Mortality in Critically Ill Patients with Liberal Versus Restrictive Transfusion Thresholds: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy and Data Extraction
2.3. Study Selection and Inclusion Criteria
- Patients and Setting: We included studies with adult critical care patients diagnosed with anemia. Patients under 18 years of age, pregnant individuals, or those with limitations of therapeutic effort were excluded.
- Interventions: Restrictive transfusion of RBCs with a target hemoglobin between 7 and 9 g/dL.
- Comparison: Liberal transfusion of RBCs with a target hemoglobin greater than 9 g/dL.
- Outcomes: Studies that evaluated mortality at 28 to 30 days as the primary outcome were included. If multiple data points were available within a study, all of the relevant data were considered.
- Study Type: Only randomized clinical trials were included. Exclusions were made for studies with standardized transfusion protocols, such as preoperative optimization in major surgery, orthopedic surgery, or cardiovascular surgery.
2.4. Study Selection and Data Collection Process
2.5. Data Items
2.6. Risk of Bias in Individual Studies
2.7. Statistical Analysis
2.7.1. Analysis of Individual Studies and Summary Measures
2.7.2. Analysis of Risk of Bias Across Studies
2.7.3. Subgroup Analysis and Trial Sequential Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Syntheses of Results
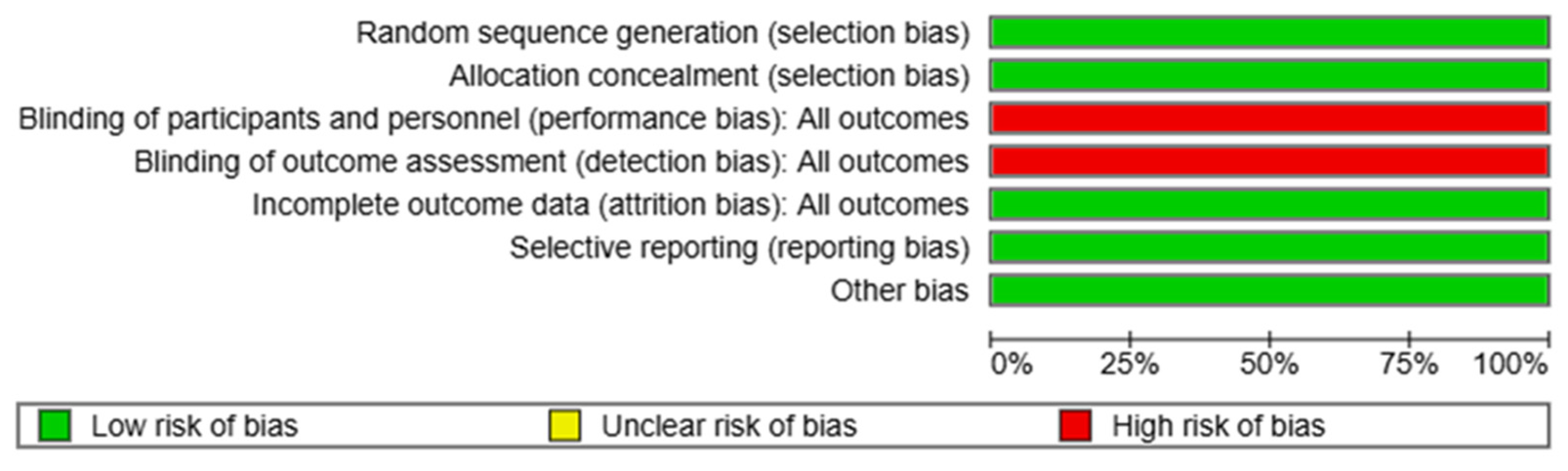
3.4. Risk of Bias
3.5. Additional Analysis
3.5.1. Sensitivity Analysis
3.5.2. Trial Sequential Analysis
3.5.3. Subgroup Analysis
3.5.4. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Warner, M.A.; Hanson, A.C.; Frank, R.D.; Schulte, P.J.; Go, R.S.; Storlie, C.B.; Kor, D.J. Prevalence of and Recovery From Anemia Following Hospitalization for Critical Illness Among Adults. JAMA Netw. Open 2020, 3, e2017843. [Google Scholar] [CrossRef] [PubMed]
- McKenna, H.T.; Murray, A.J.; Martin, D.S. Human adaptation to hypoxia in critical illness. J. Appl. Physiol. 2020, 129, 656–663. [Google Scholar] [CrossRef]
- Lin, I.-H.; Liao, P.-Y.; Wong, L.-T.; Chan, M.-C.; Wu, C.-L.; Chao, W.-C. Anaemia in the first week may be associated with long-term mortality among critically ill patients: Propensity score-based analyses. BMC Emerg. Med. 2023, 23, 32. [Google Scholar] [CrossRef]
- Carson, J.L.; Stanworth, S.J.; Alexander, J.H.; Roubinian, N.; Fergusson, A.D.; Triulzi, D.J.; Goodman, S.G.; Rao, S.V.; Doree, C.; Hebert, P.C. Clinical trials evaluating red blood cell transfusion thresholds: An updated systematic review and with additional focus on patients with cardiovascular disease. Am. Heart J. 2018, 200, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Blet, A.; McNeil, J.B.; Josse, J.; Cholley, B.; Cinotti, R.; Cotter, G.; Dauvergne, A.; Davison, B.; Duarte, K.; Duranteau, J.; et al. Association between in-ICU red blood cells transfusion and 1-year mortality in ICU survivors. Crit. Care 2022, 26, 307. [Google Scholar] [CrossRef]
- Trentino, K.M.; Farmer, S.L.; Swain, S.G.; Burrows, S.A.; Hofmann, A.; Ienco, R.; Pavey, W.; Daly, F.F.; Van Niekerk, A.; Webb, S.A.; et al. Increased hospital costs associated with red blood cell transfusion. Transfusion 2015, 55, 1082–1089. [Google Scholar] [CrossRef]
- Willems, S.A.; Kranenburg, F.J.; Le Cessie, S.; Marang- van de Mheen, P.J.; Kesecioglu, J.; van der Bom, J.G.; Arbous, M.S. Variation in red cell transfusion practice in the intensive care unit—An international survey. J. Crit. Care 2020, 55, 140–144. [Google Scholar] [CrossRef]
- Vlaar, A.P.; Oczkowski, S.; de Bruin, S.; Wijnberge, M.; Antonelli, M.; Aubron, C.; Aries, P.; Duranteau, J.; Juffermans, N.P.; Meier, J.; et al. Transfusion strategies in non-bleeding critically ill adults: A clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2020, 46, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, Y.; Yu, K.; Gu, J. Liberal Transfusion versus Restrictive Transfusion and Outcomes in Critically Ill Adults: A Meta-Analysis. Transfus. Med. Hemotherapy 2020, 48, 60–68. [Google Scholar] [CrossRef]
- Guglielmi, A.; Graziano, F.; Bogossian, E.G.; Turgeon, A.F.; Taccone, F.S.; Citerio, G.; The CENTER-TBI Participants and Investigators. Haemoglobin values, transfusion practices, and long-term outcomes in critically ill patients with traumatic brain injury: A secondary analysis of CENTER-TBI. Crit. Care 2024, 28, 199. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Hébert, P.C.; Wells, G.; Marshall, J.; Martin, C.; Tweeddale, M.; Pagliarello, G.; Blajchman, M. Transfusion Requirements in Critical Care: A Pilot Study. JAMA 1995, 273, 1439–1444. [Google Scholar] [CrossRef]
- Hébert, P.C.; Wells, G.; Blajchman, M.A.; Marshall, J.; Martin, C.; Pagliarello, G.; Tweeddale, M.; Schweitzer, I.; Yetisir, E. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. N. Engl. J. Med. 2024, 340, 409–417. [Google Scholar] [CrossRef]
- Hébert, P.; Yetisir, E.; Martin, C.; Blajchman, M.; Wells, G.; Marshall, J.; Tweeddale, M.; Pagliarello, G.; Schweitzer, I. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit. Care Med. 2001, 29, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, F.S.; Almeida, J.P.; Landoni, G.; Galas, F.R.B.G.; Fukushima, J.T.; Fominskiy, E.; Park, C.H.L.; Osawa, E.A.; Diz, M.P.E.; Oliveira, G.Q.; et al. Liberal Versus Restrictive Transfusion Strategy in Critically Ill Oncologic Patients: The Transfusion Requirements in Critically Ill Oncologic Patients Randomized Controlled Trial*. Crit. Care Med. 2017, 45, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Brooks, M.M.; Abbott, J.D.; Chaitman, B.; Kelsey, S.F.; Triulzi, D.J.; Srinivas, V.; Menegus, M.A.; Marroquin, O.C.; Rao, S.V.; et al. Liberal Versus Restrictive Transfusion Thresholds for Patients with Symptomatic Coronary Artery Disease. Am. Heart J. 2013, 165, 964–971.e1. [Google Scholar] [CrossRef]
- Walsh, T.S.; Boyd, J.A.; Watson, D.; Hope, D.; Lewis, S.; Krishan, A.; Forbes, J.F.; Ramsay, P.; Pearse, R.; Wallis, C.; et al. Restrictive Versus Liberal Transfusion Strategies for Older Mechanically Ventilated Critically Ill Patients. Crit. Care Med. 2013, 41, 2354–2363. [Google Scholar] [CrossRef]
- Holst, L.B.; Haase, N.; Wetterslev, J.; Wernerman, J.; Guttormsen, A.B.; Karlsson, S.; Johansson, P.I.; Åneman, A.; Vang, M.L.; Winding, R.; et al. Lower versus Higher Hemoglobin Threshold for Transfusion in Septic Shock. N. Engl. J. Med. 2014, 371, 1381–1391. [Google Scholar] [CrossRef]
- Mazer, C.D.; Whitlock, R.P.; Fergusson, D.A.; Hall, J.; Belley-Cote, E.; Connolly, K.; Khanykin, B.; Gregory, A.J.; de Médicis, É.; McGuinness, S.; et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N. Engl. J. Med. 2024, 377, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, T.L.; Holmes, J.H., 4th; Arnoldo, B.; Peck, M.; Potenza, B.; Cochran, A.; King, B.T.; Dominic, W.; Cartotto, R.; Bhavsar, D.; et al. Transfusion Requirement in Burn Care Evaluation (TRIBE): A Multicenter Randomized Prospective Trial of Blood Transfusion in Major Burn Injury. Ann. Surg. 2017, 266, 595–602. [Google Scholar] [CrossRef]
- Ducrocq, G.; Gonzalez-Juanatey, J.R.; Puymirat, E.; Lemesle, G.; Cachanado, M.; Durand-Zaleski, I.; Arnaiz, J.A.; Martínez-Sellés, M.; Silvain, J.; Ariza-Solé, A.; et al. Effect of a Restrictive vs Liberal Blood Transfusion Strategy on Major Cardiovascular Events Among Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Clinical Trial. JAMA 2021, 325, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, A.F.; Fergusson, D.A.; Clayton, L.; Patton, M.P.; Neveu, X.; Walsh, T.S.; Docherty, A.; Malbouisson, L.M.; Pili-Floury, S.; English, S.W.; et al. Liberal or Restrictive Transfusion Strategy in Patients with Traumatic Brain Injury. N. Engl. J. Med. 2024, 391, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Brooks, M.M.; Hébert, P.C.; Goodman, S.G.; Bertolet, M.; Glynn, S.A.; Chaitman, B.R.; Simon, T.; Lopes, R.D.; Goldsweig, A.M.; et al. Restrictive or Liberal Transfusion Strategy in Myocardial Infarction and Anemia. N. Engl. J. Med. 2023, 389, 2446–2456. [Google Scholar] [CrossRef]
- Taccone, F.S.; Rynkowski Bittencourt, C.; Møller, K.; Lormans, P.; Quintana-Díaz, M.; Caricato, A.; Cardoso Ferreira, M.A.; Badenes, R.; Kurtz, P.; Søndergaard, C.B.; et al. Restrictive vs Liberal Transfusion Strategy in Patients with Acute Brain Injury: The TRAIN Randomized Clinical Trial. JAMA 2024, 332, 1623–1633. [Google Scholar] [CrossRef]
- Siggaard-Andersen, O.; Fogh-Andersen, N.; Gothgen, I.H.; Larsen, V.H. Oxygen status of arterial and mixed venous blood. Crit. Care Med. 1995, 23, 1284–1293. [Google Scholar] [CrossRef]
- Shoemaker, W.C.; Appel, P.L.; Kram, H.B. Role of Oxygen Debt in the Development of Organ Failure Sepsis, and Death in High-Risk Surgical Patients. Chest 1992, 102, 208–215. [Google Scholar] [CrossRef]
- Perez-Garzon, M.; Poveda-Henao, C.; Bastidas-Goyes, A.; Robayo-Amortegui, H. Oxygen Debt as Predictor of Mortality and Multiple Organ Dysfunction Syndrome in Severe COVID-19 Patients: A Retrospective Study. J. Intensive Care Med. 2023, 39, 358–367. [Google Scholar] [CrossRef]
- Bunn, H. Oxygen Delivery in the Treatment of Anemia. N. Engl. J. Med. 2022, 387, 2362–2365. [Google Scholar] [CrossRef]
- Trentino, K.M.; Farmer, S.L.; Leahy, M.F.; Sanfilippo, F.M.; Isbister, J.P.; Mayberry, R.; Hofmann, A.; Shander, A.; French, C.; Murray, K. Systematic reviews and meta-analyses comparing mortality in restrictive and liberal haemoglobin thresholds for red cell transfusion: An overview of systematic reviews. BMC Med. 2020, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Braïk, R.; Jebali, S.; Blot, P.-L.; Egbeola, J.; James, A.; Constantin, J.-M. Liberal versus restrictive transfusion strategies in acute myocardial infarction: A systematic review and comparative frequentist and Bayesian meta-analysis of randomized controlled trials. Ann. Intensive Care 2024, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Hussein, G.; Liu, B.; Vojjala, N.; Warsame, M.; El Labban, M.; Rauf, I.; Hassan, M.; Zareen, T.; Usama, S.M.; et al. A Contemporary Review of Blood Transfusion in Critically Ill Patients. Medicina 2024, 60, 1247. [Google Scholar] [CrossRef] [PubMed]
- De Cassai, A.; Tassone, M.; Geraldini, F.; Sergi, M.; Sella, N.; Boscolo, A.; Munari, M. Explanation of trial sequential analysis: Using a post-hoc analysis of meta-analyses published in Korean Journal of Anesthesiology. Korean J. Anesthesiol. 2021, 74, 383–393. [Google Scholar] [CrossRef]
- Riberholt, C.G.; Olsen, M.H.; Milan, J.B.; Hafliðadóttir, S.H.; Svanholm, J.H.; Pedersen, E.B.; Lew, C.C.H.; Asante, M.A.; Ribeiro, J.P.; Wagner, V.; et al. Major mistakes or errors in the use of trial sequential analysis in systematic reviews or meta-analyses—The METSA systematic review. BMC Med. Res. Methodol. 2024, 24, 196. [Google Scholar] [CrossRef]
- Claire, R.; Gluud, C.; Berlin, I.; Coleman, T.; Leonardi-Bee, J. Using Trial Sequential Analysis for estimating the sample sizes of further trials: Example using smoking cessation intervention. BMC Med. Res. Methodol. 2020, 20, 284. [Google Scholar] [CrossRef]



| Study | Year | Sample Size | Age in Years, Mean ± SD, Median (IQR) | Sex, Male/Female | Population | Restrictive Thresholds | n= | Liberal Thresholds | n= | 30-Day Mortality (Restrictive/Liberal) no (%). |
|---|---|---|---|---|---|---|---|---|---|---|
| Hébert et al. [14] | 1995 | 69 | 58.6 ± 15 | 33/36 | Medical ICU | 7–9 g/dL | 33 | 10–12 g/dL | 36 | 8 (24%)/9 (25%) |
| Hébert et al. [15] | 1999 | 838 | 57.1 ± 18.1 | 524/314 | Medical ICU | 7–9 g/dL | 418 | 10–12 g/dL | 420 | 78 (18.7%)/98 (23%) |
| Hebert et al. [16] | 2001 | 357 | 64.0 ± 14.1 | 216/141 | Cardiovascular | 7–9 g/dL | 160 | 10–12 g/dL | 197 | 36 (23%)/45 (23%) |
| Bergamin et al. [17] | 2017 | 300 | 61.6 ± 12.9 | 154/146 | Septic shock | <7 g/dL | 151 | <9 g/dL | 149 | 67 (45%)/84 (55.6%) |
| Carson et al. [18] | 2013 | 110 | 70.8 ± 12.8 | 55/55 | Cardiovascular | <8 g/dL | 55 | <10 g/dL | 55 | 7 (13%)/1 (1.8%) |
| Walsh et al. [19] | 2013 | 100 | 67 ± 7 | 60/40 | Medical ICU | 7–9 g/dL | 51 | 9–11 g/dL | 49 | 12 (23.5%)/16 (32.7%) |
| Holst et al. [20] | 2014 | 998 | 67 (57–73) | 531/467 | Septic shock | ≤7 g/dL | 502 | <9 g/dL | 436 | 168 (31.6%)/175 (34.8%) |
| Mazer et al. [21] | 2017 | 4856 | 72 ± 10 | 3139/1717 | Cardiovascular | ≤7.5 g/dL | 2427 | ≤9.5 g/dL | 2429 | 74 (3%)/87 (3.6%) |
| Palmieri et al. [22] | 2017 | 345 | 41 (30–55) | 273/72 | Burns | 7 g/dL | 168 | ≥10 g/dL | 177 | 16 (9.5%)/15 (8.5%) |
| Ducrocq et al. [23] | 2021 | 666 | 78 (69–85) | 201/184 | Cardiovascular | 8–10 g/dL | 342 | >11 g/dL | 324 | 19 (5.6%)/25 (7.7%) |
| Turgeon et al. [24] | 2024 | 736 | 48.9 ± 18.8 | 937/201 | Acute brain injury | ≤7 g/dL | 371 | <10 g/dL | 371 | NA |
| Carson et al. [25] | 2020 | 3504 | 72.1 ± 11.6 | 1137/2367 | Cardiovascular | 7–8 g/dL | 1749 | 9–10 g/dL | 1755 | 173 (9.9%)/146 (8.3%) |
| Taccone et al. [26] | 2024 | 820 | 52 ± 16 | 436/384 | Acute brain injury | <7 g/dL | 423 | <9 g/dL | 397 | 82 (20.7%)/94 (22.5%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez Franco, D.A.; Pérez Velásquez, C.A.; Rodríguez Lima, D.R. Mortality in Critically Ill Patients with Liberal Versus Restrictive Transfusion Thresholds: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. J. Clin. Med. 2025, 14, 2049. https://doi.org/10.3390/jcm14062049
Jiménez Franco DA, Pérez Velásquez CA, Rodríguez Lima DR. Mortality in Critically Ill Patients with Liberal Versus Restrictive Transfusion Thresholds: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. Journal of Clinical Medicine. 2025; 14(6):2049. https://doi.org/10.3390/jcm14062049
Chicago/Turabian StyleJiménez Franco, Daniel Arturo, Camilo Andrés Pérez Velásquez, and David Rene Rodríguez Lima. 2025. "Mortality in Critically Ill Patients with Liberal Versus Restrictive Transfusion Thresholds: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis" Journal of Clinical Medicine 14, no. 6: 2049. https://doi.org/10.3390/jcm14062049
APA StyleJiménez Franco, D. A., Pérez Velásquez, C. A., & Rodríguez Lima, D. R. (2025). Mortality in Critically Ill Patients with Liberal Versus Restrictive Transfusion Thresholds: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. Journal of Clinical Medicine, 14(6), 2049. https://doi.org/10.3390/jcm14062049








