Systemic Lupus Erythematosus with Refractory Immune Thrombocytopenia Progressing to Catastrophic Anti-Phospholipid Syndrome During Thrombopoietin Receptor Agonist Therapy: A Case Report
Abstract
1. Introduction
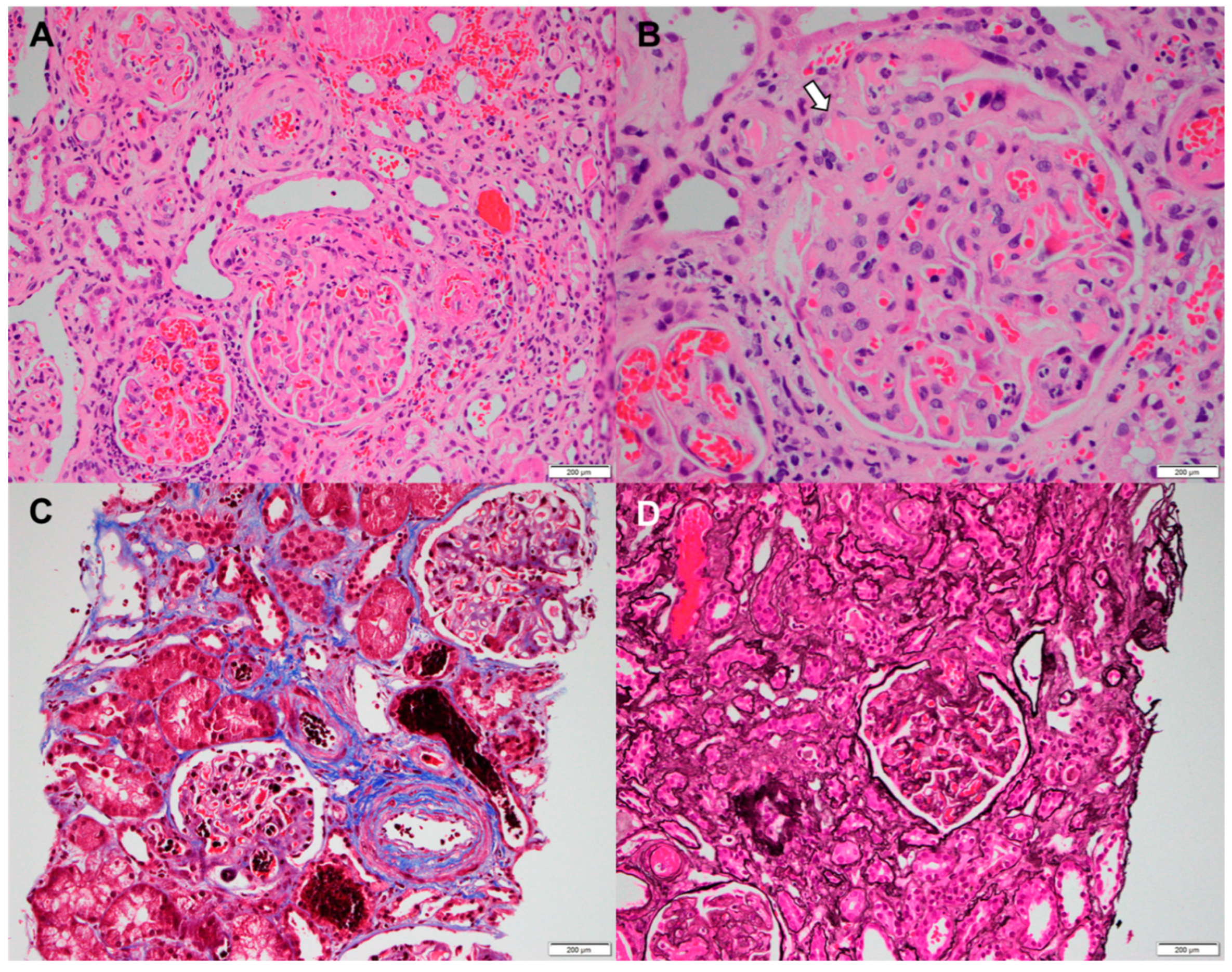
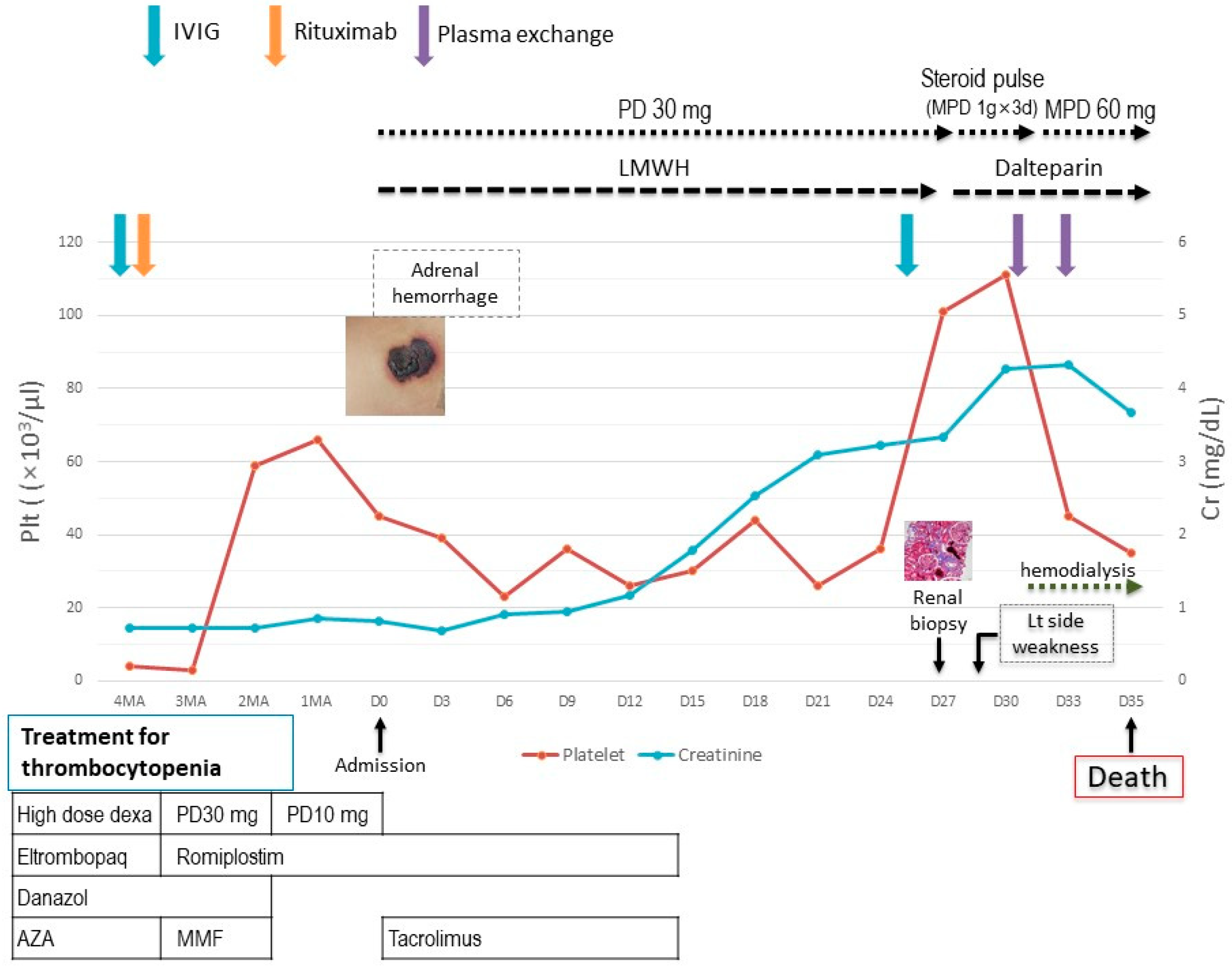
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fayyaz, A.; Igoe, A.; Kurien, B.T.; Danda, D.; James, J.A.; Stafford, H.A.; Scofield, R.H. Haematological manifestations of lupus. Lupus Sci. Med. 2015, 2, e000078. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.M.; Begum, S.; Isenberg, D.A. Prevalence, patterns of disease and outcome in patients with systemic lupus erythematosus who develop severe haematological problems. Rheumatology 2003, 42, 230–234. [Google Scholar] [CrossRef]
- Galanopoulos, N.; Christoforidou, A.; Bezirgiannidou, Z. Lupus thrombocytopenia: Pathogenesis and therapeutic implications. Mediterr. J. Rheumatol. 2017, 28, 20–26. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Tziolos, N.; Bertsia, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, P.; Aboulafia, D.M. Long-term safety and efficacy of romiplostim for treatment of immune thrombocytopenia. J. Blood Med. 2016, 7, 99–106. [Google Scholar] [PubMed]
- Kuter, D.J.; Bussel, J.B.; Newland, A.; Baker, R.I.; Lyons, R.M.; Wasser, J.; Viallard, J.; Macik, G.; Rummel, M.; Nie, K.; et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: Safety and efficacy. Br. J. Haematol. 2013, 161, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Guitton, Z.; Terriou, L.; Lega, J.-C.; Nove-Josserand, R.; Hie, M.; Amoura, Z.; Bussel, J.B.; Hamidou, M.; Rosenthal, E.; Lioger, B.; et al. Risk of thrombosis with anti-phospholipid syndrome in systemic lupus erythematosus treated with thrombopoietin-receptor agonists. Rheumatology 2018, 57, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- LaMoreaux, B.; Barbar-Smiley, F.; Ardoin, S.; Madhoun, H. Two cases of thrombosis in patients with anti-phospholipid antibodies during treatment of immune thrombocytopenia with romiplostim, a thrombopoietin receptor agonist. Semin. Arthritis Rheum. 2016, 45, e10–e12. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Perricone, C.; Ortega-Hernandez, O.-D.; Perricone, R.; Shoenfeld, Y. Immune thrombocytopaenic purpura: An autoimmune cross-link between infections and vaccines. Lupus 2014, 23, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Maroun, M.C.; Ososki, R.; Andersen, J.C.; Dhar, J.P. Eltrombopag as steroid sparing therapy for immune thrombocytopenic purpura in systemic lupus erythematosus. Lupus 2015, 24, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Santos, E.; Cervera, R.; Piette, J.C.; de la Red, G.; Gil, V.; Font, J.; Couch, R.; Ingelmo, M.; Asherson, R.A. Adrenal involvement in the anti-phospholipid syndrome: Clinical and immunologic characteristics of 86 patients. Medicine 2003, 82, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.; Wauters, N.; Lablad, Y.; Morelle, J.; Taghavi, M. Diagnosis and management of catastrophic antiphospholipid syndrome and the potential impact of the 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Antibodies 2024, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Rodríguez-Pintó, I.; Espinosa, G. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: A comprehensive review. J. Autoimmun. 2018, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Tomov, S.; Lazarchick, J.; Self, S.E.; Bruner, E.T.; Budisavljevic, M.N. Kidney-limited thrombotic microangiopathy in patients with SLE treated with romiplostim. Lupus 2013, 22, 504–509. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, S.W.; Ha, Y.-J. Systemic Lupus Erythematosus with Refractory Immune Thrombocytopenia Progressing to Catastrophic Anti-Phospholipid Syndrome During Thrombopoietin Receptor Agonist Therapy: A Case Report. J. Clin. Med. 2025, 14, 3091. https://doi.org/10.3390/jcm14093091
Chung SW, Ha Y-J. Systemic Lupus Erythematosus with Refractory Immune Thrombocytopenia Progressing to Catastrophic Anti-Phospholipid Syndrome During Thrombopoietin Receptor Agonist Therapy: A Case Report. Journal of Clinical Medicine. 2025; 14(9):3091. https://doi.org/10.3390/jcm14093091
Chicago/Turabian StyleChung, Sang Wan, and You-Jung Ha. 2025. "Systemic Lupus Erythematosus with Refractory Immune Thrombocytopenia Progressing to Catastrophic Anti-Phospholipid Syndrome During Thrombopoietin Receptor Agonist Therapy: A Case Report" Journal of Clinical Medicine 14, no. 9: 3091. https://doi.org/10.3390/jcm14093091
APA StyleChung, S. W., & Ha, Y.-J. (2025). Systemic Lupus Erythematosus with Refractory Immune Thrombocytopenia Progressing to Catastrophic Anti-Phospholipid Syndrome During Thrombopoietin Receptor Agonist Therapy: A Case Report. Journal of Clinical Medicine, 14(9), 3091. https://doi.org/10.3390/jcm14093091




