Risk Factors and Prevention of Cancer and CVDs: A Chicken and Egg Situation
Abstract
1. Introduction
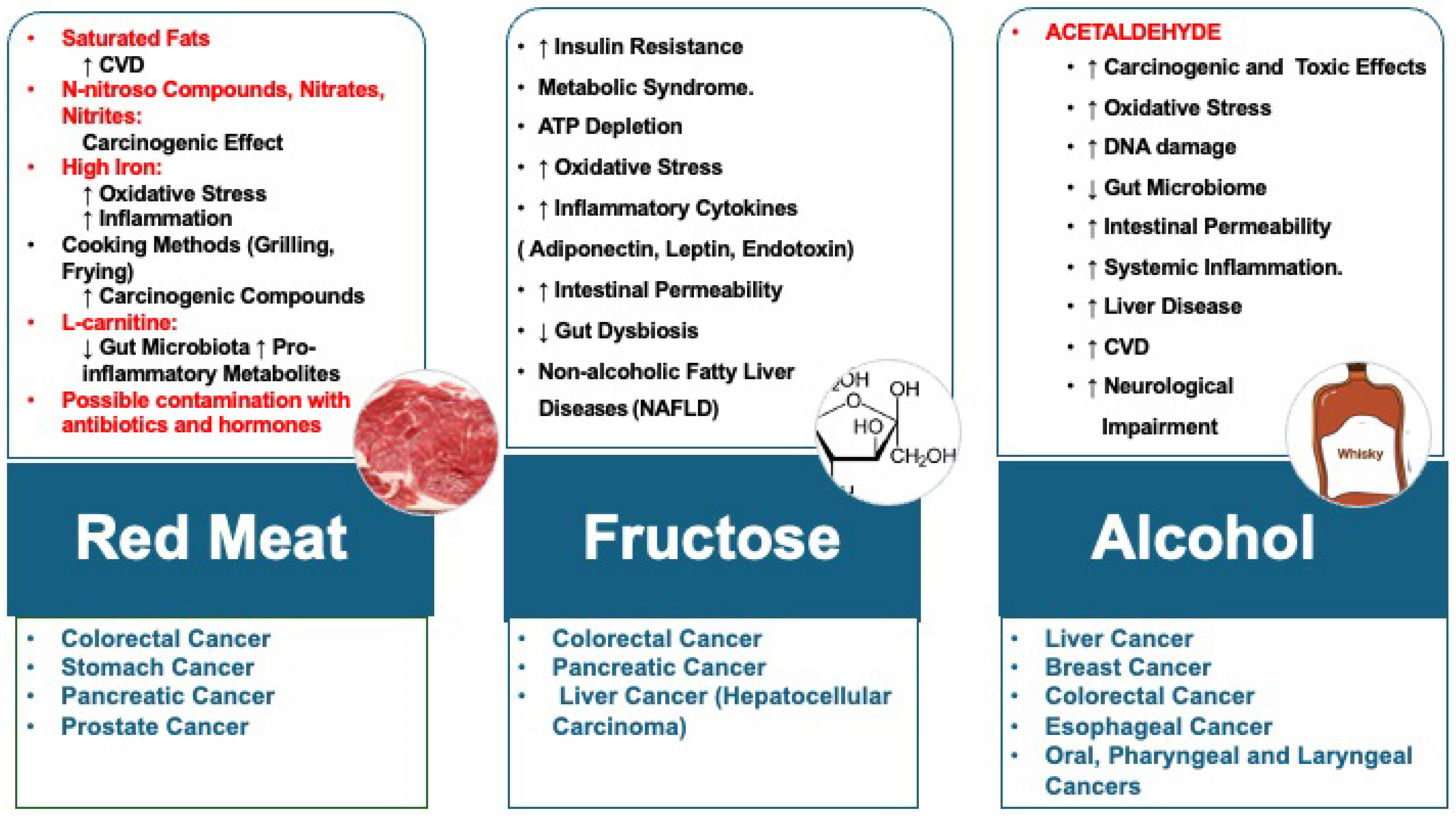
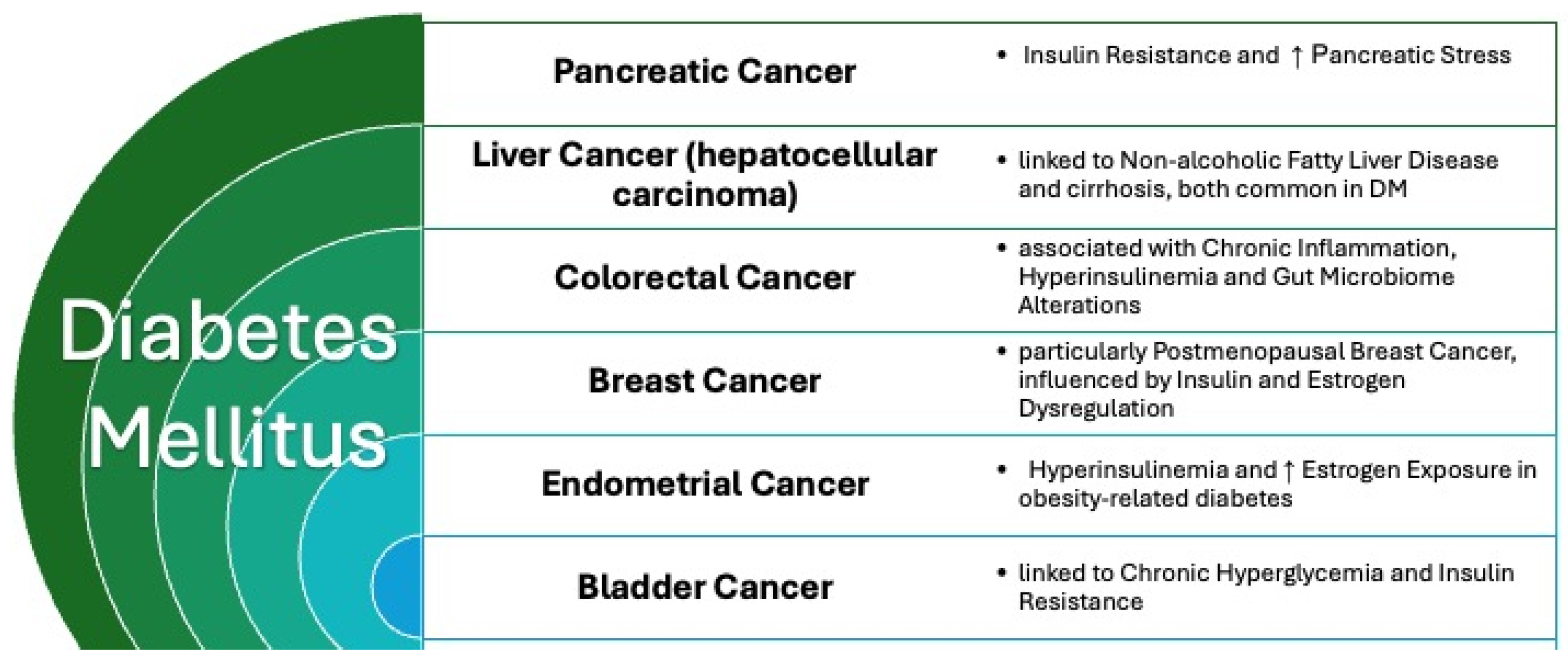
2. CV Risk Factors and Cancer
3. Tumors in Patients with CVD
4. Primary Prevention of Malignancies
5. Potential Preventive Role of Pharmacological Correction of CV Risk Factors in Neoplastic Diseases
6. Lifestyle, Risk Factor Correction, and Prevention of CV Complications and Tumor Recidivism in Neoplastic Patients
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tarantini, L.; Gulizia, M.M.; Di Lenarda, A.; Maurea, N.; Abrignani, M.G.; Bisceglia, I.; Bovelli, D.; De Gennaro, L.; Del Sindaco, D.; Macera, F.; et al. ANMCO/AIOM/AICO Consensus Document on clinical and management pathways of cardio-oncology: Executive summary. Eur. Heart J. Suppl. 2017, 19, D370–D379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ReFaey, K.; Tripathi, S.; Grewal, S.S.; Bhargav, A.G.; Quinones, D.J.; Chaichana, K.L.; Antwi, S.O.; Cooper, L.T.; Meyer, F.B.; Dronca, R.S.; et al. Cancer Mortality Rates Increasing vs Cardiovascular Disease Mortality Decreasing in the World: Future Implications. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.F.; Lei, X.; Haas, A.; Baylis, R.A.; Gao, H.; Luo, L.; Giordano, S.H.; Wehner, M.R.; Nead, K.T.; Leeper, N.J. Risk of Cancer After Diagnosis of Cardiovascular Disease. JACC CardioOncol. 2023, 5, 431–440. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Newman, A.A.; Dalman, J.M.; Moore, K.J. Cardiovascular Disease and Cancer: A Dangerous Liaison. Arter. Thromb. Vasc. Biol. 2025, 45, 359–371. [Google Scholar] [CrossRef]
- Bai, T.; Wu, C. Association of cardiovascular disease on cancer: Observational and mendelian randomization analyses. Sci. Rep. 2024, 14, 28465. [Google Scholar] [CrossRef]
- Makram, O.M.; Okwuosa, T.; Addison, D.; Cortes, J.; Dent, S.; Bevel, M.; Ganatra, S.; Al-Kindi, S.; Hedrick, C.C.; Weintraub, N.L.; et al. Cardiovascular Diseases Increase Cancer Mortality in Adults: NHANES-Continuous Study. J. Am. Heart Assoc. 2024, 13, e035500. [Google Scholar] [CrossRef]
- Daly, A.A.; Rolph, R.; Cutress, R.I.; Copson, E.R. A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer. Breast Cancer Targets Ther. 2021, 13, 241–257. [Google Scholar] [CrossRef]
- Hu, J.; Dong, H.; Dong, Y.; Zhou, R.; Teixeira, W.; He, X.; Ye, D.-W.; Ti, G. Cancer burden attributable to risk factors, 1990–2019: A comparative risk assessment. iScience 2024, 27, 109430. [Google Scholar] [CrossRef]
- Yu, S.; Cai, X.; Wang, X.; Lin, X.; Cai, S. Disease burden of breast cancer and risk factors in Europe 44 countries, 1990-2019: Findings of the global burden of disease study 2019. Front. Endocrinol. 2024, 15, 1405204. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zheng, L.; Fang, K.; Zheng, Y.; Wu, J.; Zheng, M. Proportion of liver cancer cases and deaths attributable to potentially modifiable risk factors in China. Int. J. Epidemiol. 2023, 52, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, D.N.; Miles, R.C.; Guevara, A.E.; Flores, E.J.; Narayan, A.K. Prevalence of Modifiable Breast Cancer Risk Factors and Potential Opportunities for Primary Prevention Among Women Engaged in Screening Mammography: National Health Interview Survey Results. J. Breast Imaging 2023, 5, 538–545. [Google Scholar] [CrossRef]
- Tybjerg, A.J.; Friis, S.; Brown, K.; Nilbert, M.C.; Morch, L.; Køster, B. Updated fraction of cancer attributable to lifestyle and environmental factors in Denmark in 2018. Sci. Rep. 2022, 12, 549. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, H.; Liu, J.; Zhang, Y.; Zhou, T.; Yang, Y.; Fang, W.; Huang, Y.; Zhang, L. A modifiable risk factors atlas of lung cancer: A Mendelian randomization study. Cancer Med. 2021, 10, 4587–4603. [Google Scholar] [CrossRef]
- Lau, E.S.; Paniagua, S.M.; Liu, E.; Jovani, M.; Li, S.X.; Takvorian, K.; Suthahar, N.; Cheng, S.; Splansky, G.L.; Januzzi, J.L.; et al. Cardiovascular Risk Factors Are Associated With Future Cancer. JACC CardioOncol. 2021, 3, 48–58. [Google Scholar] [CrossRef]
- Qi, H.; Xia, D.; Xu, X. Dietary glycemic index, glycemic load, and renal cancer risk: Findings from prostate, lung, colorectal, and ovarian cancer trial. Front. Nutr. 2023, 10, 1073373. [Google Scholar] [CrossRef]
- Ke, J.; Lin, T.; Liu, X.; Wu, K.; Ruan, X.; Ding, Y.; Liu, W.; Qiu, H.; Tan, X.; Wang, X.; et al. Glucose Intolerance and Cancer Risk: A Community-Based Prospective Cohort Study in Shanghai, China. Front. Oncol. 2021, 11, 726672. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and added sugar intakes, sugar types, and cancer risk: Results from the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279. [Google Scholar] [CrossRef]
- McRobbie, H.; Kwan, B. Tobacco use disorder and the lungs. Addiction 2021, 116, 2559–2571. [Google Scholar] [CrossRef]
- Sharma, R.; Rakshit, B. Global burden of cancers attributable to tobacco smoking, 1990–2019: An ecological study. EPMA J. 2023, 14, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, N.; Wang, M.; Liu, Y.; Ma, L.; Wang, Q.; Yang, Q.; Liu, X.; Zhou, F.; Wei, Y. Distributions and Trends of the Global Burden of Colorectal Cancer Attributable to Dietary Risk Factors over the Past 30 Years. Nutrients 2023, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, A.; Yang, S.; Fang, H.; Wang, Q.; Li, H.; Liu, S.; Liu, A. The Burden of Gastric Cancer Attributable to High Sodium Intake: A Longitudinal Study from 1990 to 2019 in China. Nutrients 2023, 15, 5088. [Google Scholar] [CrossRef] [PubMed]
- Selinger, E.; Rylander, C.; Skeie, G. Dietary patterns in relation to incidence rate of pancreatic cancer—The Norwegian women and cancer cohort study. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef]
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16, 800. [Google Scholar] [CrossRef]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef]
- Aveta, A.; Cacciapuoti, C.; Barone, B.; Di Zazzo, E.; Del Giudice, F.; Maggi, M.; Ferro, M.; Terracciano, D.; Busetto, G.M.; Lucarelli, G.; et al. The Impact of Meat Intake on Bladder Cancer Incidence: Is It Really a Relevant Risk? Cancers 2022, 14, 4775. [Google Scholar] [CrossRef]
- Ko, K.-P. Risk Factors of Gastric Cancer and Lifestyle Modification for Prevention. J. Gastric Cancer 2024, 24, 99–107. [Google Scholar] [CrossRef]
- Turner, N.D.; Lloyd, S.K. Association between red meat consumption and colon cancer: A systematic review of experimental results. Exp. Biol. Med. 2017, 242, 813–839. [Google Scholar] [CrossRef]
- Tan, J.; Chen, Y.X. Dietary and Lifestyle Factors Associated with Colorectal Cancer Risk and Interactions with Microbiota: Fiber, Red or Processed Meat and Alcoholic Drinks. Gastrointest Tumors 2016, 3, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zmysłowski, A.; Szterk, A. Current knowledge on the mechanism of atherosclerosis and pro-atherosclerotic properties of oxysterols. Lipids Health Dis. 2017, 16, 188. [Google Scholar] [CrossRef] [PubMed]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Abrignani, V.; Salvo, A.; Pacinella, G.; Tuttolomondo, A. The Mediterranean Diet, Its Microbiome Connections, and Cardiovascular Health: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4942. [Google Scholar] [CrossRef]
- Rehm, J.; Shield, K.D.; Weiderpass, E. Alcohol consumption. A leading risk factor for cancer. Chem. Biol. Interact. 2020, 331, 109280. [Google Scholar] [CrossRef]
- Cai, X.; Li, X.; Liang, C.; Zhang, M.; Dong, Z.; Yu, W. The effect of metabolism-related lifestyle and clinical risk factors on digestive system cancers in East Asian populations: A two-sample Mendelian randomization analysis. Sci. Rep. 2024, 14, 9474. [Google Scholar] [CrossRef]
- Jun, S.; Park, H.; Kim, U.-J.; Lee, H.A.; Park, B.; Lee, S.Y.; Jee, S.H.; Park, H. The Combined Effects of Alcohol Consumption and Smoking on Cancer Risk by Exposure Level: A Systematic Review and Meta-analysis. J. Korean Med. Sci. 2024, 39, e185. [Google Scholar] [CrossRef]
- Mansour, R.; Al-Ani, A.; Al-Hussaini, M.; Abdel-Razeq, H.; Al-Ibraheem, A.; Mansour, A.H. Modifiable risk factors for cancer in the middle East and North Africa: A scoping review. BMC Public Health 2024, 24, 223. [Google Scholar] [CrossRef]
- Chantaprasopsuk, S.; Rees-Punia, E.; Patel, A.V. Physical activity, obesity, and bladder cancer incidence. Cancer Causes Control. 2023, 34, 715–724. [Google Scholar] [CrossRef]
- Shinoda, S.; Nakamura, N.; Roach, B.; Bernlohr, D.A.; Ikramuddin, S.; Yamamoto, M. Obesity and Pancreatic Cancer: Recent Progress in Epidemiology, Mechanisms and Bariatric Surgery. Biomedicines 2022, 10, 1284. [Google Scholar] [CrossRef]
- Papavasileiou, G.; Tsilingiris, D.; Spyrou, N.; Vallianou, N.G.; Karampela, I.; Magkos, F.; Dalamaga, M. Obesity and main urologic cancers: Current systematic evidence, novel biological mechanisms, perspectives and challenges. Semin. Cancer Biol. 2023, 91, 70–98. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Maiello, M.; Cecere, A.; Ciccone, M.M.; Palmiero, P. Metabolic syndrome and breast cancer: A dangerous association for postmenopausal women. Acta Biomed. 2021, 92, e2021177. [Google Scholar]
- Lampimukhi, M.; Qassim, T.; Venu, R.; Pakhala, N.; Mylavarapu, S.; Perera, T.; Sathar, B.S.; Nair, A. A Review of Incidence and Related Risk Factors in the Development of Hepatocellular Carcinoma. Cureus 2023, 15, e49429. [Google Scholar] [CrossRef] [PubMed]
- Staley, S.A.; Tucker, K.R.; Clark, L.H. The Role of Obesity in the Development and Management of Gynecologic Cancer. Obstet. Gynecol. Surv. 2020, 75, 308–316. [Google Scholar] [CrossRef]
- Liu, E.E.; Suthahar, N.; Paniagua, S.M.; Wang, D.; Lau, E.S.; Li, S.X.; Jovani, M.; Takvorian, K.S.; Kreger, B.E.; Benjamin, E.J.; et al. Association of Cardiometabolic Disease With Cancer in the Community. JACC CardioOncol. 2022, 4, 69–81. [Google Scholar] [CrossRef]
- Chen, J.; Terry, M.B.; Dalerba, P.; Hur, C.; Hu, J.; Yang, W. Environmental drivers of the rising incidence of early-onset colorectal cancer in the United States. Int. J. Cancer 2024, 154, 1930–1939. [Google Scholar] [CrossRef]
- Hazelwood, E.; Sanderson, E.; Tan, V.Y.; Ruth, K.S.; Frayling, T.M.; Dimou, N.; Gunter, M.J.; Dossus, L.; Newton, C.; Ryan, N.; et al. Identifying molecular mediators of the relationship between body mass index and endometrial cancer risk: A Mendelian randomization analysis. BMC Med. 2022, 20, 125. [Google Scholar] [CrossRef]
- Loosen, S.H.; Roderburg, C.; Jördens, M.S.; Fluegen, G.; Luedde, T.; Kostev, K. Overweight and Obesity Determine the Risk for Gastrointestinal Cancer in a Sex-Dependent Manner: A Retrospective Cohort Study of 287,357 Outpatients in Germany. Cancers 2022, 14, 931. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Vallianou, N.G.; Spyrou, N.; Kounatidis, D.; Christodoulatos, G.S.; Karampela, I.; Dalamaga, M. Obesity and Leukemia: Biological Mechanisms, Perspectives, and Challenges. Curr. Obes. Rep. 2024, 13, 1–34. [Google Scholar] [CrossRef]
- Li, Z.; Shen, G.; Shi, M.; Zheng, Y.; Guan, Y.; Xin, Y.; Wang, M.; Zhao, F.; Ren, D.; Zhao, J. Association between high body mass index and prognosis of patients with early-stage breast cancer: A systematic review and meta-analysis. Cancer Pathog. Ther. 2023, 1, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kotara, K.; Dakowska, D. Impact of obesity on risk of cancer. Cent. Eur. J. Public Health 2021, 29, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.F.; Garcia, C.; Jacox, J.B.; Lawres, L.; Muzumdar, M.D. Decoding the obesity–cancer connection: Lessons from preclinical models of pancreatic adenocarcinoma. Life Sci. Alliance 2023, 6, e202302228. [Google Scholar] [CrossRef]
- Capuozzo, M.; Celotto, V.; Landi, L.; Ferrara, F.; Sabbatino, F.; Perri, F.; Cascella, M.; Granata, V.; Santorsola, M.; Ottaiano, A. Beyond Body Size: Adiponectin as a Key Player in Obesity-Driven Cancers. Nutr. Cancer 2023, 75, 1848–1862. [Google Scholar] [CrossRef]
- Bitzur, R.; Brenner, R.; Maor, E.; Antebi, M.; Ziv-Baran, T.; Segev, S.; Sidi, Y.; Kivity, S. Metabolic syndrome, obesity, and the risk of cancer development. Eur. J. Intern. Med. 2016, 34, 89–93. [Google Scholar] [CrossRef]
- Grundy, S.M. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation 2002, 105, 2696–2698. [Google Scholar] [CrossRef]
- Zhan, Z.Q.; Chen, Y.Z.; Huang, Z.M.; Luo, Y.H.; Zeng, J.J.; Wang, Y.; Tan, J.; Chen, Y.X.; Fang, J.Y. Metabolic syndrome, its components, and gastrointestinal cancer risk: A meta-analysis of 31 prospective cohorts and Mendelian randomization study. J. Gastroenterol. Hepatol. 2024, 39, 630–641. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef]
- Ta, H.D.K.; Nguyen, N.N.; Ho, D.K.N.; Nguyen, H.D.; Ni, Y.-C.; Yee, K.X.; Pan, S.-R.; Nguyen, H.S.; Phuoc, T.T.H.; Chen, M.-J.; et al. Association of diabetes mellitus with early-onset colorectal cancer: A systematic review and meta-analysis of 19 studies including 10 million individuals and 30,000 events. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102828. [Google Scholar] [CrossRef]
- Yue, Y.; Hur, J.; Cao, Y.; Tabung, F.; Wang, M.; Wu, K.; Song, M.; Zhang, X.; Liu, Y.; Meyerhardt, J.; et al. Prospective evaluation of dietary and lifestyle pattern indices with risk of colorectal cancer in a cohort of younger women. Ann. Oncol. 2021, 32, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B.; Cohen, R.A.; Williams, C.R.; Lu, X.; Sutliff, R.L.; Hart, C.M.; Leo, C.H.; Joshi, A.; Woodman, O.L.; Bell, T.D.; et al. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am. J. Physiol. Circ. Physiol. 1992, 263 Pt 2, H321–H326. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, S.; Zhang, Y.; Ji, Y.; Wu, J.; Duan, H.; Liu, X.; Li, J.; Zhang, Y.; Lyu, Z.; et al. Severe obesity, high inflammation, insulin resistance with risks of all-cause mortality and all-site cancers, and potential modification by healthy lifestyles. Sci. Rep. 2025, 15, 1472. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study. Int. J. Cancer 2017, 141, 102–111. [Google Scholar] [CrossRef]
- Zhang, A.M.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021, 45, 285–311. [Google Scholar] [CrossRef]
- Grossman, E.; Messerli, F.H.; Boyko, V.; Goldbourt, U. Is there an association between hypertension and cancer mortality? Am. J. Med. 2002, 112, 479–486. [Google Scholar] [CrossRef]
- Kang, Y.S.; Park, Y.G.; Kim, B.K.; Han, S.Y.; Jee, Y.H.; Han, K.H.; Lee, M.H.; Song, H.K.; Cha, D.R.; Kang, S.W.; et al. Angiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytes. J. Mol. Endocrinol. 2006, 36, 377–388. [Google Scholar] [CrossRef][Green Version]
- Chan, I.I.; Kwok, M.K.; Schooling, C.M. Blood pressure and risk of cancer: A Mendelian randomization study. BMC Cancer 2021, 21, 1338. [Google Scholar] [CrossRef]
- Warner, M.; Gustafsson, J.A. On estrogen, cholesterol metabolism, and breast cancer. N. Engl. J. Med. 2014, 370, 572–573. [Google Scholar] [CrossRef]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol Links Hypercholesterolemia and Breast Cancer Pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Biggers, A.; Oddo, V.M.; Yanez, B.; Booms, E.; Sharp, L.; Naylor, K.; Wolf, P.G.; Tussing-Humphreys, L. A Perspective Review on Diet Quality, Excess Adiposity, and Chronic Psychosocial Stress and Implications for Early-Onset Colorectal Cancer. J. Nutr. 2024, 154, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.; Yap, S.; Goldsbury, D.; Nair-Shalliker, V.; Banks, E.; Canfell, K.; O’connell, D.L. Large-scale systematic analysis of exposure to multiple cancer risk factors and the associations between exposure patterns and cancer incidence. Sci. Rep. 2021, 11, 2343. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Kim, J.; Wang, Q.; Lee, A.; Babic, A.; Amundadottir, L.; Klein, A.; Li, D.; McCullough, M.; Petersen, G.; et al. The age-dependent association of risk factors with pancreatic cancer. Ann. Oncol. 2022, 33, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Hyun, K.; Hafiz, N.; Knight, A.; Hespe, C.; Chow, C.K.; Briffa, T.; Gallagher, R.; Reid, C.M.; Hare, D.L.; et al. Utilisation of Chronic Disease and Mental Health Management Services and Cardioprotective Medication Prescriptions in Primary Care for Patients With Cardiovascular Diseases and Cancer: A Cross-Sectional Study. Heart Lung Circ. 2024, 33, 738–746. [Google Scholar] [CrossRef]
- Hasin, T.; Gerber, Y.; McNallan, S.M.; Weston, S.A.; Kushwaha, S.S.; Nelson, T.J.; Cerhan, J.R.; Roger, V.L. Patients With Heart Failure Have an Increased Risk of Incident Cancer. J. Am. Coll. Cardiol. 2013, 62, 881–886. [Google Scholar] [CrossRef]
- Banke, A.B.S.; Schou, M.; Videbaek, L.; Møller, J.E.; Torp-Pedersen, C.; Gustafsson, F.; Dahl, J.S.; Køber, L.; Hildebrandt, P.R.; Gislason, G.H. Incidence of cancer in patients with chronic heart failure: A long-term follow-up study. Eur. J. Heart Fail. 2016, 18, 260–266. [Google Scholar] [CrossRef]
- Sayed, A.; Munir, M.; Addison, D.; Abushouk, A.I.; Dent, S.F.; Neilan, T.G.; Blaes, A.; Fradley, M.G.; Nohria, A.; Moustafa, K.; et al. The underutilization of preventive cardiovascular measures in patients with cancer: An analysis of the Behavioural Risk Factor Surveillance System, 2011–2022. Eur. J. Prev. Cardiol. 2023, 30, 1325–1332. [Google Scholar] [CrossRef]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef]
- Tojjari, A.; Choucair, K.; Sadeghipour, A.; Saeed, A.; Saeed, A. Anti-Inflammatory and Immune Properties of Polyunsaturated Fatty Acids (PUFAs) and Their Impact on Colorectal Cancer (CRC) Prevention and Treatment. Cancers 2023, 15, 4294. [Google Scholar] [CrossRef]
- Bojková, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary Fat and Cancer-Which Is Good, Which Is Bad, and the Body of Evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef]
- Teng, C.; Zheng, S.; Wan, W.; Liu, L.; Yu, S.; Cao, M.; Lu, W.; Shan, Y. Fatty foods and the risk of bladder cancer: A case-control study. Nutrition 2023, 106, 111868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-M.; Jiao, R.-Q.; Kong, L.-D. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Nutrition Concerns and Health Effects of Vegetarian Diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef]
- Huang, T.; Yang, B.; Zheng, J.; Li, G.; Wahlqvist, M.L.; Li, D. Cardiovascular Disease Mortality and Cancer Incidence in Vegetarians: A Meta-Analysis and Systematic Review. Ann. Nutr. Metab. 2012, 60, 233–240. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Chao, X.; Su, T.; Fu, X.; Tse, A.K.W.; Fong, W.F.; Yu, Z.-L. The anticancer and antiobesity effects of Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2017, 57, 82–94. [Google Scholar] [CrossRef]
- Stamler, J. Reprint of: Toward a Modern Mediterranean Diet for the 21st Century. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 767–769. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Tang, W.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Woodside, J.V.; Nugent, A.P.; Moore, R.E.; McKinley, M.C. Fruit and vegetable consumption as a preventative strategy for non-communicable diseases. Proc. Nutr. Soc. 2023, 82, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Kahlmeier, S.; the Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Franco-García, J.M.; Castillo-Paredes, A.; Rodríguez-Redondo, Y.; Carlos-Vivas, J.; García-Carrillo, R.M.; Denche-Zamorano, Á. Greater physical activity levels are associated with lower prevalence of tumors and risk of cancer in Spanish population: A cross-sectional study. Heliyon 2024, 10, e29191. [Google Scholar] [CrossRef]
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; de Gonzalez, A.B.; Hartge, P.; et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Ceci, C.; García-Chico, C.; Atzori, M.G.; Lacal, P.M.; Lista, S.; Santos-Lozano, A.; Graziani, G.; Pinto-Fraga, J. Impact of Physical Exercise on Melanoma Hallmarks: Current Status of Preclinical and Clinical Research. J. Cancer 2024, 15, 1–19. [Google Scholar] [CrossRef]
- Giganti, M.G.; Tresoldi, I.; Sorge, R.; Melchiorri, G.; Triossi, T.; Masuelli, L.; Lido, P.; Albonici, L.; Foti, C.; Modesti, A.; et al. Physical exercise modulates the level of serum MMP-2 and MMP-9 in patients with breast cancer. Oncol. Lett. 2016, 12, 2119–2126. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Parrini, I.; Grosseto, D.; Lestuzzi, C.; Mistrangelo, M.; Passaretti, B. Stili di vita, fattori di rischio e prevenzione delle malattie oncologiche: Il ruolo del cardiologo. G. Ital. Cardiol. 2019, 20, 20–31. [Google Scholar] [CrossRef]
- Joo, M.K.; Park, J.-J.; Chun, H.J. Additional Benefits of Routine Drugs on Gastrointestinal Cancer: Statins, Metformin, and Proton Pump Inhibitors. Dig. Dis. 2018, 36, 1–14. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef]
- Abdelgadir, E.; Ali, R.; Rashid, F.; Bashier, A. Effect of Metformin on Different Non-Diabetes Related Conditions, a Special Focus on Malignant Conditions: Review of Literature. J. Clin. Med. Res. 2017, 9, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Rains, S.L.; Amaya, C.N.; Bryan, B.A. Beta-adrenergic receptors are expressed across diverse cancers. Oncoscience 2017, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Momi, S.; Malvestiti, M.; Sebastiano, M. Platelet-targeted pharmacologic treatments as anti-cancer therapy. Cancer Metastasis Rev. 2017, 36, 331–355. [Google Scholar] [CrossRef]
- Laeeq, T.; Ahmed, M.; Sattar, H.; Zeeshan, M.H.; Ali, M.B. Role of SGLT2 Inhibitors, DPP-4 Inhibitors, and Metformin in Pancreatic Cancer Prevention. Cancers 2024, 16, 1325. [Google Scholar] [CrossRef]
- Oza, P.P.; Kashfi, K. The evolving landscape of PCSK9 inhibition in cancer. Eur. J. Pharmacol. 2023, 949, 175721. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.G.; Karampela, I.; Rebelos, E.; Kouveletsou, M.; Dalopoulos, V.; Koufopoulos, P.; Diakoumopoulou, E.; Tentolouris, N.; Dalamaga, M. Anti-Diabetic Therapies and Cancer: From Bench to Bedside. Biomolecules 2024, 14, 1479. [Google Scholar] [CrossRef]
- Aydiner, A.; Ciftci, R.; Karabulut, S.; Kilic, L. Does beta-blocker therapy improve the survival of patients with metastatic non-small cell lung cancer? Asian Pac. J. Cancer Prev. 2013, 14, 6109–6114. [Google Scholar] [CrossRef]
- Chang, A.; Yeung, S.; Thakkar, A.; Huang, K.M.; Liu, M.M.; Kanassatega, R.-S.; Parsa, C.; Orlando, R.; Jackson, E.K.; Andresen, B.T.; et al. Prevention of Skin Carcinogenesis by the β-Blocker Carvedilol. Cancer Prev. Res. 2015, 8, 27–36. [Google Scholar] [CrossRef]
- Hole, D.J.; Gillis, C.R.; McCallum, I.R.; McInnes, G.T.; MacKinnon, P.L.; Meredith, P.A.; Murray, L.S.; Robertson, J.W.; Lever, A.F. Cancer risk of hypertensive patients taking calcium antagonists. J. Hypertens. 1998, 16, 119–124. [Google Scholar] [CrossRef]
- Azoulay, L.; Assimes, T.L.; Yin, H.; Bartels, D.B.; Schiffrin, E.L.; Suissa, S. Long-Term Use of Angiotensin Receptor Blockers and the Risk of Cancer. PLoS ONE 2012, 7, e50893. [Google Scholar] [CrossRef]
- López-Fernández, T.; Mitroi, C.; Chaparro-Muñoz, M.; Moliner, P.; Martin-Garcia, A.C.; Martinez-Monzonis, A.; Castro, A.; Lopez-Sendon, J.L.; Sanchez, P.L. Effectiveness of sacubitril–valsartan in cancer patients with heart failure. ESC Heart Fail. 2020, 7, 763–767. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Hu, B.; Shi, J.; Chen, Y.; Huang, K. New insights into the therapeutic potentials of statins in cancer. Front. Pharmacol. 2023, 14, 1188926. [Google Scholar] [CrossRef] [PubMed]
- Gazzerro, P.; Proto, M.C.; Gangemi, G.; Malfitano, A.M.; Ciaglia, E.; Pisanti, S.; Santoro, A.; Laezza, C.; Bifulco, M. Pharmacological Actions of Statins: A Critical Appraisal in the Management of Cancer. Pharmacol. Rev. 2012, 64, 102–146. [Google Scholar] [CrossRef] [PubMed]
- Florensa, D.; Mateo, J.; Solsona, F.; Galván, L.; Mesas, M.; Piñol, R.; Espinosa-Leal, L.; Godoy, P. Low-dose acetylsalicylic acid for cancer prevention considering risk factors: A retrospective cohort study. Ann. Epidemiol. 2023, 84, 60–66. [Google Scholar] [CrossRef]
- Largent, J.A.; McEligot, A.J.; Ziogas, A.; Reid, C.; Hess, J.; Leighton, N.; Peel, D.; Anton-Culver, H. Hypertension, diuretics and breast cancer risk. J. Hum. Hypertens. 2006, 20, 727–732. [Google Scholar] [CrossRef]
- Chen, L.-C.; Yang, H.-J.; Yu, B.-H.; Lee, M.-S.; Lin, H.-Y.; Chiou, W.-Y.; Liu, D.-W.; Hsu, F.-C.; Chew, C.-H.; Hung, S.-K. Association of spironolactone use with risk of urinary tract cancer in the general population: A matched population-based cohort study. PLoS ONE 2024, 19, e0300391. [Google Scholar] [CrossRef]
- Numbere, B.; Fleming, K.M.; Walker, A.; Card, T.R. Adrenergic blockers and the risk for common solid cancers: A case–control study. Eur. J. Cancer Prev. 2017, 26, 86–93. [Google Scholar] [CrossRef]
- Huang, T.; Poole, E.M.; Eliassen, A.H.; Okereke, O.I.; Kubzansky, L.D.; Sood, A.K.; Forman, J.P.; Tworoger, S.S. Hypertension, use of antihypertensive medications, and risk of epithelial ovarian cancer. Int. J. Cancer 2016, 139, 291–299. [Google Scholar] [CrossRef]
- Yoon, C.; Yang, H.-S.; Jeon, I.; Chang, Y.; Park, S.M. Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: A meta-analysis of observational studies. Can. Med. Assoc. J. 2011, 183, E1073–E1084. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Li, P.Y.; Zhang, J.Q.; Wang, L.; Yi, Z. Angiotensin II Receptor Blockers and Cancer Risk: A Meta-Analysis of Randomized Controlled Trials. Medicine 2016, 95, e3600. [Google Scholar] [CrossRef]
- Harris, A.M.; Warner, B.W.; Wilson, J.M.; Becker, A.; Rowland, R.G.; Conner, W.; Lane, M.; Kimbler, K.; Durbin, E.B.; Baron, A.T.; et al. Effect of α1-Adrenoceptor Antagonist Exposure on Prostate Cancer Incidence: An Observational Cohort Study. J. Urol. 2007, 178, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, N.V.; Tafreshi, J.; Hauschild, C.L.; Pai, R.G. Cardiovascular medications and risk of cancer. Am. J. Cardiol. 2011, 108, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.W.; Yu, M.C.; Huang, S.T.; Yang, C.K.; Chen, C.H.; Lo, Y.C.; Lin, C.L.; Shu, K.H.; Yu, T.M.; Kao, C.H. Spironolactone and the risk of urinary tract cancer in patients with hypertension: A nationwide population-based retrospective case-control study. J. Hypertens. 2017, 35, 170–177. [Google Scholar] [CrossRef]
- Farag, M. Can Aspirin and Cancer Prevention be Ageless Companions? J. Clin. Diagn. Res. 2015, 9, Xe01–xe03. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Nishihara, R.; Wu, K.; Wang, M.; Ogino, S.; Willett, W.C.; Spiegelman, D.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Population-wide Impact of Long-term Use of Aspirin and the Risk for Cancer. JAMA Oncol. 2016, 2, 762–769. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016, 68, 967–976. [Google Scholar] [CrossRef]
- Soriano, L.C.; Soriano-Gabarró, M.; Rodríguez, L.A.G. The Protective Effect of Low-Dose Aspirin against Colorectal Cancer Is Unlikely Explained by Selection Bias: Results from Three Different Study Designs in Clinical Practice. PLoS ONE 2016, 11, e0159179. [Google Scholar] [CrossRef]
- Downer, M.K.; Allard, C.B.; Preston, M.A.; Gaziano, J.M.; Stampfer, M.J.; Mucci, L.A.; Batista, J.L. Regular Aspirin Use and the Risk of Lethal Prostate Cancer in the Physicians’ Health Study. Eur. Urol. 2017, 72, 821–827. [Google Scholar] [CrossRef]
- Cherepanov, V.; Cabrera-Fuentes, H.A.; Kim, M.H.; Serebruany, V.L. Solid cancers after antiplatelet therapy: Confirmations, controversies, and challenges. Thromb. Haemost. 2015, 114, 1104–1112. [Google Scholar] [CrossRef]
- Belayneh, Y.M.; Amare, G.G.; Meharie, B.G. Updates on the molecular mechanisms of aspirin in the prevention of colorectal cancer: Review. J. Oncol. Pharm. Pract. 2021, 27, 954–961. [Google Scholar] [CrossRef]
- Kotronias, R.A.; Kwok, C.S.; Wong, C.W.; Kinnaird, T.; Zaman, A.; Mamas, M.A. Cancer Event Rate and Mortality with Thienopyridines: A Systematic Review and Meta-Analysis. Drug Saf. 2016, 40, 229–240. [Google Scholar] [CrossRef]
- Leader, A.; Zelikson-Saporta, R.; Pereg, D.; Spectre, G.; Rozovski, U.; Raanani, P.; Hermoni, D.; Lishner, M. The Effect of Combined Aspirin and Clopidogrel Treatment on Cancer Incidence. Am. J. Med. 2017, 130, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Frouws, M.; Rademaker, E.; Bastiaannet, E.; van Herk-Sukel, M.; Lemmens, V.; Van de Velde, C.; Portielje, J.; Liefers, G. The difference in association between aspirin use and other thrombocyte aggregation inhibitors and survival in patients with colorectal cancer. Eur. J. Cancer 2017, 77, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Haaland, G.S.; Falk, R.S.; Straume, O.; Lorens, J.B. Association of Warfarin Use With Lower Overall Cancer Incidence Among Patients Older Than 50 Years. JAMA Intern. Med. 2017, 177, 1774–1780. [Google Scholar] [CrossRef]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef]
- Tseng, C.-H. Metformin and lung cancer risk in patients with type 2 diabetes mellitus. Oncotarget 2017, 8, 41132–41142. [Google Scholar] [CrossRef]
- Liu, F.; Yan, L.; Wang, Z.; Lu, Y.; Chu, Y.; Li, X.; Liu, Y.; Rui, D.; Nie, S.; Xiang, H. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Oncotarget 2017, 8, 16017–16026. [Google Scholar] [CrossRef]
- Weltermann, T.; Schulz, C.; Macke, L. Effect of frequently prescribed drugs on gastric cancer risk. Best. Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101741. [Google Scholar] [CrossRef]
- Amin, S.; Boffetta, P.; Lucas, A.L. The Role of Common Pharmaceutical Agents on the Prevention and Treatment of Pancreatic Cancer. Gut Liver 2016, 10, 665–671. [Google Scholar] [CrossRef]
- Kobo, O.; Michos, E.D.; Roguin, A.; Bagur, R.; Gulati, M.; Mamas, M.A. Recommended and observed statin use among US adults with and without cancer. Eur. J. Prev. Cardiol. 2024, 31, 1251–1257. [Google Scholar] [CrossRef]
- Liu, H.W.; Bian, S.Y.; Zhu, Q.W.; Zhao, Y.X. Cancer risk in older people receiving statin therapy: A meta-analysis of randomized controlled trials. J. Geriatr. Cardiol. 2016, 13, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Brugts, J.; Ades, T. Comparative tolerability and harms of individual statins: A study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Emberson, J.R.; Kearney, P.M.; Blackwell, L.; Newman, C.; Reith, C.; Bhala, N.; Holland, L.; Peto, R.; Keech, A.; Collins, R.; et al. Lack of effect of lowering LDL cholesterol on cancer: Meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 2012, 7, e29849. [Google Scholar] [CrossRef]
- Fujimoto, M.; Higuchi, T.; Hosomi, K.; Takada, M. Association between Statin Use and Cancer: Data Mining of a Spontaneous Reporting Database and a Claims Database. Int. J. Med. Sci. 2015, 12, 223–233. [Google Scholar] [CrossRef]
- Wang, A.; Wakelee, H.A.; Aragaki, A.K.; Tang, J.Y.; Kurian, A.W.; Manson, J.E.; Stefanick, M.L. Protective Effects of Statins in Cancer: Should They Be Prescribed for High-Risk Patients? Curr. Atheroscler. Rep. 2016, 18, 72. [Google Scholar] [CrossRef]
- Alfaqih, M.A.; Allott, E.H.; Hamilton, R.J.; Freeman, M.R.; Freedland, S.J. The current evidence on statin use and prostate cancer prevention: Are we there yet? Nat. Rev. Urol. 2017, 14, 107–119. [Google Scholar] [CrossRef]
- Pottegård, A.; Clark, P.; Friis, S.; Hallas, J.; Lund, L. Long-term Use of Statins and Risk of Renal Cell Carcinoma: A Population-based Case–Control Study. Eur. Urol. 2016, 69, 877–882. [Google Scholar] [CrossRef]
- Liu, J.-C.; Yang, T.-Y.; Hsu, Y.-P.; Hao, W.-R.; Kao, P.-F.; Sung, L.-C.; Chen, C.-C.; Wu, S.-Y. Statins dose-dependently exert a chemopreventive effect against lung cancer in COPD patients: A population-based cohort study. Oncotarget 2016, 7, 59618–59629. [Google Scholar] [CrossRef][Green Version]
- Mamtani, R.; Lewis, J.D.; Scott, F.I.; Ahmad, T.; Goldberg, D.S.; Datta, J.; Yang, Y.-X.; Boursi, B. Disentangling the Association between Statins, Cholesterol, and Colorectal Cancer: A Nested Case-Control Study. PLOS Med. 2016, 13, e1002007. [Google Scholar] [CrossRef]
- Lytras, T.; Nikolopoulos, G. Statins and the risk of colorectal cancer: An updated systematic review and meta-analysis of 40 studies. World J. Gastroenterol. 2014, 20, 1858–1870. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.B.; Chen, Q. Statin use is not associated with reduced risk of skin cancer: A meta-analysis. Br. J. Cancer 2014, 110, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Arnspang, S.; Pottegård, A.; Friis, S.; Clemmensen, O.; Andersen, K.E.; Hallas, J.; Gaist, D. Statin use and risk of nonmelanoma skin cancer: A nationwide study in Denmark. Br. J. Cancer 2015, 112, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Marley, A.; Tang, H.; Song, Y.; Tang, J.Y.; Han, J. Statin use and non-melanoma skin cancer risk: A meta-analysis of randomized controlled trials and observational studies. Oncotarget 2017, 8, 75411–75417. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.S.; McCoy, L.; Neugut, A.I.; Wrensch, M.; Lai, R. HMG CoA reductase inhibitors, NSAIDs and risk of glioma. Int. J. Cancer 2012, 131, E1031–E1037. [Google Scholar] [CrossRef]
- Sperling, C.D.; Verdoodt, F.; Friis, S.; Dehlendorff, C.; Kjaer, S.K. Statin use and risk of endometrial cancer: A nationwide registry-based case–control study. Acta Obstet. Gynecol. Scand. 2017, 96, 144–149. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Q.; Liu, Q.; Wang, Y.; Xie, W.; Hu, L. Statin use and endometrial cancer risk: A meta-analysis. Oncotarget 2017, 8, 62425–62434. [Google Scholar] [CrossRef]
- Friedman, G.D.; Achacoso, N.; Fireman, B.; Habel, L.A. Statins and Reduced Risk of Liver Cancer: Evidence for Confounding. JNCI J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Shi, M.; Zheng, H.; Nie, B.; Gong, W.; Cui, X. Statin use and risk of liver cancer: An update meta-analysis. BMJ Open 2014, 4, e005399. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhu, G.-Q.; Wang, Y.; Zheng, J.-N.; Ruan, L.-Y.; Cheng, Z.; Hu, B.; Fu, S.-W.; Zheng, M.-H. Systematic review with network meta-analysis: Statins and risk of hepatocellular carcinoma. Oncotarget 2016, 7, 21753–21762. [Google Scholar] [CrossRef]
- Thomas, T.; Loke, Y.; Beales, I.L.P. Systematic Review and Meta-analysis: Use of Statins Is Associated with a Reduced Incidence of Oesophageal Adenocarcinoma. J. Gastrointest. Cancer 2018, 49, 442–454. [Google Scholar] [CrossRef]
- Alexandre, L.; Clark, A.B.; Cheong, E.; Lewis, M.P.N.; Hart, A.R. Systematic review: Potential preventive effects of statins against oesophageal adenocarcinoma. Aliment. Pharmacol. Ther. 2012, 36, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-F.; Yang, Y.-H.; Liu, Y.-C.; Hsiao, H.-H.; Huang, C.-T.; Wu, C.-H.; Tsai, Y.-F.; Wang, H.-C.; Liu, T.-C. Previous Exposure to Statin May Reduce the Risk of Subsequent Non-Hodgkin Lymphoma: A Nationwide Population-Based Case-Control Study. PLoS ONE 2015, 10, e0139289. [Google Scholar] [CrossRef]
- Islam, M.; Yang, H.-C.; Nguyen, P.-A.; Poly, T.N.; Huang, C.-W.; Kekade, S.; Khalfan, A.M.; Debnath, T.; Li, Y.-C.J.; Abdul, S.S. Exploring association between statin use and breast cancer risk: An updated meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 1043–1053. [Google Scholar] [CrossRef]
- Ahern, T.P.; Pedersen, L.; Tarp, M.; Cronin-Fenton, D.P.; Garne, J.P.; Silliman, R.A.; Sørensen, H.T.; Lash, T.L. Statin Prescriptions and Breast Cancer Recurrence Risk: A Danish Nationwide Prospective Cohort Study. J. Natl. Cancer Inst. 2011, 103, 1461–1468. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Udomsubpayakul, U.; McEvoy, M.; Lerdsitthichai, P.; Attia, J.; Thakkinstian, A. Effect of Lipophilic and Hydrophilic Statins on Breast Cancer Risk in Thai Women: A Cross-sectional Study. J. Cancer 2016, 7, 1163–1168. [Google Scholar] [CrossRef]
- McDougall, J.A.; Malone, K.E.; Daling, J.R.; Cushing-Haugen, K.L.; Porter, P.L.; Li, C.I. Long-Term Statin Use and Risk of Ductal and Lobular Breast Cancer among Women 55 to 74 Years of Age. Cancer Epidemiology Biomarkers Prev. 2013, 22, 1529–1537. [Google Scholar] [CrossRef]
- Desai, P.; Chlebowski, R.; Cauley, J.A.; Manson, J.E.; Wu, C.; Martin, L.W.; Jay, A.; Bock, C.; Cote, M.; Petrucelli, N.; et al. Prospective Analysis of Association between Statin Use and Breast Cancer Risk in the Women's Health Initiative. Cancer Epidemiology Biomarkers Prev. 2013, 22, 1868–1876. [Google Scholar] [CrossRef][Green Version]
- Boudreau, D.M.; Yu, O.; Chubak, J.; Wirtz, H.S.; Bowles, E.J.A.; Fujii, M.; Buist, D.S.M. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res. Treat. 2014, 144, 405–416. [Google Scholar] [CrossRef]
- Epstein, M.M.; Divine, G.; Chao, C.R.; Wells, K.E.; Feigelson, H.S.; Scholes, D.; Roblin, D.; Yood, M.U.; Engel, L.S.; Taylor, A.; et al. Statin use and risk of multiple myeloma: An analysis from the cancer research network. Int. J. Cancer 2017, 141, 480–487. [Google Scholar] [CrossRef]
- Baandrup, L.; Dehlendorff, C.; Friis, S.; Olsen, J.H.; Kjær, S.K. Statin use and risk for ovarian cancer: A Danish nationwide case–control study. Br. J. Cancer 2015, 112, 157–161. [Google Scholar] [CrossRef]
- Kho, P.F.; Fawcett, J.; Fritschi, L.; Risch, H.; Webb, P.M.; Whiteman, D.C.; Neale, R.E. Nonsteroidal anti-inflammatory drugs, statins, and pancreatic cancer risk: A population-based case-control study. Cancer Causes Control 2016, 27, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Song, X.; Zhang, G.; Peng, A.; Li, X.; Li, M.; Liu, Y.; Wang, C. Statins and the Risk of Lung Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e57349. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Tangen, C.M.; Goodman, P.J.; Till, C.; Parnes, H.L.; Figg, W.D.; Albanes, D.; Neuhouser, M.L.; Klein, E.A.; Lucia, M.S.; et al. Statin Drug Use is Not Associated with Prostate Cancer Risk in Men Who are Regularly Screened. J. Urol. 2014, 192, 379–384. [Google Scholar] [CrossRef]
- Bansal, D.; Undela, K.; D'Cruz, S.; Schifano, F. Statin Use and Risk of Prostate Cancer: A Meta-Analysis of Observational Studies. PLoS ONE 2012, 7, e46691. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Liu, M.; Qian, J.; Zheng, J.-H.; Zhang, X.P.; Guo, C.-C.; Geng, J.; Peng, B.; Che, J.-P.; Wu, Y. Statin use and risk of kidney cancer: A meta-analysis of observational studies and randomized trials. Br. J. Clin. Pharmacol. 2014, 77, 458–465. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, C.; Hsu, W.; Chang, C.; Yeh, H.; Tung, C.; Wu, Y.; Sung, F.; Kao, C. Statins are associated with a reduced risk of cholangiocarcinoma: A population-based case–control study. Br. J. Clin. Pharmacol. 2015, 80, 755–761. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Wiviott, S.D.; Blazing, M.A.; De Ferrari, G.M.; Park, J.G.; Murphy, S.A.; White, J.A.; Tershakovec, A.M.; Cannon, C.P.; Braunwald, E. Long-term Safety and Efficacy of Achieving Very Low Levels of Low-Density Lipoprotein Cholesterol : A Prespecified Analysis of the IMPROVE-IT Trial. JAMA Cardiol. 2017, 2, 547–555. [Google Scholar] [CrossRef]
- Bonovas, S.; Nikolopoulos, G.K.; Bagos, P.G. Use of Fibrates and Cancer Risk: A Systematic Review and Meta-Analysis of 17 Long-Term Randomized Placebo-Controlled Trials. PLoS ONE 2012, 7, e45259. [Google Scholar] [CrossRef]
- Quagliariello, V.; Bisceglia, I.; Berretta, M.; Iovine, M.; Canale, M.L.; Maurea, C.; Giordano, V.; Paccone, A.; Inno, A.; Maurea, N. PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology. Cancers 2023, 15, 1397. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. New Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Mahboobnia, K.; Pirro, M.; Marini, E.; Grignani, F.; Bezsonov, E.E.; Jamialahmadi, T.; Sahebkar, A. PCSK9 and cancer: Rethinking the link. Biomed. Pharmacother. 2021, 140, 111758. [Google Scholar] [CrossRef]
- Liu, X.; Bao, X.; Hu, M.; Chang, H.; Jiao, M.; Cheng, J.; Xie, L.; Huang, Q.; Li, F.; Li, C.-Y. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 2020, 588, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, M.; Yuan, H.; Wang, Z.; Yu, L. A novel insight into cancer therapy: Lipid metabolism in tumor-associated macrophages. Int. Immunopharmacol. 2024, 135, 112319. [Google Scholar] [CrossRef]
- Bao, X.; Liang, Y.; Chang, H.; Cai, T.; Feng, B.; Gordon, K.; Zhu, Y.; Shi, H.; He, Y.; Xie, L. Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): From bench to bedside. Signal Transduct. Target. Ther. 2024, 9, 13. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, X.; Liu, J.; Lv, X.; Lu, K.; Lu, Y.; Jiang, Y. PCSK9 in T-cell function and the immune response. Biomark. Res. 2024, 12, 163. [Google Scholar] [CrossRef]
- Ishida, J.; Konishi, M.; Ebner, N.; Springer, J. Repurposing of approved cardiovascular drugs. J. Transl. Med. 2016, 14, 269. [Google Scholar] [CrossRef]
- Kwan, M.L.; Cheng, R.K.; Iribarren, C.; Neugebauer, R.; Rana, J.S.; Nguyen-Huynh, M.; Shi, Z.; Laurent, C.A.; Lee, V.S.; Roh, J.M.; et al. Risk of Cardiometabolic Risk Factors in Women With and Without a History of Breast Cancer: The Pathways Heart Study. J. Clin. Oncol. 2022, 40, 1635–1646. [Google Scholar] [CrossRef]
- Brenner, D.R.; Brockton, N.T.; Kotsopoulos, J.; Cotterchio, M.; Boucher, B.A.; Courneya, K.S.; Knight, J.A.; Olivotto, I.A.; Quan, M.L.; Friedenreich, C.M. Breast cancer survival among young women: A review of the role of modifiable lifestyle factors. Cancer Causes Control. 2016, 27, 459–472. [Google Scholar] [CrossRef]
- Smith, S.L.; Singh-Carlson, S.; Downie, L.; Payeur, N.; Wai, E.S. Survivors of breast cancer: Patient perspectives on survivorship care planning. J. Cancer Surviv. 2011, 5, 337–344. [Google Scholar] [CrossRef]
- Meade, E.; McIlfatrick, S.; Groarke, A.M.; Butler, E.; Dowling, M. Survivorship care for postmenopausal breast cancer patients in Ireland: What do women want? Eur. J. Oncol. Nurs. 2017, 28, 69–76. [Google Scholar] [CrossRef]
- Sturgeon, K.M.; Ky, B.; Libonati, J.R.; Schmitz, K.H. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res. Treat. 2014, 143, 219–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.; Gotay, C. The Effectiveness of Exercise Interventions for Improving Health-Related Quality of Life From Diagnosis Through Active Cancer Treatment. Oncol. Nurs. Forum 2015, 42, E33–E53. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L. Weight Loss Interventions and Breast Cancer Survival: The Time Is Now. J. Clin. Oncol. 2014, 32, 2197–2199. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Ballard-Barbash, R.; Friedenreich, C.M.; Courneya, K.S.; Siddiqi, S.M.; McTiernan, A.; Alfano, C.M. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. J. Natl. Cancer Inst. 2012, 104, 815–840. [Google Scholar] [CrossRef]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef]
- Barbaric, M.; Brooks, E.; Moore, L.; Cheifetz, O. Effects of Physical Activity on Cancer Survival: A Systematic Review. Physiother. Can. 2010, 62, 25–34. [Google Scholar] [CrossRef]
- Bourke, L.; Homer, K.E.; Thaha, M.A.; Steed, L.; Rosario, D.J.; Robb, K.A.; Saxton, J.M.; Taylor, S.J. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst. Rev. 2013, CD010192. [Google Scholar] [CrossRef]
- de Haas, E.C.; Oosting, S.F.; Lefrandt, J.D.; Wolffenbuttel, B.H.; Sleijfer, D.T.; Gietema, J.A. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010, 11, 193–203. [Google Scholar] [CrossRef]
- Pan, A.; Yu, D.; Demark-Wahnefried, W.; Franco, O.H.; Lin, X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am. J. Clin. Nutr. 2009, 90, 288–297. [Google Scholar] [CrossRef]
- Dueregger, A.; Heidegger, I.; Ofer, P.; Perktold, B.; Ramoner, R.; Klocker, H.; Eder, I.E. The Use of Dietary Supplements to Alleviate Androgen Deprivation Therapy Side Effects during Prostate Cancer Treatment. Nutrients 2014, 6, 4491–4519. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Pearce, L.S.; Wilkins, J.T.; Overington, J.P.; Hingorani, A.D.; Casas, J.P. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2017, 4, CD011748. [Google Scholar] [CrossRef]
- Thomas, J.A.; Miller, E.R.; Ward, P.R. Lifestyle Interventions through Participatory Research: A Mixed-Methods Systematic Review of Alcohol and Other Breast Cancer Behavioural Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 980. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Albini, A.; Fossile, E.; Pessi, M.A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. Speckle-Tracking Echocardiography for Cardioncological Evaluation in Bevacizumab-Treated Colorectal Cancer Patients. Cardiovasc. Toxicol. 2020, 20, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Murphy, A.C.; Ramalingam, S.; Scherrer-Crosbie, M.; Lopez-Fernandez, T.; Reynolds, K.L.; Aznar, M.; Lin, A.E.; Libby, P.; Cordoba, R.; et al. Cardiovascular Considerations Before Cancer Therapy: Gaps in Evidence and JACC: CardioOncology Expert Panel Recommendations. JACC CardioOncol. 2024, 6, 631–654. [Google Scholar] [CrossRef]
- Nechita, L.C.; Tutunaru, D.; Nechita, A.; Voipan, A.E.; Voipan, D.; Tupu, A.E.; Musat, C.L. AI and Smart Devices in Cardio-Oncology: Advancements in Cardiotoxicity Prediction and Cardiovascular Monitoring. Diagnostics 2025, 15, 787. [Google Scholar] [CrossRef]
- Armoundas, A.A.; Narayan, S.M.; Arnett, D.K.; Spector-Bagdady, K.; Bennett, D.A.; Celi, L.A.; Friedman, P.A.; Gollob, M.H.; Hall, J.L.; Kwitek, A.E.; et al. Use of Artificial Intelligence in Improving Outcomes in Heart Disease: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e1028–e1050. [Google Scholar] [CrossRef]
- Khera, R.; Oikonomou, E.K.; Nadkarni, G.N.; Morley, J.R.; Wiens, J.; Butte, A.J.; Topol, E.J. Transforming Cardiovascular Care With Artificial Intelligence: From Discovery to Practice. Circ. 2024, 84, 97–114. [Google Scholar] [CrossRef]
- Goldfarb, M.J.; Saylor, M.A.; Bozkurt, B.; Code, J.; Di Palo, K.E.; Durante, A.; Flanary, K.; Creber, R.M.; Ogunniyi, M.O.; Rodriguez, F.; et al. Patient-Centered Adult Cardiovascular Care: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e1176–e1188. [Google Scholar] [CrossRef]

| Drug Class | Potential Role in Cancer Prevention | Mechanism of Action | Evidence/Findings |
|---|---|---|---|
| SGLT2 Inhibitors (e.g., empagliflozin, dapagliflozin) (Various dosages) (2018–2023) | Antineoplastic properties, including reduced pancreatic, breast, and prostate cancer risks. | Modulates metabolic reprogramming, reduces glucose and insulin availability in tumor microenvironment, reduces oxidative stress. | Preclinical studies suggest inhibition of cancer cell proliferation, induction of apoptosis, and reduced oxidative stress [104]. Clinical trials are needed for validation. |
| PCSK9 Inhibitors (Various dosages) (2015–2023) | Potential reduction in cancer risk, especially related to tumor growth and immune response. | Modulates lipid metabolism, enhances immune cell function by increasing LDL receptor expression, may reduce cancer cell proliferation. | Early preclinical studies suggest potential anticancer effects through immune modulation and altered cholesterol availability in tumors [105]. Clinical trials are needed for further investigation. |
| GLP-1 Receptor Agonists (e.g., liraglutide, semaglutide) (Various dosages) (2016–2024) | Potential reduction in colorectal, pancreatic, and breast cancer risks. | Modulates insulin secretion, reduces systemic inflammation, promotes apoptosis, inhibits tumor cell proliferation, and improves mitochondrial function. | Epidemiological and preclinical data suggest these agents could inhibit tumor cell growth and reduce oxidative stress, potentially lowering cancer incidence [106]. Further clinical studies are needed to confirm these findings. |
| Beta-Blockers (Various dosages) (1990–2015) | Protective effects against melanomas and hepatocellular carcinoma, particularly in patients with cirrhosis and esophageal varices. | Reduces sympathetic nervous system activity, may inhibit cancer cell proliferation. | No strong evidence for a reduction in incidence of common cancers (e.g., ovarian, colorectal) [107]. Potential protective effect seen in specific cancers (e.g., melanoma) [108]. |
| Calcium Antagonists (Various dosages) (1985–1998) | Neutral or slightly protective role in ovarian neoplasms, but possible risk increase in pulmonary cancers. | Block calcium channels, may affect cellular growth and survival. | Evidence indicates no significant effect on overall cancer incidence, though some studies suggest reduced ovarian and increased pulmonary cancer risks [109]. |
| Renin–Angiotensin System Inhibitors (e.g., ACE inhibitors, ARBs) (Various dosages) (1991–2012) | Reduction in esophageal, pulmonary, prostatic, and colorectal cancers, with potential increase in melanomas and kidney cancers. | Modulates blood pressure, impacts cell growth, and inhibits tumor progression via antiangiogenic effects. | Mixed results, with some studies showing a protective effect, while others suggest potential harm in certain cancers (e.g., kidney, melanoma) [110]. |
| Sacubitril/Valsartan (Various dosages) (2016–2020) | Potential cardioprotective effects in cancer survivors, reducing chemotherapy-induced cardiotoxicity. | Enhances endothelial function, reduces myocardial fibrosis, modulates inflammation. | Shows promise in reducing heart damage caused by cancer treatment, with some evidence suggesting protective effects against tumor progression [111]. |
| Statins (Various molecules and dosages) (2001–2023) | Possible reduction in gastric, hepatic, hematological, and prostate cancers; no significant effect on colorectal, pancreatic, bladder, or lung cancers. | Inhibits HMG-CoA reductase, which is involved in cholesterol biosynthesis, modulates angiogenesis, and promotes apoptosis. | Long-term use linked to reduced incidence in some cancers [112]. Their pleiotropic effects may help suppress tumor metastasis and improve cancer treatment efficacy [113]. |
| Aspirin (100–325 mg) (2007–2016) | Potential reduction in gastrointestinal, breast (hormone receptor-positive), and prostatic cancers. | COX inhibition, suppresses prostaglandins, potentially reducing cancer cell growth. | Long-term use (5+ years) shown to reduce risks in certain cancers, especially colorectal and gastric cancers [114]. |
| Diuretics (Various dosages) (1990–2013) | Possible correlation with increased cancer risk, particularly in mammary, ovarian, and renal cancers. | Alters fluid balance, impacts renal function. | Potential link to cancer risk, but likely due to reverse causality or confounding factors [115]. Spironolactone may reduce prostate cancer risk in men and bladder cancer in women [116]. |
| Cancer Type | Effect | Study Design | Sample Size | Author |
|---|---|---|---|---|
| Colorectal | ||||
| ≈ | Case–control | 4606 | Pottegard A [147] | |
| ↓ | Prospective cohort | 783 | Liu JC [148] | |
| ↓ | Case–control | 22,163 | Mamtani R [149] | |
| ↓ | Meta-analysis | >100,000 | Lytras T [150] | |
| Skin | ||||
| ≈ | Meta-analysis | >11,000 | Li X [151] | |
| ≈ | Retrospective cohort | 1099 | Jagtap D [151] | |
| ↑ | Case–control | > 40,000 | Arnspang S [152] | |
| ↑ | Meta-analysis | 57,004 | Yang K [153] | |
| Brain | ||||
| ↓ | Case–control | 517 | Ferris J [154] | |
| Endometrial | ||||
| ≈ | Case–control | 5382 | Sperling CD [155] | |
| ≈ | Meta-analysis | 9517 | Yang J [156] | |
| Liver | ||||
| ≈ | Case–control | 2877 | Friedman GD [157] | |
| ↓ | Meta-analysis | 35,756 | Shi M [158] | |
| ↓ | Meta-analysis | 10,993 | Zhou YY [159] | |
| Esophageal | ||||
| ↓ | Meta-analysis | 1057 | Thomas T [160] | |
| ↓ | Systematic review | 35,214 | Alexandre L [161] | |
| Non-Hodgkin Lymphoma | ||||
| ↓ | Case–control | 1715 | Cho SF [162] | |
| Breast | ||||
| ≈ | Meta-analysis | 121,399 | Islam MM [163] | |
| ↓ | Prospective cohort | 18,769 | Ahern TP [164] | |
| ↓ | Retrospective cohort | 15,718 | Anothaisintawee T [165] | |
| ↑ | Case–control | 1582 | McDougall JA [166] | |
| ≈ | Prospective cohort | 7430 | Desai D [167] | |
| ↓ | Prospective cohort | 4216 | Boudreau DM [168] | |
| Multiple Myeloma | ||||
| ↓ | Case–control | 2532 | Epstein MM [169] | |
| Ovarian | ||||
| ≈ | Case–control | 4103 | Baandrup L [170] | |
| Pancreatic | ||||
| ↓ | Case–control | 704 | Kho P [171] | |
| ↓ | Retrospective cohort | 2341 | Chen MJ [148] | |
| Lung | ||||
| ↓ | Prospective cohort | 1225 | Liu JC [148] | |
| ≈ | Meta-analysis | 38,013 | Tan M [172] | |
| Prostate | ||||
| ≈ | Prospective cohort | 9457 | Platz E [173] | |
| ↓ | Meta-analysis | 61,958 | Bansal D [174] | |
| Kidney | ||||
| ≈ | Meta-analysis | 870 | Zhang XJ [175] | |
| Biliary | ||||
| ↓ | Case–control | 3174 | Peng YC [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrignani, M.G.; Lucà, F.; Abrignani, V.; Nucara, M.; Grosseto, D.; Lestuzzi, C.; Mistrangelo, M.; Passaretti, B.; Rao, C.M.; Parrini, I. Risk Factors and Prevention of Cancer and CVDs: A Chicken and Egg Situation. J. Clin. Med. 2025, 14, 3083. https://doi.org/10.3390/jcm14093083
Abrignani MG, Lucà F, Abrignani V, Nucara M, Grosseto D, Lestuzzi C, Mistrangelo M, Passaretti B, Rao CM, Parrini I. Risk Factors and Prevention of Cancer and CVDs: A Chicken and Egg Situation. Journal of Clinical Medicine. 2025; 14(9):3083. https://doi.org/10.3390/jcm14093083
Chicago/Turabian StyleAbrignani, Maurizio Giuseppe, Fabiana Lucà, Vincenzo Abrignani, Mariacarmela Nucara, Daniele Grosseto, Chiara Lestuzzi, Marinella Mistrangelo, Bruno Passaretti, Carmelo Massimiliano Rao, and Iris Parrini. 2025. "Risk Factors and Prevention of Cancer and CVDs: A Chicken and Egg Situation" Journal of Clinical Medicine 14, no. 9: 3083. https://doi.org/10.3390/jcm14093083
APA StyleAbrignani, M. G., Lucà, F., Abrignani, V., Nucara, M., Grosseto, D., Lestuzzi, C., Mistrangelo, M., Passaretti, B., Rao, C. M., & Parrini, I. (2025). Risk Factors and Prevention of Cancer and CVDs: A Chicken and Egg Situation. Journal of Clinical Medicine, 14(9), 3083. https://doi.org/10.3390/jcm14093083





