Can Thrombosed Abdominal Aortic Dissecting Aneurysm Cause Mesenteric Artery Thrombosis and Ischemic Colitis?—A Case Report and a Review of Literature
Abstract
:1. Introduction
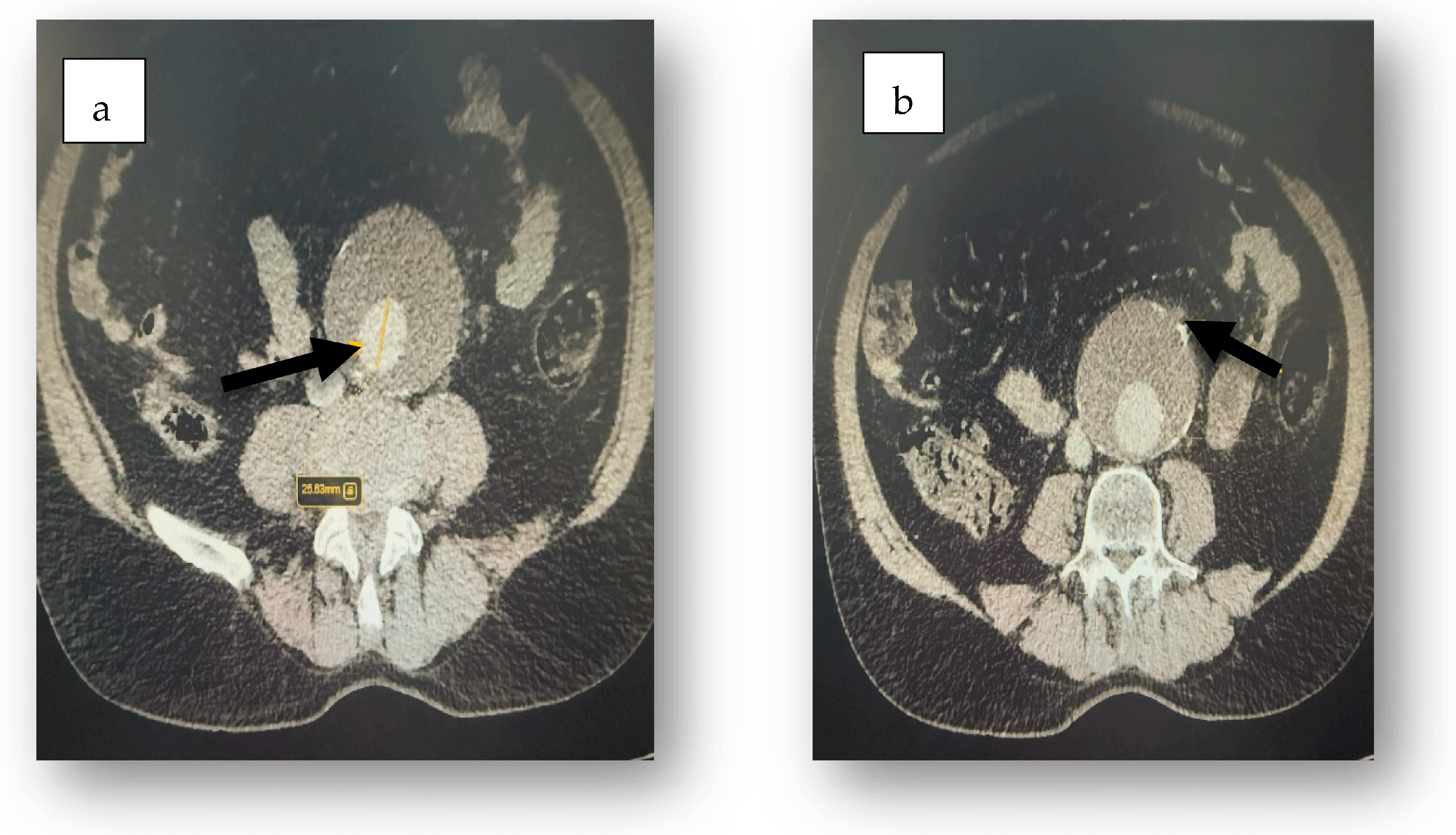
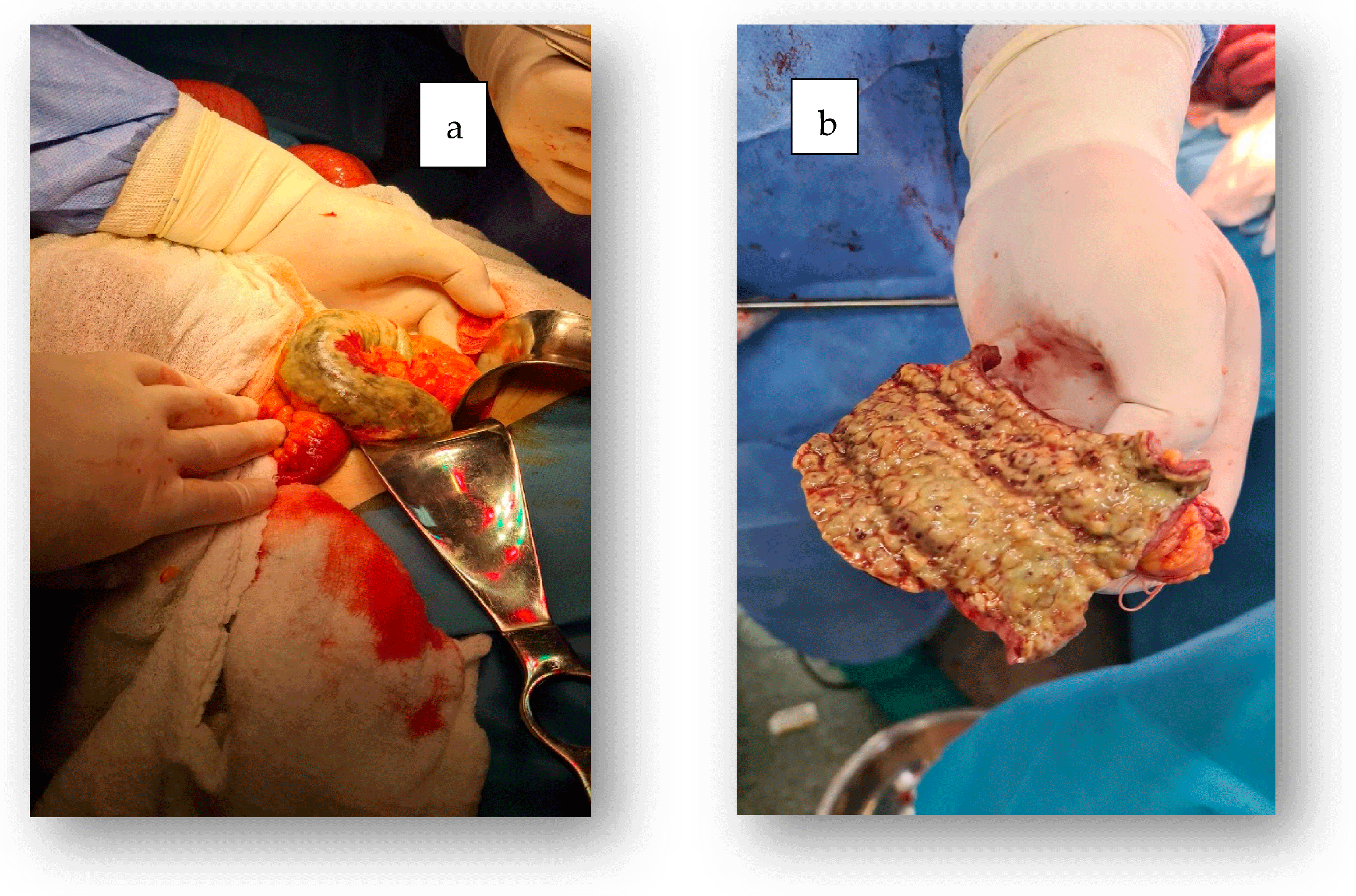
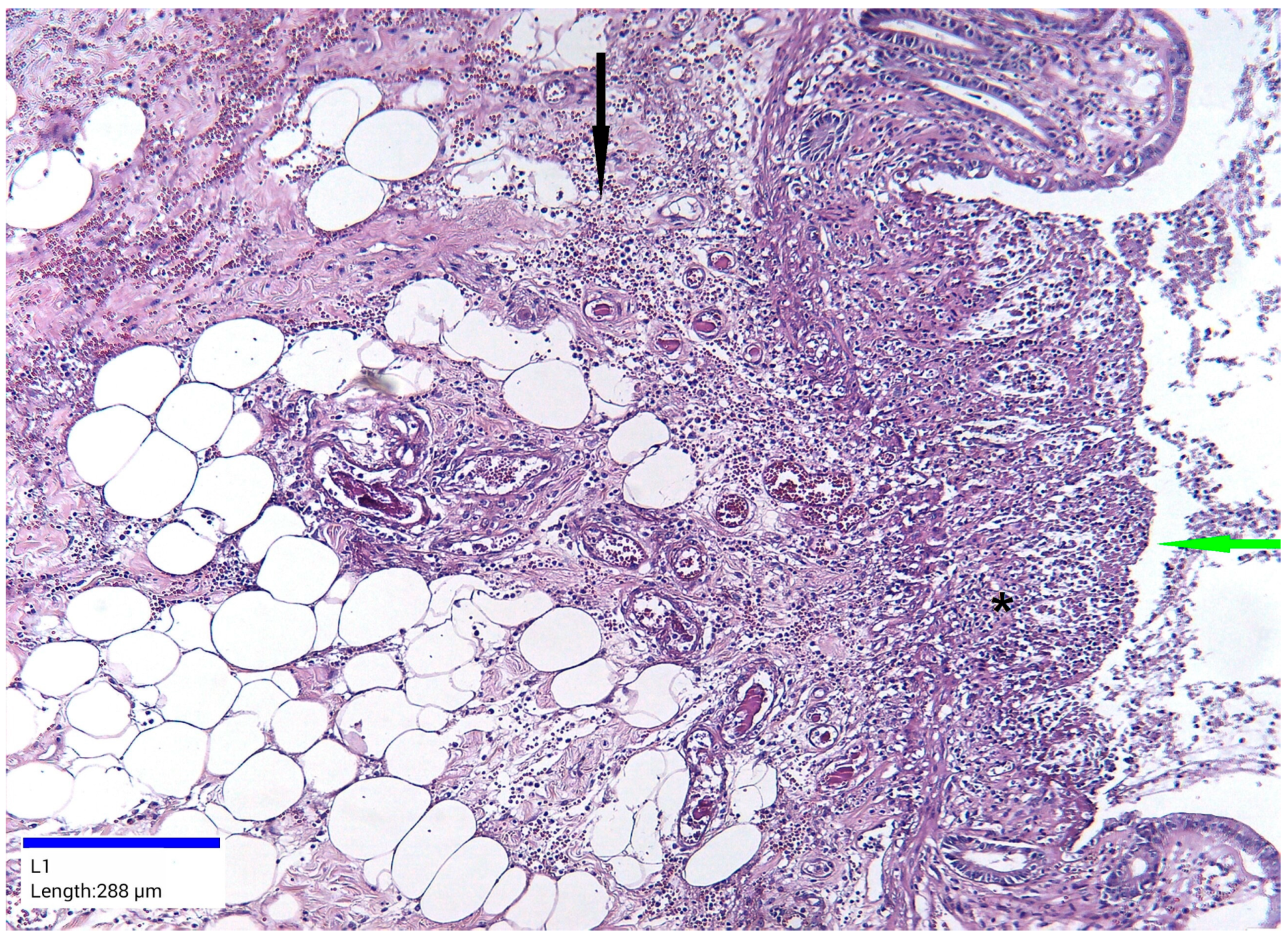
2. Case Report
3. Discussion
- Patient with ulcerative colitis → pro-thrombotic status → thrombosis in a pre-existing aneurysm → embolization → superimposed ischemic colitis.
- Elderly patient with thrombosed aortic aneurysm → mesenteric emboli → ischemic colitis → severe symptoms → accidental discovery of coexisting ulcerative colitis.
- Systemic autoimmune disease (e.g., lupus, vasculitis) → predisposition to all three entities.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, W.M.; Gloviczki, P.; Cherry, K.J., Jr.; Hallett, J.W., Jr.; Bower, T.C.; Panneton, J.M.; Schleck, C.; Ilstrup, D.; Harmsen, W.S.; Noel, A.A. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J. Vasc. Surg. 2002, 35, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Rhee, R.Y.; Gloviczki, P.; Mendonca, C.T.; Petterson, T.M.; Serry, R.D.; Sarr, M.G.; Johnson, C.M.; Bower, T.C.; Hallett, J.W., Jr.; Cherry, K.J., Jr. Mesenteric venous thrombosis: Still a lethal disease in the 1990s. J. Vasc. Surg. 1994, 20, 688–697. [Google Scholar] [CrossRef]
- Bergman, R.T.; Gloviczki, P.; Welch, T.J.; Naessens, J.M.; Bower, T.C.; Hallett, J.W., Jr.; Pairolero, P.C.; Cherry, K.J., Jr. The role of intravenous fluorescein in the detection of colon ischemia during aortic reconstruction. Ann. Vasc. Surg. 1992, 6, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, A.; Koutroubakis, I.E. Ischemic colitis: Clinical practice in diagnosis and treatment. World J. Gastroenterol. 2008, 14, 7302–7308. [Google Scholar] [CrossRef]
- Greenwald, D.A.; Brandt, L.J. Colonic ischemia. J. Clin. Gastroenterol. 1998, 27, 122–128. [Google Scholar] [CrossRef]
- Zhu, M.; Ran, Z. Clinical characteristics of ulcerative colitis in elderly patients. JGH Open 2021, 5, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Rozich, J.J.; Dulai, P.S.; Fumery, M.; Sandborn, W.J.; Singh, S. Progression of Elderly Onset Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2020, 18, 2437–2447.e6. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G. Inflammatory bowel disease in the elderly is associated with worse outcomes: A national study of hospitalizations. Inflamm. Bowel Dis. 2009, 15, 182–189. [Google Scholar] [CrossRef]
- Cottone, M.; Kohn, A.; Daperno, M.; Armuzzi, A.; Guidi, L.; D’Inca, R.; Bossa, F.; Angelucci, E.; Biancone, L.; Gionchetti, P.; et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2011, 9, 30–35. [Google Scholar] [CrossRef]
- Mak, J.W.Y.; Lok Tung Ho, C.; Wong, K.; Cheng, T.Y.; Yip, T.C.F.; Leung, W.K.; Li, M.; Lo, F.H.; Ng, K.M.; Sze, S.F.; et al. Epidemiology and Natural History of Elderly-onset Inflammatory Bowel Disease: Results from a Territory-wide Hong Kong IBD Registry. J. Crohns Colitis 2021, 15, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Bontekoe, J.; Matsumura, J.; Liu, B. Thrombosis in the pathogenesis of abdominal aortic aneurysm. JVS Vasc. Sci. 2023, 4, 100106. [Google Scholar] [CrossRef]
- Labruto, F.; Blomqvist, L.; Swedenborg, J. Imaging the intraluminal thrombus of abdominal aortic aneurysms: Techniques, findings, and clinical implications. J. Vasc. Interv. Radiol. 2011, 22, 1069–1075. [Google Scholar] [CrossRef]
- Di Martino, E.; Mantero, S.; Inzoli, F.; Melissano, G.; Astore, D.; Chiesa, R.; Fumero, R. Biomechanics of abdominal aortic aneurysm in the presence of endoluminal thrombus: Experimental characterisation and structural static computational analysis. Eur. J. Vasc. Endovasc. Surg. 1998, 15, 290–299. [Google Scholar] [CrossRef]
- Schanzer, A.; Greenberg, R.K.; Hevelone, N.; Robinson, W.P.; Eslami, M.H.; Goldberg, R.J.; Messina, L. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011, 123, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, J.; Ferreira, R.S.; Oliveira, N.F.G.; Hoeks, S.; Van Rijn, M.J.; Raa, S.T.; Mansilha, A.; Verhagen, H.J.M.; Gonçalves, F.B. Total Luminal Volume Predicts Risk after Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.; Loper, B.; Jain, M.; Lewis, A.V.; Margulies, D.R.; Alban, R.F. Predictive factors of mortality after colectomy in ischemic colitis: An ACS-NSQIP database study. Trauma Surg. Acute Care Open 2017, 2, e000126. [Google Scholar] [CrossRef]
- Moszkowicz, D.; Mariani, A.; Trésallet, C.; Menegaux, F. Ischemic colitis: The ABCs of diagnosis and surgical management. J. Visc. Surg. 2013, 150, 19–28. [Google Scholar] [CrossRef]
- Huguier, M.; Barrier, A.; Boelle, P.Y.; Houry, S.; Lacaine, F. Ischemic colitis. Am. J. Surg. 2006, 192, 679–684. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Yao, J.F. Epidemiology, clinical features, high-risk factors, and outcome of acute large bowel ischemia. Clin. Gastroenterol. Hepatol. 2009, 7, e1–e2. [Google Scholar] [CrossRef]
- Paterno, F.; McGillicuddy, E.A.; Schuster, K.M.; Longo, W.E. Ischemic colitis: Risk factors for eventual surgery. Am. J. Surg. 2010, 200, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Añón, R.; Boscá, M.M.; Sanchiz, V.; Tosca, J.; Almela, P.; Amorós, C.; Benages, A. Factors predicting poor prognosis in ischemic colitis. World J. Gastroenterol. 2006, 12, 4875–4878. [Google Scholar] [PubMed]
- Green, B.T.; Tendler, D.A. Ischemic colitis: A clinical review. South. Med. J. 2005, 98, 217–222. [Google Scholar] [CrossRef]
- Hass, D.J.; Kozuch, P.; Brandt, L.J. Pharmacologically mediated colon ischemia. Am. J. Gastroenterol. 2007, 102, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.; Bories, P.; Donadio, D.; Parelon, G.; Rouanet, C.; Paleirac, G.; Michel, H. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol. Clin. Biol. 1989, 13, 945. [Google Scholar]
- Verger, P.; Blanc, C.; Feydy, P.; Boey, S. Colite ischémique due à un déficit en protéine S [Ischemic colitis caused by protein S deficiency]. Presse Med. 1996, 25, 1350. [Google Scholar]
- Ludwig, D.; Stahl, M.; David-Walek, T.; Brüning, A.; Siemens, A.; Zwaan, M.; Schmücker, G.; Stange, E.F. Ischemic colitis, pulmonary embolism, and right atrial thrombosis in a patient with inherited resistance to activated protein C. Dig. Dis. Sci. 1998, 43, 1362–1367. [Google Scholar] [CrossRef]
- Yee, N.S.; Guerry, D., 4th; Lichtenstein, G.R. Ischemic colitis associated with factor V Leiden mutation. Ann. Intern. Med. 2000, 132, 595–596. [Google Scholar] [CrossRef]
- Balian, A.; Veyradier, A.; Naveau, S.; Wolf, M.; Montembault, S.; Giraud, V.; Borotto, E.; Henry, C.; Meyer, D.; Chaput, J.C. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig. Dis. Sci. 1999, 44, 1910–1913. [Google Scholar] [CrossRef]
- Cervera, R.; Espinosa, G.; Cordero, A.; Oltra, M.R.; Unzurrunzaga, A.; Rossiñol, T.; Plaza, J.; Bucciarelli, S.; Ramos-Casals, M.; Ingelmo, M.; et al. Catastrophic Antiphospholipid Syndrome (CAPS) Registry Project Group. Intestinal involvement secondary to the antiphospholipid syndrome (APS): Clinical and immunologic characteristics of 97 patients: Comparison of classic and catastrophic APS. Semin. Arthritis Rheum. 2007, 36, 287–296. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Theodoropoulou, A.; Sfiridaki, A.; Kouroumalis, E.A. Low plasma protein Z levels in patients with ischemic colitis. Dig. Dis. Sci. 2003, 48, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Sfiridaki, A.; Theodoropoulou, A.; Kouroumalis, E.A. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001, 121, 561–565. [Google Scholar] [CrossRef]
- Theodoropoulou, A.; Sfiridaki, A.; Oustamanolakis, P.; Vardas, E.; Livadiotaki, A.; Boumpaki, A.; Paspatis, G.; Koutroubakis, I.E. Genetic risk factors in young patients with ischemic colitis. Clin. Gastroenterol. Hepatol. 2008, 6, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Midian-Singh, R.; Polen, A.; Durishin, C.; Crock, R.D.; Whittier, F.C.; Fahmy, N. Ischemic colitis revisited: A prospective study identifying hypercoagulability as a risk factor. South. Med. J. 2004, 97, 120–123. [Google Scholar] [CrossRef]
- Baixauli, J.; Kiran, R.P.; Delaney, C.P. Investigation and management of ischemic colitis. Cleve Clin. J. Med. 2003, 70, 920–921. [Google Scholar] [CrossRef]
- Brandt, L.J.; Boley, S.J. Colonic ischemia. Surg. Clin. N. Am. 1992, 72, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.K.; Hanson, M.M.; Vernava, A.M.; Kaminski, D.L.; Longo, W.E. Ischemic colitis. Dis. Colon Rectum 1996, 39, 88–100. [Google Scholar] [CrossRef]
- Longo, W.E.; Ballantyne, G.H.; Gusberg, R.J. Ischemic colitis: Patterns and prognosis. Dis. Colon Rectum 1992, 35, 726–730. [Google Scholar] [CrossRef]
- Medina, C.; Vilaseca, J.; Videla, S.; Fabra, R.; Armengol-Miro, J.R.; Malagelada, J.R. Outcome of patients with ischemic colitis: Review of fifty-three cases. Dis. Colon Rectum 2004, 47, 180–184. [Google Scholar] [CrossRef]
- Ilie, D.S.; Mitroi, G.; Păun, I.; Ţenea-Cojan, T.Ş.; Neamţu, C.; Totolici, B.D.; Sapalidis, K.; Mogoantă, S.Ş.; Murea, A. Pathological and immunohistochemical study of colon cancer. Evaluation of markers for colon cancer stem cells. Rom. J. Morphol. Embryol. 2021, 62, 117–124. [Google Scholar] [CrossRef]
- Boyd, A.J.; Kuhn, D.C.; Lozowy, R.J.; Kulbisky, G.P. Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J. Vasc. Surg. 2016, 63, 1613–1619. [Google Scholar] [CrossRef]
- Biasetti, J.; Hussain, F.; Gasser, T.C. Blood flow and coherent vortices in the normal and aneurysmatic aortas: A fluid dynamical approach to intra-luminal thrombus formation. J. R. Soc. Interface 2011, 8, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Biasetti, J.; Gasser, T.C.; Auer, M.; Hedin, U.; Labruto, F. Hemodynamics of the normal aorta compared to fusiform and saccular abdominal aortic aneurysms with emphasis on a potential thrombus formation mechanism. Ann. Biomed. Eng. 2010, 38, 380–390. [Google Scholar] [CrossRef]
- Soudah, E.; Ng, E.Y.; Loong, T.H.; Bordone, M.; Pua, U.; Narayanan, S. CFD modelling of abdominal aortic aneurysm on hemodynamic loads using a realistic geometry with CT. Comput. Math. Methods Med. 2013, 2013, 472564. [Google Scholar] [CrossRef] [PubMed]
- Holme, P.A.; Orvim, U.; Hamers, M.J.; Solum, N.O.; Brosstad, F.R.; Barstad, R.M.; Sakariassen, K.S. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arter. Thromb. Vasc. Biol. 1997, 17, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M.; Orje, J.N.; Habermann, R.; Federici, A.B.; Reininger, A.J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 2006, 108, 1903–1910. [Google Scholar] [CrossRef]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion mechanisms in platelet function. Circ. Res. 2007, 100, 1673–1685. [Google Scholar] [CrossRef]
- Maxwell, M.J.; Westein, E.; Nesbitt, W.S.; Giuliano, S.; Dopheide, S.M.; Jackson, S.P. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood 2007, 109, 566–576. [Google Scholar] [CrossRef]
- Hourmand-Ollivier, I.; Bouin, M.; Saloux, E.; Morello, R.; Rousselot, P.; Piquet, M.A.; Dao, T.; Verwaerde, J.C. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am. J. Gastroenterol. 2003, 98, 1573–1577. [Google Scholar] [CrossRef]
- Su, C.; Brandt, L.J.; Sigal, S.H.; Alt, E.; Steinberg, J.J.; Patterson, K.; Tarr, P.I. The immunohistological diagnosis of E. coli O157:H7 colitis: Possible association with colonic ischemia. Am. J. Gastroenterol. 1998, 93, 1055–1059. [Google Scholar] [CrossRef]



| Laboratory Tests | Laboratory Tests upon Admission | Laboratory Tests After Surgery |
|---|---|---|
| White blood cell | 17.20 × 103 cells/μL | 28 × 103 cells/μL |
| Neutrophil proportion | 89.4% | 94% |
| Hemoglobin | 14.2 g/dL | 12.8 g/dL |
| Platelet count | 147 × 103 cells/μL | 125 × 103 cells/μL |
| Creatinine | 1.9 mg/dL | 2.5 mg/dL |
| Urea | 107 mg/dL | 180 mg/dL |
| INR | 1.34 | 1.15 |
| Prothrombin time | 14.7 s | 14 s |
| Fibrinogen | 1335 mg/dL | 1500 mg/dL |
| Erythrocyte sedimentation rate | 55/mm | 50/mm |
| Parameter | Upon Admission to the Intensive Care Unit | 6 h After Admission | 10 h After Admission |
|---|---|---|---|
| pH | 7.38 | 7.25 | 7.20 |
| PaCO2 | 25 mmHg | 60 mmHg | 38 mmHg |
| HCO3⁻ | 15 mEq/L | 18 mEq/L | 14 mEq/L |
| PaO2 | 95 mmHg | 70 mmHg | 75 mmHg |
| SaO2 | 98% | 92% | 94% |
| BE | −8 mmol/L | −6 mmol/L | −15 mmol/L |
| Lactat | 1.5 mmol/L | 2.0 mmol/L | 3 mmol/L |
| Comments | Totally compensated respiratory metabolic acidosis | Ph is predominantly acidified by respiration | Severe pure metabolic acidosis |
| 1. Address Risk Factors |
|
| 2. Medication Assessment |
|
| 3. Surgical Prevention (in Rare Cases) |
|
| 4. Management of Mild Ischemic Colitis |
|
| 5. Management of Severe Cases |
|
| 1. Clinical Suspicion |
|
| 2. Laboratory Tests |
|
| 3. Imaging |
|
| 4. Colonoscopy or Flexible Sigmoidoscopy |
|
| 5. Doppler Ultrasound/MRA/CTA |
|
| Approach | Examples |
|---|---|
| Preventive Treatment | Cardiovascular control, medication adjustment, hydration |
| Medical Management | IV fluids, bowel rest, antibiotics |
| Surgical Intervention | For complications or mesenteric vascular disease |
| Clinical Monitoring | Recognize pain, bleeding, risk factors |
| Imaging | CT of abdomen with contrast (first line) |
| Endoscopy | Visual and biopsy confirmation (if stable) |
| Vascular Studies | CTA, MRA, Doppler for chronic/recurrent or high-risk patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbu, L.A.; Mărgăritescu, N.-D.; Cercelaru, L.; Caragea, D.-C.; Vîlcea, I.-D.; Șurlin, V.; Mogoantă, S.-Ș.; Mogoș, G.F.R.; Vasile, L.; Țenea Cojan, T.Ș. Can Thrombosed Abdominal Aortic Dissecting Aneurysm Cause Mesenteric Artery Thrombosis and Ischemic Colitis?—A Case Report and a Review of Literature. J. Clin. Med. 2025, 14, 3092. https://doi.org/10.3390/jcm14093092
Barbu LA, Mărgăritescu N-D, Cercelaru L, Caragea D-C, Vîlcea I-D, Șurlin V, Mogoantă S-Ș, Mogoș GFR, Vasile L, Țenea Cojan TȘ. Can Thrombosed Abdominal Aortic Dissecting Aneurysm Cause Mesenteric Artery Thrombosis and Ischemic Colitis?—A Case Report and a Review of Literature. Journal of Clinical Medicine. 2025; 14(9):3092. https://doi.org/10.3390/jcm14093092
Chicago/Turabian StyleBarbu, Laurențiu Augustus, Nicolae-Dragoș Mărgăritescu, Liliana Cercelaru, Daniel-Cosmin Caragea, Ionică-Daniel Vîlcea, Valeriu Șurlin, Stelian-Ștefaniță Mogoantă, Gabriel Florin Răzvan Mogoș, Liviu Vasile, and Tiberiu Ștefăniță Țenea Cojan. 2025. "Can Thrombosed Abdominal Aortic Dissecting Aneurysm Cause Mesenteric Artery Thrombosis and Ischemic Colitis?—A Case Report and a Review of Literature" Journal of Clinical Medicine 14, no. 9: 3092. https://doi.org/10.3390/jcm14093092
APA StyleBarbu, L. A., Mărgăritescu, N.-D., Cercelaru, L., Caragea, D.-C., Vîlcea, I.-D., Șurlin, V., Mogoantă, S.-Ș., Mogoș, G. F. R., Vasile, L., & Țenea Cojan, T. Ș. (2025). Can Thrombosed Abdominal Aortic Dissecting Aneurysm Cause Mesenteric Artery Thrombosis and Ischemic Colitis?—A Case Report and a Review of Literature. Journal of Clinical Medicine, 14(9), 3092. https://doi.org/10.3390/jcm14093092






