Sex- and Stage-Specific Predictors of Anemia in Chronic Kidney Disease: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Variables and Definitions
2.3. Statistical Analysis
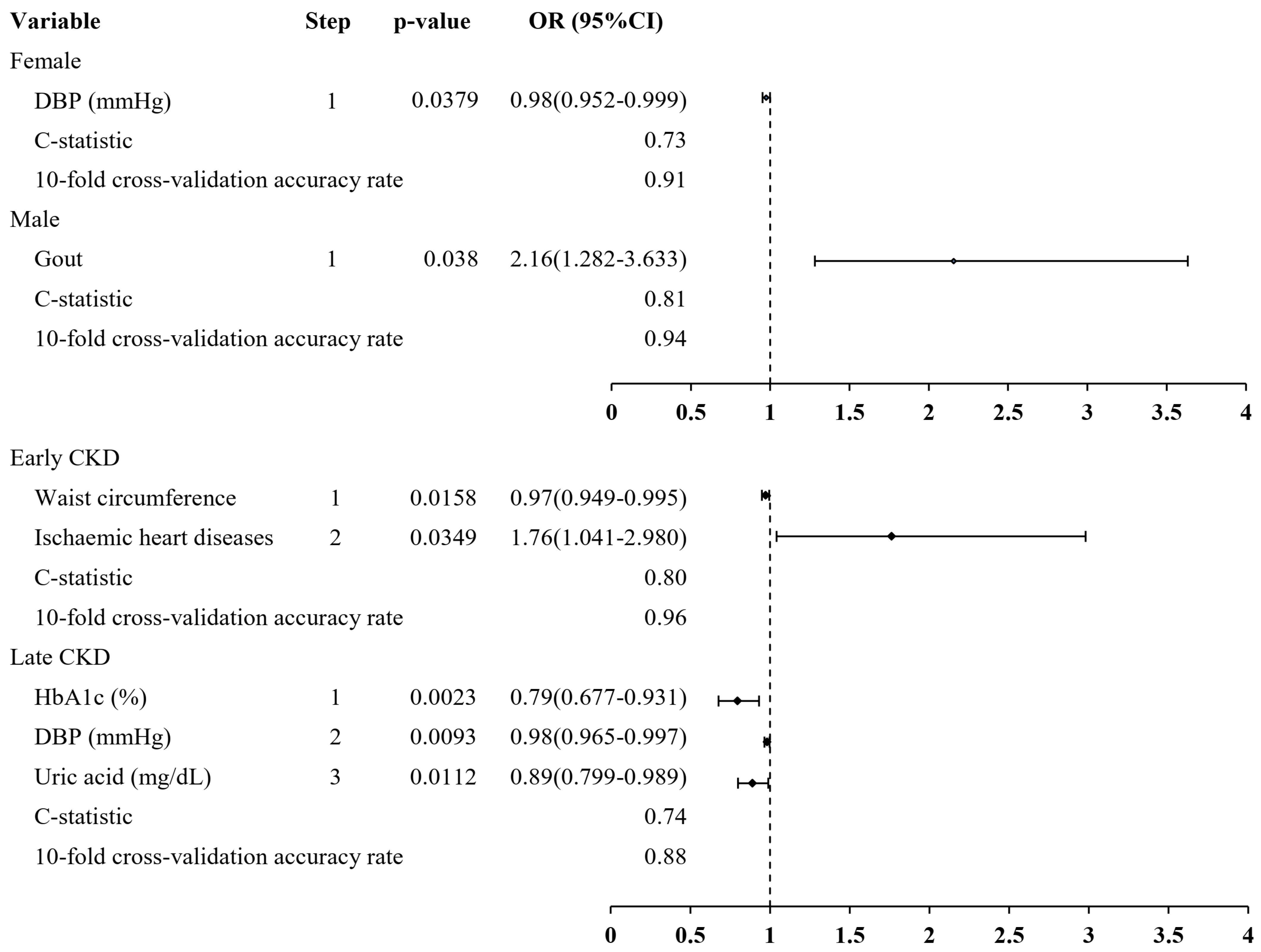
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, M.E.; Fan, T. Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE 2014, 9, e84943. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Muntner, P.; Levin, A.; Eustace, J.A.; Coresh, J. Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch. Intern. Med. 2002, 162, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Sofue, T.; Nakagawa, N.; Kanda, E.; Nagasu, H.; Matsushita, K.; Nangaku, M.; Maruyama, S.; Wada, T.; Terada, Y.; Yamagata, K.; et al. Prevalence of anemia in patients with chronic kidney disease in Japan: A nationwide, cross-sectional cohort study using data from the Japan Chronic Kidney Disease Database (J-CKD-DB). PLoS ONE 2020, 15, e0236132. [Google Scholar] [CrossRef]
- Awan, A.A.; Walther, C.P.; Richardson, P.A.; Shah, M.; Winkelmayer, W.C.; Navaneethan, S.D. Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2021, 36, 129–136. [Google Scholar] [CrossRef]
- Smith, R.E., Jr. The clinical and economic burden of anemia. Am. J. Manag. Care 2010, 16, S59–S66. [Google Scholar]
- Mehdi, U.; Toto, R.D. Anemia, diabetes, and chronic kidney disease. Diabetes Care 2009, 32, 1320–1326. [Google Scholar] [CrossRef]
- van Nooten, F.E.; Green, J.; Brown, R.; Finkelstein, F.O.; Wish, J. Burden of illness for patients with non-dialysis chronic kidney disease and anemia in the United States: Review of the literature. J. Med. Econ. 2010, 13, 241–256. [Google Scholar] [CrossRef]
- Farrington, D.K.; Sang, Y.; Grams, M.E.; Ballew, S.H.; Dunning, S.; Stempniewicz, N.; Coresh, J. Anemia Prevalence, Type, and Associated Risks in a Cohort of 5.0 Million Insured Patients in the United States by Level of Kidney Function. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2023, 81, 201–209.e201. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in CKD. J. Am. Soc. Nephrol. JASN 2012, 23, 1631–1634. [Google Scholar] [CrossRef]
- Cotes, P.M. Immunoreactive erythropoietin in serum. I. Evidence for the validity of the assay method and the physiological relevance of estimates. Br. J. Haematol. 1982, 50, 427–438. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, R.J.; Wallin, J.D.; Shadduck, R.K.; Fisher, J.W. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int. 1984, 25, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.F.; Ebbe, S.N.; Hollander, L.; Cutting, H.O.; Miller, M.E.; Cronkite, E.P. Radioimmunoassay of erythropoietin: Circulating levels in normal and polycythemic human beings. J. Lab. Clin. Med. 1982, 99, 624–635. [Google Scholar] [PubMed]
- Shiferaw, W.S.; Akalu, T.Y.; Aynalem, Y.A. Risk Factors for Anemia in Patients with Chronic Renal Failure: A Systematic Review and Meta-Analysis. Ethiop. J. Health Sci. 2020, 30, 829–842. [Google Scholar]
- Li, Y.; Shi, H.; Wang, W.M.; Peng, A.; Jiang, G.R.; Zhang, J.Y.; Ni, Z.H.; He, L.Q.; Niu, J.Y.; Wang, N.S.; et al. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: First multicenter, cross-sectional study. Medicine 2016, 95, e3872. [Google Scholar] [CrossRef]
- Nalado, A.M.; Mahlangu, J.N.; Waziri, B.; Duarte, R.; Paget, G.; Olorunfemi, G.; Naicker, S. Ethnic prevalence of anemia and predictors of anemia among chronic kidney disease patients at a tertiary hospital in Johannesburg, South Africa. Int. J. Nephrol. Renov. Dis. 2019, 12, 19–32. [Google Scholar] [CrossRef]
- Lau, B.C.V.; Ong, K.Y.; Yap, C.W.; Vathsala, A.; How, P. Predictors of anemia in a multi-ethnic chronic kidney disease population: A case–control study. SpringerPlus 2015, 4, 233. [Google Scholar] [CrossRef]
- Adera, H.; Hailu, W.; Adane, A.; Tadesse, A. Prevalence Of Anemia And Its Associated Factors Among Chronic Kidney Disease Patients At University Of Gondar Hospital, Northwest Ethiopia: A Hospital-Based Cross Sectional Study. Int. J. Nephrol. Renov. Dis. 2019, 12, 219–228. [Google Scholar] [CrossRef]
- Yin, P.; Wu, Q.; Shou, L.; Dong, X. Risk factors for anemia in patients with chronic kidney disease: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e27371. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 39 (Suppl. S1), S1–S266. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.I.; Guh, J.Y.; Wu, K.D.; Chen, Y.M.; Kuo, M.C.; Hwang, S.J.; Chen, T.H.; Chen, H.C. Modification of diet in renal disease (MDRD) study and CKD epidemiology collaboration (CKD-EPI) equations for Taiwanese adults. PLoS ONE 2014, 9, e99645. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Warren-Gash, C.; Smeeth, L.; Chen, P.C. Data resource profile: The National Health Insurance Research Database (NHIRD). Epidemiol. Health 2018, 40, e2018062. [Google Scholar] [CrossRef]
- Vupputuri, S.; Sandler, D.P. Lifestyle risk factors and chronic kidney disease. Ann. Epidemiol. 2003, 13, 712–720. [Google Scholar] [CrossRef]
- Chang, P.Y.; Li, Y.L.; Chuang, T.W.; Chen, S.Y.; Lin, L.Y.; Lin, Y.F.; Chiou, H.Y. Exposure to ambient air pollutants with kidney function decline in chronic kidney disease patients. Environ. Res. 2022, 215, 114289. [Google Scholar] [CrossRef]
- Jing, J.; Kielstein, J.T.; Schultheiss, U.T.; Sitter, T.; Titze, S.I.; Schaeffner, E.S.; McAdams-DeMarco, M.; Kronenberg, F.; Eckardt, K.U.; Köttgen, A.; et al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: The German Chronic Kidney Disease (GCKD) study. Nephrol. Dial. Transplant. 2014, 30, 613–621. [Google Scholar] [CrossRef]
- Cavalcanti, N.G.; Marques, C.D.L.; Lins e Lins, T.U.; Pereira, M.C.; Rêgo, M.J.B.D.M.; Duarte, A.L.B.P.; Pitta, I.D.R.; Pitta, M.G.D.R. Cytokine Profile in Gout: Inflammation Driven by IL-6 and IL-18? Immunol. Investig. 2016, 45, 383–395. [Google Scholar] [CrossRef]
- Begum, S.; Latunde-Dada, G.O. Anemia of Inflammation with An Emphasis on Chronic Kidney Disease. Nutrients 2019, 11, 2424. [Google Scholar] [CrossRef]
- Silverberg, D.S.; Wexler, D.; Iaina, A.; Steinbruch, S.; Wollman, Y.; Schwartz, D. Anemia, chronic renal disease and congestive heart failure--the cardio renal anemia syndrome: The need for cooperation between cardiologists and nephrologists. Int. Urol. Nephrol. 2006, 38, 295–310. [Google Scholar] [CrossRef]
- Tim Goodnough, L.; Comin-Colet, J.; Leal-Noval, S.; Ozawa, S.; Takere, J.; Henry, D.; Javidroozi, M.; Hohmuth, B.; Bisbe, E.; Gross, I.; et al. Management of anemia in patients with congestive heart failure. Am. J. Hematol. 2017, 92, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Idris, I.; Tohid, H.; Muhammad, N.A.; Rashid, M.R.A.; Ahad, A.M.; Ali, N.; Sharifuddin, N.; Aris, J.H. Anaemia among primary care patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD): A multicentred cross-sectional study. BMJ Open 2018, 8, e025125. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.H.; Kim, G.S.; Kim, Y.J.; Hwang, E.Y.; Park, C.E.; Park, J. The relationship between anemia and pulse pressure and hypertension: The Korea National Health and Nutrition Examination Survey 2010–2012. Clin. Exp. Hypertens. 2018, 40, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Lundby, C.; Ponte, B.; Lundby, A.K.; Robach, P.; de Seigneux, S. Red blood cell volume is not decreased in ESA-naive anemic chronic kidney disease patients. Physiol. Rep. 2018, 6, e13900. [Google Scholar] [CrossRef]
- Jacob, G.; Biaggioni, I.; Mosqueda-Garcia, R.; Robertson, R.M.; Robertson, D. Relation of blood volume and blood pressure in orthostatic intolerance. Am. J. Med. Sci. 1998, 315, 95–100. [Google Scholar]
- Downs, B.W.; Corbier, J.R.; Speight, N.; Kushner, S.; Aloisio, T.; Bagchi, M.; Bagchi, D. Chapter 5—Anemia: Influence of dietary fat, sugar, and salt on hemoglobin and blood health. In Dietary Sugar, Salt and Fat in Human Health; Preuss, H.G., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 103–127. [Google Scholar]
- Chang, J.T.; Liang, Y.J.; Hsu, C.Y.; Chen, C.Y.; Chen, P.J.; Yang, Y.F.; Chen, Y.L.; Pei, D.; Chang, J.B.; Leu, J.G. Glucagon-like peptide receptor agonists attenuate advanced glycation end products-induced inflammation in rat mesangial cells. BMC Pharmacol. Toxicol. 2017, 18, 67. [Google Scholar] [CrossRef]
- Chang, J.-T.; Liang, Y.-J.; Leu, J.-G. Proinflammatory Cytokines Inhibit Expression of Erythropoietin Receptor Messenger Ribonucleic Acid. Acta Nephrol. 2013, 27, 160–165. [Google Scholar]
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; DeFilippi, C.R.; Cleland, J.G.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef]
- Hao, Y.; Li, X.; Zhu, Y.; Ke, J.; Lou, T.; Li, M.; Wang, C. Effect of age and isolated systolic or diastolic hypertension on target organ damage in non-dialysis patients with chronic kidney disease. Aging 2021, 13, 6144–6155. [Google Scholar] [CrossRef]
- Okada, K.; Yanai, M.; Takeuchi, K.; Matsuyama, K.; Nitta, K.; Hayashi, K.; Takahashi, S. Sex differences in the prevalence, progression, and improvement of chronic kidney disease. Kidney Blood Press. Res. 2014, 39, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Melse-Boonstra, A.; Pan, X.; Yuan, B.; Dai, Y.; Zhao, J.; Zimmermann, M.B.; Kok, F.J.; Zhou, M.; Shi, Z. Anemia in relation to body mass index and waist circumference among chinese women. Nutr. J. 2013, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Gillum, R.F. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men--the Third National Health and Nutrition Examination Survey. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2001, 25, 639–645. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Kirwan, J.P.; Arrigain, S.; Schreiber, M.J.; Sarnak, M.J.; Schold, J.D. Obesity, anthropometric measures and chronic kidney disease complications. Am. J. Nephrol. 2012, 36, 219–227. [Google Scholar] [CrossRef]
- Ford, E.S.; Cowie, C.C.; Li, C.; Handelsman, Y.; Bloomgarden, Z.T. Iron-deficiency anemia, non-iron-deficiency anemia and HbA1c among adults in the US. J. Diabetes 2011, 3, 67–73. [Google Scholar] [CrossRef]
- Bindayel, I.A. Influence of iron deficiency anemia on glycated hemoglobin levels in non-diabetic Saudi women. J. Int. Med. Res. 2021, 49, 0300060521990157. [Google Scholar] [CrossRef]
- Choi, H.K.; Ford, E.S. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am. J. Med. 2007, 120, 442–447. [Google Scholar] [CrossRef]
- Su, P.; Hong, L.; Zhao, Y.; Sun, H.; Li, L. The Association Between Hyperuricemia and Hematological Indicators in a Chinese Adult Population. Medicine 2016, 95, e2822. [Google Scholar] [CrossRef]
- Guo, L.P.; Wang, Q.; Pan, Y.; Wang, Y.L.; Zhang, Z.J.; Hu, C.; Ding, F.; Peng, A.; Liu, J.Y. A retrospective cross-sectional study of the associated factors of hyperuricemia in patients with chronic kidney disease. J. Int. Med. Res. 2020, 48, 0300060520919224. [Google Scholar] [CrossRef]
- Kedzierska-Kapuza, K.; Safranow, K.; Niewinski, K.; Niewinski, G.; Durlik, M.; Szczuko, M. Indices of Nutrition Status of Kidney and Pancreas Transplant Candidates. Transpl. Proc 2024, 56, 813–821. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Sabatino, A.; Barazzoni, R.; Carrero, J.J.; Cupisti, A.; De Waele, E.; Jonckheer, J.; Singer, P.; Cuerda, C. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin. Nutr. 2021, 40, 1644–1668. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Cheng, T.Y.D.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Horton, N.J.; Lipsitz, S.R.; Parzen, M. A Potential for Bias When Rounding in Multiple Imputation. Am. Stat. 2003, 57, 229–232. [Google Scholar] [CrossRef]
| Characteristic | Overall | Anemia | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Without (n = 5137) | With (n = 519) | ||||||
| n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | ||
| Age (years) | 62.57 | 14.00 | 62.18 | 14.01 | 66.52 | 13.32 | <0.0001 |
| Sex, % | 0.0001 | ||||||
| Female | 2354 | 41.62 | 2096 | 40.8 | 258 | 49.71 | |
| Male | 3302 | 58.38 | 3041 | 59.2 | 261 | 50.29 | |
| Retire | 3525 | 62.99 | 3153 | 62.02 | 372 | 72.66 | <0.0001 |
| Receive nutrition education | 2939 | 52.17 | 2619 | 51.18 | 320 | 61.9 | <0.0001 |
| Receive low-sodium diet education | 1700 | 30.17 | 1519 | 29.69 | 181 | 35.01 | 0.0138 |
| Receive low phosphorus diet education | 1242 | 22.04 | 1097 | 21.44 | 145 | 28.05 | 0.0007 |
| Receive low-protein diet education | 2032 | 36.07 | 1795 | 35.08 | 237 | 45.84 | <0.0001 |
| Physical examination | |||||||
| Height (cm) | 161.54 | 8.33 | 161.69 | 8.34 | 160.01 | 8.08 | <0.0001 |
| Weight (kg) | 66.10 | 12.95 | 66.52 | 12.99 | 61.90 | 11.69 | <0.0001 |
| Waist (cm) | 87.04 | 10.71 | 87.22 | 10.58 | 85.21 | 11.75 | 0.0012 |
| SBP (mmHg) | 132.30 | 17.24 | 132.39 | 17.32 | 131.39 | 16.44 | 0.2405 |
| DBP (mmHg) | 76.42 | 11.57 | 76.66 | 11.60 | 74.01 | 11.01 | <0.0001 |
| BMI (kg/m2) | 25.27 | 4.14 | 25.38 | 4.14 | 24.13 | 3.91 | <0.0001 |
| Baseline eGFR (ml/min per 1.73 m2) | 46.73 | 26.06 | 48.10 | 25.94 | 33.08 | 23.20 | <0.0001 |
| BUN (mg/dL) | 28.61 | 18.11 | 27.76 | 17.67 | 36.58 | 20.20 | <0.0001 |
| Serum creatinine (mg/dL) | 1.96 | 1.65 | 1.88 | 1.57 | 2.74 | 2.12 | <0.0001 |
| Total cholesterol (mg/dL) | 185.35 | 42.70 | 186.40 | 42.71 | 175.09 | 41.24 | <0.0001 |
| Triglyceride (mg/dL) | 139.16 | 78.77 | 140.54 | 79.27 | 125.68 | 72.39 | <0.0001 |
| Na (Sodium) (mmol/L) | 139.36 | 3.43 | 139.41 | 3.39 | 138.99 | 3.72 | 0.0371 |
| P (Phosphorus) (mg/dL) | 3.87 | 0.88 | 3.85 | 0.88 | 4.00 | 0.82 | 0.0020 |
| Uric acid (mg/dL) | 6.88 | 1.77 | 6.87 | 1.76 | 6.98 | 1.86 | 0.1807 |
| HbA1c (%) | 6.79 | 1.51 | 6.82 | 1.52 | 6.51 | 1.45 | 0.0003 |
| Hemoglobin/Hb (g/dL) | 12.49 | 2.62 | 12.68 | 2.59 | 10.77 | 2.26 | <0.0001 |
| Proteinuria | 0.0106 | ||||||
| None | 1512 | 42.57 | 1391 | 43.08 | 121 | 37.46 | |
| Trace | 437 | 12.3 | 406 | 12.57 | 31 | 9.6 | |
| ≥1+ | 1603 | 45.13 | 1432 | 44.35 | 171 | 52.94 | |
| Health-related behaviors, % | |||||||
| Cigarette smoking | 1434 | 25.71 | 1341 | 26.5 | 93 | 18.02 | <0.0001 |
| Alcohol consumption | 598 | 10.74 | 556 | 11.0 | 42 | 8.16 | 0.0558 |
| Comorbidities, % | |||||||
| Hypertension | 3661 | 64.73 | 3302 | 64.28 | 359 | 69.17 | 0.0296 |
| Diabetes mellitus | 2392 | 42.29 | 2177 | 42.38 | 215 | 41.43 | 0.7097 |
| Dyslipidemia | 1395 | 24.66 | 1270 | 24.72 | 125 | 24.08 | 0.7888 |
| Gout | 997 | 17.63 | 885 | 17.23 | 112 | 21.58 | 0.0156 |
| Ischemic heart disease | 1356 | 23.97 | 1194 | 23.24 | 162 | 31.21 | <0.0001 |
| Stroke | 1000 | 17.68 | 892 | 17.36 | 108 | 20.81 | 0.0574 |
| Congestive heart failure | 476 | 8.42 | 391 | 7.61 | 85 | 16.38 | <0.0001 |
| Urinary Tract Infection | 1024 | 18.1 | 901 | 17.54 | 123 | 23.7 | 0.0006 |
| Depression | 398 | 7.04 | 346 | 6.74 | 52 | 10.02 | 0.0070 |
| Cancer | 942 | 16.65 | 817 | 15.9 | 125 | 24.08 | <0.0001 |
| Characteristic | Anemia | p-Value | ||
|---|---|---|---|---|
| OR | 95%CI | |||
| Receive low-sodium diet education | 0.66 | 0.446 | 0.975 | 0.0368 |
| DBP (mmHg) | 0.98 | 0.965 | 0.999 | 0.0372 |
| Gout | 1.86 | 1.175 | 2.937 | 0.0081 |
| Congestive heart failure | 1.85 | 1.131 | 3.028 | 0.0143 |
| C statistic | 0.78 | |||
| The 10-fold cross-validation accuracy rate | 0.92 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-T.; Lin, C.-J.; Yeh, J.-H.; Tsai, C.-H.; Hsieh, I.-S.; Chang, P.-Y. Sex- and Stage-Specific Predictors of Anemia in Chronic Kidney Disease: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 3088. https://doi.org/10.3390/jcm14093088
Chang J-T, Lin C-J, Yeh J-H, Tsai C-H, Hsieh I-S, Chang P-Y. Sex- and Stage-Specific Predictors of Anemia in Chronic Kidney Disease: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(9):3088. https://doi.org/10.3390/jcm14093088
Chicago/Turabian StyleChang, Jui-Ting, Chun-Ji Lin, Jiann-Horng Yeh, Chin-Hung Tsai, I-Shan Hsieh, and Po-Ya Chang. 2025. "Sex- and Stage-Specific Predictors of Anemia in Chronic Kidney Disease: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 9: 3088. https://doi.org/10.3390/jcm14093088
APA StyleChang, J.-T., Lin, C.-J., Yeh, J.-H., Tsai, C.-H., Hsieh, I.-S., & Chang, P.-Y. (2025). Sex- and Stage-Specific Predictors of Anemia in Chronic Kidney Disease: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(9), 3088. https://doi.org/10.3390/jcm14093088







