbiadini, R.; Rattazzi, M.; Armuzzi, A. Cytokines in spondyloarthritis and inflammatory bowel diseases: From pathogenesis to therapeutic implications. Int. J. Mol. Sci. 2023, 24, 3957. [Google Scholar] [CrossRef]
- Gulino, G.R.; Van Mechelen, M.; Lories, R. Cellular and molecular diversity in spondyloarthritis. Semin. Immunol. 2021, 58, 101521. [Google Scholar] [CrossRef]
- Smith, J.A.; Colbert, R.A. Review: The Interleukin-23/Interleukin-17 axis in Spondyloarthritis Pathogenesis: TH17 and Beyond. Arthritis Rheumatol. 2013, 66, 231–241. [Google Scholar] [CrossRef]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Falciani, F.; Curnow, S.J.; Ross, E.J.; Lee, C.-Y.; Akbar, A.N.; Lord, J.M.; Gordon, C.; Buckley, C.D.; Salmon, M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 2005, 7, R784–R795. [Google Scholar] [CrossRef]
- Cargill, M.; Schrodi, S.J.; Chang, M.; Garcia, V.E.; Brandon, R.; Callis, K.P.; Matsunami, N.; Ardlie, K.G.; Civello, D.; Catanese, J.J.; et al. A Large-Scale genetic association study confirms IL12B and leads to the identification of IL23R as Psoriasis-Risk genes. Am. J. Hum. Genet. 2007, 80, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.P.; Duffin, K.C.; Helms, C.; Ding, J.; Stuart, P.E.; Goldgar, D.; Gudjonsson, J.E.; Li, Y.; Tejasvi, T.; Feng, B.-J.; et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat. Genet. 2009, 41, 199–204. [Google Scholar] [CrossRef]
- Russell, T.; Bridgewood, C.; Rowe, H.; Altaie, A.; Jones, E.; McGonagle, D. Cytokine “fine tuning” of enthesis tissue homeostasis as a pointer to spondyloarthritis pathogenesis with a focus on relevant TNF and IL-17 targeted therapies. Semin. Immunopathol. 2021, 43, 193–206. [Google Scholar] [CrossRef]
- Spadaro, A. Interleukin 13 in synovial fluid and serum of patients with psoriatic arthritis. Ann. Rheum. Dis. 2002, 61, 174–176. [Google Scholar] [CrossRef]
- Zhang, X.; Olsen, N.; Zheng, S.G. The progress and prospect of regulatory T cells in autoimmune diseases. J. Autoimmun. 2020, 111, 102461. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, S.; Davidson, C.; Vecellio, M. Regulatory T cells in spondyloarthropathies: Genetic evidence, functional role, and therapeutic possibilities. Front. Immunol. 2024, 14, 1303640. [Google Scholar] [CrossRef]
- Appel, H.; Wu, P.; Scheer, R.; Kedor, C.; Sawitzki, B.; Thiel, A.; Radbruch, A.; Sieper, J.; Syrbe, U. Synovial and peripheral blood CD4+FoxP3+ T cells in spondyloarthritis. J. Rheumatol. 2011, 38, 2445–2451. [Google Scholar] [CrossRef]
- Zhuang, Z.; Wang, Y.; Zhu, G.; Gu, Y.; Mao, L.; Hong, M.; Li, Y.; Zheng, M. Imbalance of Th17/Treg cells in pathogenesis of patients with human leukocyte antigen B27 associated acute anterior uveitis. Sci. Rep. 2017, 7, 40414. [Google Scholar] [CrossRef]
- Yang, W.-C.; Hwang, Y.-S.; Chen, Y.-Y.; Liu, C.-L.; Shen, C.-N.; Hong, W.-H.; Lo, S.-M.; Shen, C.-R. Interleukin-4 supports the suppressive immune responses elicited by regulatory T cells. Front. Immunol. 2017, 8, 1508. [Google Scholar] [CrossRef]
- Maerten, P.; Shen, C.; Bullens, D.M.A.; Van Assche, G.; Van Gool, S.; Geboes, K.; Rutgeerts, P.; Ceuppens, J.L. Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J. Autoimmun. 2005, 25, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the pathophysiology of severe asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Strugariu, G.; Pomîrleanu, C.; Bran, C.; Costea, A.; Vicovan, A.; Tatarciuc, D.; Eșanu, I.; Ancuța, E.; Chirieac, R.; Ancuța, C. The prevalence of Atopy in biologically treated spondyloarthropathies: A retrospective study of 200 patients. J. Clin. Med. 2021, 11, 55. [Google Scholar] [CrossRef]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.M.; Van den Bosch, F.E.; Boteva, B.; Bremander, A.; et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2023, 82, 19–34. [Google Scholar] [CrossRef]
- Gossec, L.; Andreas Kerschbaumer, A.; Ferreira, R.J.O.; Aletaha, D.; Baraliakos, X.; Bertheussen, H.; Wolf-Henning Boehncke, W.H.; Esbensen, B.A.; McInnes, J.B.; McGonagle, D.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 2024, 83, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyy, D.; Navarro-Compán, V.; Torgutalp, M.; Arends, S.; Sibel Zehra Aydin, S.Z.; Battista, S.; van den Bosch, F.; Bundy, C.; Cauli, A.; Davies, J.; et al. The Assessment of SpondyloArthritis International Society (ASAS) Consensus-Based Expert Definition of Difficult-to-Manage, including Treatment-Refractory, Axial Spondyloarthritis. Ann. Rheum. Dis. 2025, 84, 538–546. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Krueger, J.G.; Lebwohl, M.G. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp. Dermatol. 2017, 27, 409–417. [Google Scholar] [CrossRef]
- Hosseini, P.; Khoshkhui, M.; Hosseini, R.F.; Ahanchian, H.; Ravanshad, Y.; Layegh, P.; Bakhshoudeh, B.; Ariaee, N. Investigation of the relationship between atopy and psoriasis. Adv. Dermatol. Alergol. 2019, 36, 276–281. [Google Scholar] [CrossRef]
| General Characteristics | Bio-Naïve SpA (BN, Control Group) (n = 67) | Bio-Experimented SpA (BT, n = 69) | p |
|---|---|---|---|
| Gender distribution n (%) chi-square Male Female | 37 (55) (43.35) [0.93] 30 (45) (23.65) [1.71] | 51 (74) (44.65) [0.90] 18 (26) (24.35) [1.66] | 0.0226 * |
| Mean age (years ± SD) | 50.3 ± 11.34 | 49.61 ± 10.64 | p > 0.05 |
| SPA subtype n (%) chi-square axSpA APS PsA | 41 (61) (46.31) [0.61] 26 (39) (20.69) [1.36] | 53 (77) (47.69) [0.59] 16 (23) (21.31) [1.32] | 0.0487 |
| Atopy present n (%) chi-square AR AA AD | 17 (25) 8 (47) (9.19) [0.15] 8 (47) (4.98) [1.83] 2 (11.7) (3.83) [0.87] | 22 (39) 16 (72.7) (14.81) [0.10] 5 (22.7) (8.02) [1.14] 8 (36.3) (6.17) [0.54] | 0.9834 |
| Years since onset (medium years ± DS) | 11.83 ± 8.72 | 18.01 ± 9.98 | p > 0.05 |
| Years of diagnosis (medium years ± DS) | 7.43 ± 6.66 | 13.86 ± 9.57 | p > 0.05 |
| BASDAI ≥ 4 n (%) chi-square | 20 (48.7) (13.96) [2.62] | 12 (22.6) (18.04) [2.02] | 0.007996 * |
| ASDAS ≥ 2.1 n (%) chi-square | 24 (58.5) (18.32) [1.76] | 18 (33.9) (23.68) [1.36] | 0.017475 * |
| DAPSA ≥ 16 n (%) chi-square | 12 (46) (11.82) [0.00] | 8 (44.4) (8.18) [0.00] | 0.910853 |
| CRP ≥ 2 mg% n (%) chi-square | 18 (26.8) (12.81) [2.10] | 8 (11.6) (13.19) [2.04] | 0.023557 * |
| Parameter | BT Atopic SPA (n = 22) | BN Atopic SPA (n = 17) |
|---|---|---|
| Age (mean ± SD) | 47.55 ± 8.88 | 51.47 ± 12.83 |
| Gender—Male n (%) | 16 (72.7%) | 4 (23.5%) |
| axSpA, n (%) | 17 (77.2%) | 11 (65%) |
| PsA, n (%) | 5 (22.8%) | 6 (35%) |
| Years-onset (mean ± SD) | 15.58± 5.81 | 9.85 ± 5.78 |
| Years-diagnosis (mean ± SD) | 12.37 ± 5.92 | 7.08 ± 5.28 |
| Age at atopy onset 20–30 years n (%) >30 years n (%) | 4 (22.7%) 17 (77.3%) | 7 (41.2%) 10 (58.8%) |
| AR, n (%) | 16 (73%) | 8 (47%) |
| AA, n (%) | 5 (22.7%) | 8 (47%) |
| AD, n (%) | 8 (36.3%) | 2 (12%) |
| BASDAI mean ± SD | 2.18 ± 2.05 | 2.72 ± 1.1 |
| ASDAS mean ± SD | 2.11 ± 0.97 | 2 ± 0.86 |
| DAPSA mean ± SD | 12.4 ± 9.70 | 29.6 ± 16.62 |
| CRPmg/mL mean ± SD | 0.72 ± 0.77 | 1.87 ± 4.03 |
| CK | BT Min | BT Max | BT Mean ± SD | BN Min | BN Max | BN Mean ± SD |
|---|---|---|---|---|---|---|
| IL-4 | 5.33 | 266.25 | 19.68 ± 13.36 | 12.51 | 166.87 | 20.77 ± 35.04 |
| IL-5 | 1.14 | 7.08 | 3.53 ± 2.39 | 1.44 | 9.83 | 3.36 ± 2.51 |
| IL-13 | 12.65 | 526.6 | 450.18 ± 118.0231 | 160.6 | 526.6 | 288.89 ± 173.93 |
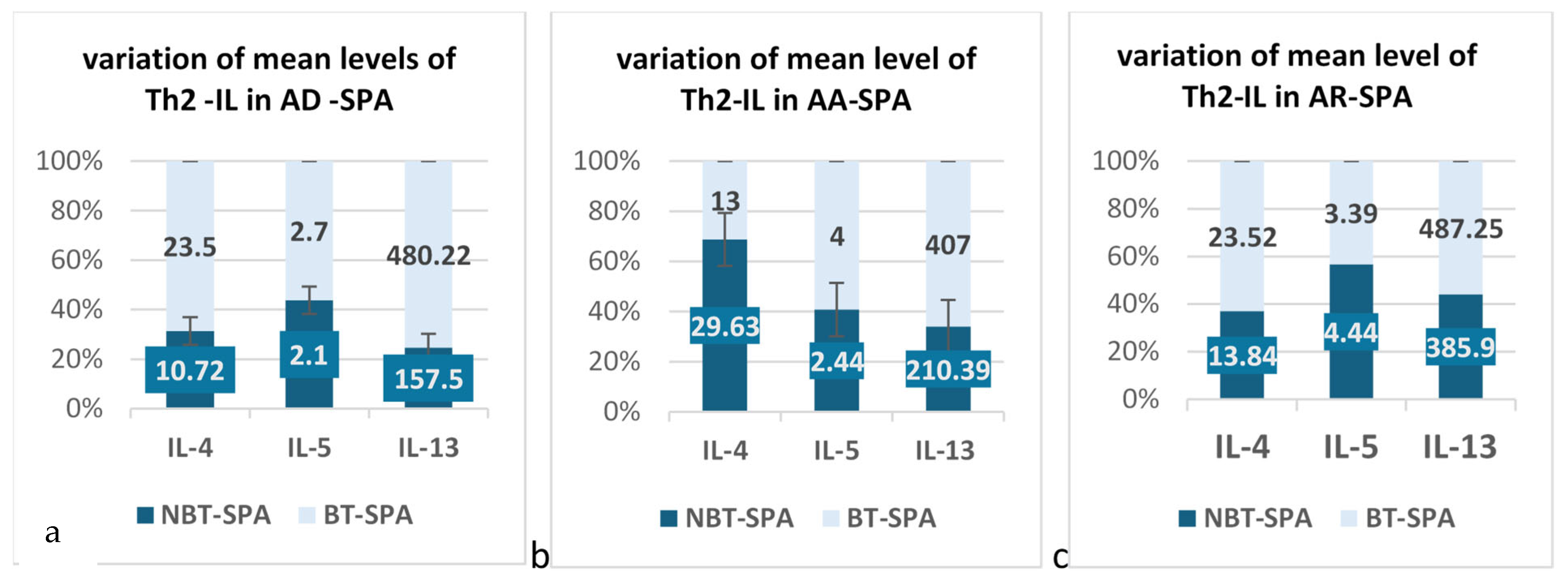
| Cytokines | Atopic Rhinitis Group | Number of Patients (n) | Mean | Std. Deviation | Std. Error Mean |
|---|---|---|---|---|---|
| IL-4 | BT | 11 | 23.52 | 17.1 | 5.15 |
| BN | 8 | 13.84 | 5.23 | 1.84 | |
| IL-5 | BT | 11 | 3.39 | 2.38 | 0.71 |
| BN | 8 | 4.44 | 2.84 | 1 | |
| IL-13 | BT | 11 | 487.25 | 25.27 | 7.61 |
| BN | 8 | 385.93 | 180.22 | 63.72 |
| Allergic Asthma Group | N Patients | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| IL-4 | BT | 4 | 13.45 | 5.42 | 2.71 |
| BN | 8 | 29.63 | 50.98 | 18.02 | |
| IL-5 | BT | 4 | 3.35 | 2.48 | 1.24 |
| BN | 8 | 2.44 | 1.96 | 0.69 | |
| IL-13 | BT | 4 | 407.27 | 168.45 | 84.23 |
| BN | 8 | 210.39 | 128.07 | 45.28 |
| Atopic Dermatitis Subgroup | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| IL-4 | BT | 7 | 23.45 | 19.50 | 7.37 |
| BN | 2 | 10.73 | 7.63 | 5.39 | |
| IL-5 | BT | 7 | 2.76 | 1.92 | 0.72 |
| BN | 2 | 2.10 | 0.000 | 0.000 | |
| IL-13 | BT | 7 | 480.22 | 20.44 | 7.72 |
| BN | 2 | 157.50 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strugariu, G.; Pomirleanu, C.; Russu, M.; Lapuste, V.; Constantinescu, D.; Cianga, P.; Ancuta, C. Anti-Inflammatory Interleukin Levels Reflect Th1/Th2 Imbalance in Spondyloarthritis Patients with Concomitant Atopy Under Biological Therapy. J. Clin. Med. 2025, 14, 3094. https://doi.org/10.3390/jcm14093094
Strugariu G, Pomirleanu C, Russu M, Lapuste V, Constantinescu D, Cianga P, Ancuta C. Anti-Inflammatory Interleukin Levels Reflect Th1/Th2 Imbalance in Spondyloarthritis Patients with Concomitant Atopy Under Biological Therapy. Journal of Clinical Medicine. 2025; 14(9):3094. https://doi.org/10.3390/jcm14093094
Chicago/Turabian StyleStrugariu, Georgiana, Cristina Pomirleanu, Mara Russu, Vladia Lapuste, Daniela Constantinescu, Petru Cianga, and Codrina Ancuta. 2025. "Anti-Inflammatory Interleukin Levels Reflect Th1/Th2 Imbalance in Spondyloarthritis Patients with Concomitant Atopy Under Biological Therapy" Journal of Clinical Medicine 14, no. 9: 3094. https://doi.org/10.3390/jcm14093094
APA StyleStrugariu, G., Pomirleanu, C., Russu, M., Lapuste, V., Constantinescu, D., Cianga, P., & Ancuta, C. (2025). Anti-Inflammatory Interleukin Levels Reflect Th1/Th2 Imbalance in Spondyloarthritis Patients with Concomitant Atopy Under Biological Therapy. Journal of Clinical Medicine, 14(9), 3094. https://doi.org/10.3390/jcm14093094






