Safety and Efficacy of Different Therapeutic Interventions for Primary Progressive Aphasia: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Outcome Measures
2.4. Selection of Studies and Extraction of Data
2.5. Assessment of Quality of the Included Studies
2.6. Statistical Analysis
3. Results
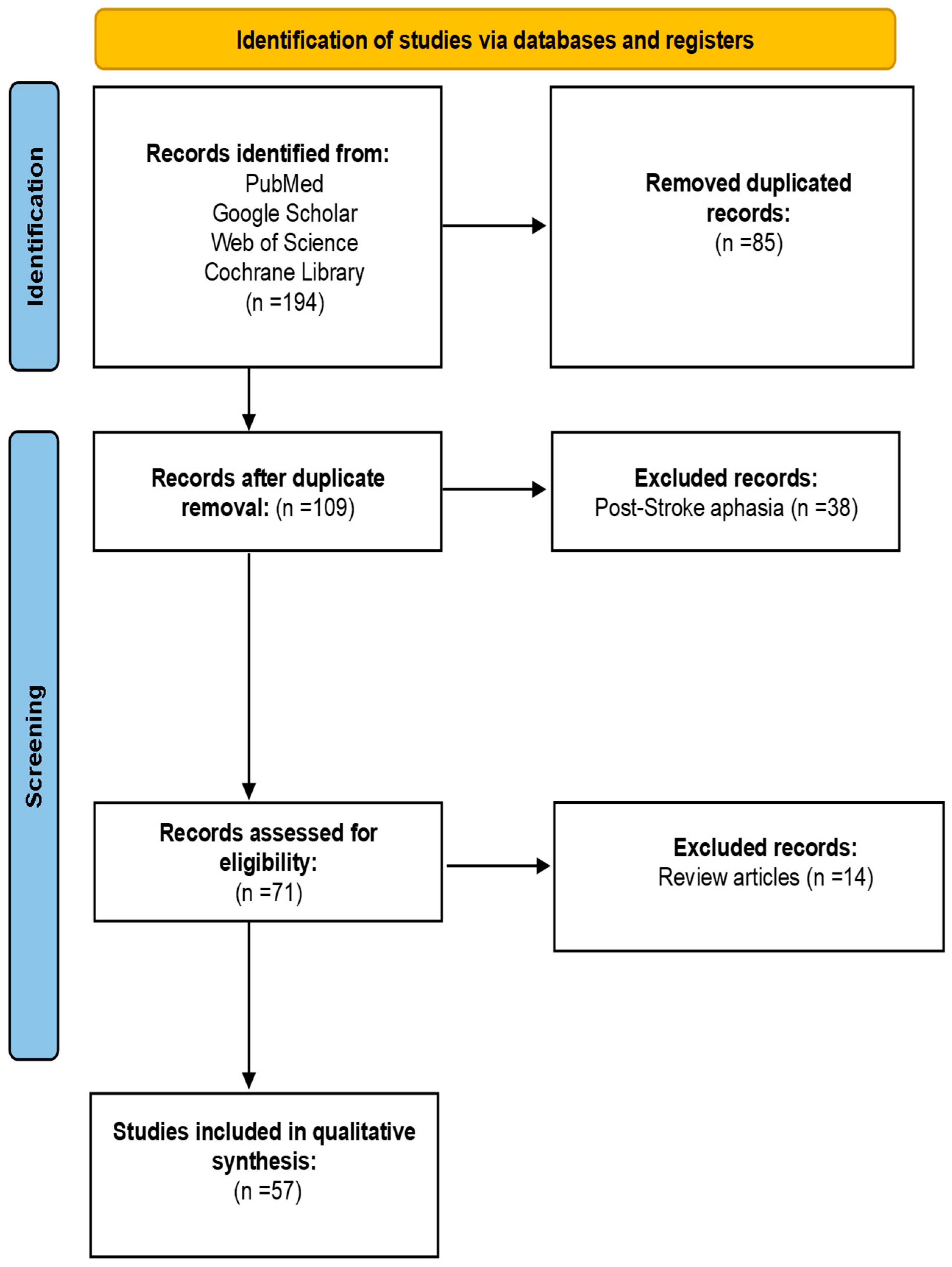
3.1. Search Results
3.2. Study Characteristics
3.3. Risk of Bias Assessment
3.4. Overall Treatment Effects
3.5. Treatment Gain
3.5.1. Naming and Word Finding for Trained and Untrained Words
3.5.2. Spontaneous Speech or Fluency
3.5.3. Auditory Verbal Comprehension
3.5.4. Repetition
3.5.5. AOS
3.5.6. Reading Abilities
3.5.7. Cognitive Function
3.5.8. Maintenance of Therapeutic Effects
3.5.9. Neuroplastic, Functional Reorganization, and Metabolic Changes
3.5.10. Quality of Life
3.5.11. Adverse Events
4. Discussion
4.1. Summary of Findings
4.2. Comparison with Previous Systematic Reviews
4.3. Clinical Implications of PPA
4.4. Pathophysiology and Treatment Considerations
4.5. Study Strengths and Limitations
4.6. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tippett, D.C.; Hillis, A.E.; Tsapkini, K. Treatment of primary progressive aphasia. Curr. Treat. Options Neurol. 2015, 17, 34. [Google Scholar] [CrossRef]
- Nickels, L.; Croot, K. Understanding and living with primary progressive aphasia: Current progress and challenges for the future. Aphasiology 2014, 28, 885–899. [Google Scholar] [CrossRef]
- Hardy, C.J.D.; Taylor-Rubin, C.; Taylor, B.; Harding, E.; Gonzalez, A.S.; Jiang, J.; Thompson, L.; Kingma, R.; Chokesuwattanaskul, A.; Walker, F.; et al. Symptom-led staging for semantic and non-fluent/agrammatic variants of primary progressive aphasia. Alzheimer’s Dement. 2024, 20, 195–210. [Google Scholar] [CrossRef]
- Moeller, S.; Sridhar, J.; Martersteck, A.; Coventry, C.; Kuang, A.; Zhang, H.; Weintraub, S.; Mesulam, M.; Rogalski, E. Functional decline in the aphasic variant of Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 1641–1648. [Google Scholar] [CrossRef]
- Morhardt, D.J.; O’Hara, M.C.; Zachrich, K.; Wieneke, C.; Rogalski, E.J. Development of a psycho-educational support program for individuals with primary progressive aphasia and their care-partners. Dementia 2019, 18, 1310–1327. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, E.; Miller, B.L. Frontotemporal lobar degeneration: A clinical approach. Semin. Neurol. 2014, 34, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, L.; Nickels, L.; Croot, K. Quality of life in primary progressive aphasia: What do we know and what can we do next? Aphasiology 2019, 33, 498–519. [Google Scholar] [CrossRef]
- Davies, K.; Howe, T. Experiences of living with primary progressive aphasia: A scoping review of qualitative studies. Am. J. Alzheimer’s Dis. Other Dement. 2020, 35, 1–10. [Google Scholar] [CrossRef]
- Montembeault, M.; Brambati, S.M.; Gorno-Tempini, M.L.; Migliaccio, R. Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: A review. Front. Neurol. 2018, 9, 692. [Google Scholar] [CrossRef]
- Moya, A.; Isaacson, R.; Sun, D. 141st Annual Meeting of the American Neurological Association. Ann. Neurol. 2016, 80, S1. [Google Scholar] [CrossRef]
- Thompson, C.K.; Mack, J.E. Grammatical impairments in PPA. Aphasiology 2014, 28, 1018–1037. [Google Scholar] [CrossRef] [PubMed]
- Tippett, D.C. Classification of primary progressive aphasia: Challenges and complexities. F1000Research 2020, 9, 64. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- UCSF Weill Institute for Neurosciences. A Healthcare Provider’s Guide to the Logopenic Variant of Primary Progressive Aphasia (lvPPA). 2024. Available online: https://memory.ucsf.edu/sites/memory.ucsf.edu/files/wysiwyg/UCSF_lvPPA_Providers_7-13-17.pdf (accessed on 12 March 2025).
- Cadório, I.; Lousada, M.; Martins, P.; Figueiredo, D. Generalization and maintenance of treatment gains in primary progressive aphasia (PPA): A systematic review. Int. J. Lang. Commun. Disord. 2017, 52, 543–560. [Google Scholar] [CrossRef]
- Fried-Oken, M.; Rowland, C.; Gibbons, C. Providing augmentative and alternative communication treatment to persons with progressive nonfluent aphasia. Perspect. Neurophysiol. Neurogenic Speech Lang. Disord. 2010, 20, 21–25. [Google Scholar] [CrossRef]
- Rogalski, E.J.; Saxon, M.; McKenna, H.; Wieneke, C.; Rademaker, A.; Corden, M.E.; Borio, K.; Mesulam, M.; Khayum, B. Communication Bridge: A pilot feasibility study of internet-based speech-language therapy for individuals with progressive aphasia. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2016, 2, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Rosenbaum, S. Speech and Language Disorders in Children: Implications for the Social Security Administration’s Supplemental Security Income Program; National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Hodges, J.R.; Piguet, O. Progress and challenges in frontotemporal dementia research: A 20-year review. J. Alzheimer’s Dis. 2018, 62, 1467–1480. [Google Scholar] [CrossRef]
- Rogalski, E.J.; Khayum, B. A life participation approach to primary progressive aphasia intervention. Semin. Speech Lang. 2018, 39, 284–296. [Google Scholar] [CrossRef]
- Croot, K.; Nickels, L.; Laurence, F.; Manning, M. Impairment- and activity/participation-directed interventions in progressive language impairment: Clinical and theoretical issues. Aphasiology 2009, 23, 125–160. [Google Scholar] [CrossRef]
- Gill, J.; Shah-Basak, P.P.; Hamilton, R. It’s the thought that counts: Examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain Stimul. 2015, 8, 253–259. [Google Scholar] [CrossRef]
- Munasinghe, T.U.; Ariyasena, A.D.K.; Siriwardhana, D.D. Speech therapy interventions for acquired apraxia of speech: An updated systematic review. Am. J. Speech-Lang. Pathol. 2023, 32, 1336–1359. [Google Scholar] [CrossRef] [PubMed]
- Volkmer, A.; Spector, A.; Warren, J.D.; Beeke, S. Speech and language therapy for primary progressive aphasia: Referral patterns and barriers to service provision across the UK. Dementia 2020, 19, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- JBI. JBI Manual for Evidence Synthesis; JBI: North Adelaide, Australia, 2024. [Google Scholar] [CrossRef]
- Tsapkini, K.; Webster, K.T.; Ficek, B.N.; Desmond, J.E.; Onyike, C.U.; Rapp, B.; Frangakis, C.E.; Hillis, A.E. Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 461–472. [Google Scholar] [CrossRef]
- Rogalski, E.; Roberts, A.; Salley, E.; Saxon, M.; Fought, A.; Esparza, M.; Blaze, E.; Coventry, C.; Mesulam, M.-M.; Weintraub, S.; et al. Communication partner engagement: A relevant factor for functional outcomes in speech-language therapy for aphasic dementia. J. Gerontol. Psychol. Sci. 2021, 77, 1017–1025. [Google Scholar] [CrossRef]
- Joubert, S.; Maquestiaux, F.; Enriquez-Rosas, A.; Villalpando, J.M.; Brodeur, C.; Bier, N. Smartphone use as an efficient tool to improve anomia in primary progressive aphasia. Neuropsychol. Rehabil. 2024, 34, 362–387. [Google Scholar] [CrossRef]
- Meyer, A.M.; Snider, S.F.; Tippett, D.C.; Saloma, R.; Turkeltaub, P.E.; Hillis, A.E.; Friedman, R.B. Baseline conceptual-semantic impairment predicts longitudinal treatment effects for anomia in primary progressive aphasia and Alzheimer’s disease. Aphasiology 2024, 38, 205–236. [Google Scholar] [CrossRef]
- de Aguiar, V.; Zhao, Y.; Ficek, B.N.; Webster, K.; Rofes, A.; Wendt, H.; Frangakis, C.; Caffo, B.; Hillis, A.E.; Rapp, B.; et al. Cognitive and language performance predicts effects of spelling intervention and tDCS in primary progressive aphasia. Cortex 2020, 124, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Nissim, N.R.; Harvey, D.Y.; Haslam, C.; Friedman, L.; Bharne, P.; Litz, G.; Phillips, J.S.; Cousins, K.A.Q.; Xie, S.X.; Grossman, M.; et al. Through thick and thin: Baseline cortical volume and thickness predict performance and response to transcranial direct current stimulation in primary progressive aphasia. Front. Hum. Neurosci. 2022, 16, 907425. [Google Scholar] [CrossRef]
- Henry, M.L.; Hubbard, H.I.; Grasso, S.M.; Mandelli, M.L.; Wilson, S.M.; Sathishkumar, M.T.; Fridriksson, J.; Daigle, W.; Boxer, A.L.; Miller, B.L.; et al. Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain 2018, 141, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Pytel, V.; Cabrera-Martín, M.N.; Delgado-Álvarez, A.; Ayala, J.L.; Balugo, P.; Delgado-Alonso, C.; Yus, M.; Carreras, M.T.; Carreras, J.L.; Matías-Guiu, J.; et al. Personalized repetitive transcranial magnetic stimulation for primary progressive aphasia. J. Alzheimer’s Dis. 2021, 84, 151–167. [Google Scholar] [CrossRef]
- Montagut, N.; Borrego-Écija, S.; Castellví, M.; Rico, I.; Reñé, R.; Balasa, M.; Lladó, A.; Sánchez-Valle, R. Errorless learning therapy in semantic variant of primary progressive aphasia. J. Alzheimer’s Dis. 2021, 79, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tan, Y.; Hao, H.; Li, J.; Liu, C.; Hu, Y.; Wu, Y.; Ding, Q.; Zhou, Y.; Li, Y.; et al. Treatment of primary progressive aphasia by repetitive transcranial magnetic stimulation: A randomized, double-blind, placebo-controlled study. J. Neural Transm. 2023, 130, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Écija, S.; Montagut, N.; Martín-Trias, P.; Vaqué-Alcázar, L.; Illán-Gala, I.; Balasa, M.; Casanova-Mollà, J.; Bargalló, N.; Valls-Solé, J.; Lleó, A.; et al. Multifocal transcranial direct current stimulation in primary progressive aphasia does not provide a clinical benefit over speech therapy. J. Alzheimer’s Dis. 2023, 93, 1169–1180. [Google Scholar] [CrossRef]
- Schaffer, K.M.; Wauters, L.; Berstis, K.; Grasso, S.M.; Henry, M.L. Modified script training for nonfluent/agrammatic with significant hearing loss: A single-case experimental design. Neuropsychol. Rehabil. 2022, 32, 306–335. [Google Scholar] [CrossRef]
- Schaffer, K.M.; Evans, W.S.; Dutcher, C.D.; Philburn, C.; Henry, M.L. Embedding aphasia-modified cognitive behavioral therapy in script training for primary progressive aphasia: A single-case pilot study. Am. J. Speech-Lang. Pathol. 2021, 30, 2053–2068. [Google Scholar] [CrossRef]
- Meyer, A.M.; Tippett, D.C.; Friedman, R.B. Prophylaxis and remediation of anomia in the semantic and logopenic variants of primary progressive aphasia. Neuropsychol. Rehabil. 2016, 28, 352. [Google Scholar] [CrossRef]
- Meyer, A.M.; Tippett, D.C.; Turner, R.S.; Friedman, R.B. Long-term maintenance of anomia treatment effects in primary progressive aphasia. Neuropsychol. Rehabil. 2019, 29, 1439–1463. [Google Scholar] [CrossRef]
- Farrajota, L.; Maruta, C.; Maroco, J.; Martins, I.P.; Guerreiro, M.; de Mendonça, A. Speech therapy in primary progressive aphasia: A pilot study. Dement. Geriatr. Cogn. Disord. Extra 2012, 2, 321. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Petesi, M.; Brambilla, M.; Cosseddu, M.; Zanetti, O.; Miniussi, C.; Padovani, A.; Borroni, B. Treatment of PPA by transcranial direct current stimulation combined with language training. J. Alzheimer’s Dis. 2014, 39, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Dial, H.R.; Hinshelwood, H.; Grasso, S.M.; Hubbard, H.I.; Gorno-Tempini, M.-L.; Henry, M.L. Investigating the utility of teletherapy in individuals with primary progressive aphasia. Clin. Interv. Aging 2019, 14, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Krajenbrink, T.; Croot, K.; Taylor-Rubin, C.; Nickels, L. Treatment for spoken and written word retrieval in the semantic variant of primary progressive aphasia. Neuropsychol. Rehabil. 2020, 30, 915–947. [Google Scholar] [CrossRef]
- Taylor-Rubin, C.; Nickels, L.; Croot, K. Exploring the effects of verb and noun treatment on verb phrase production in primary progressive aphasia: A series of single-case experimental design studies. Neuropsychol. Rehabil. 2022, 32, 1121–1163. [Google Scholar] [CrossRef]
- Gervits, F.; Ash, S.; Coslett, H.B.; Rascovsky, K.; Grossman, M.; Hamilton, R. Transcranial direct current stimulation for the treatment of PPA: An open-label pilot study. Brain Lang. 2016, 162, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jokel, R.; Kielar, A.; Anderson, N.D.; Black, S.E.; Rochon, E.; Graham, S.; Freedman, M.; Tang-Wai, D.F. Behavioural and neuroimaging changes after naming therapy for semantic variant primary progressive aphasia. Neuropsychologia 2016, 89, 191–216. [Google Scholar] [CrossRef]
- Meyer, A.M.; Snider, S.F.; Eckmann, C.B.; Friedman, R.B. Prophylactic treatments for anomia in the logopenic variant of primary progressive aphasia: Cross-language transfer. Aphasiology 2015, 29, 1062–1081. [Google Scholar] [CrossRef]
- Trebbastoni, A.; Raccah, R.; de Lena, C.; Zangen, A.; Inghilleri, M. Repetitive deep transcranial magnetic stimulation improves verbal fluency and written language in a patient with logopenic variant (lvPPA). Brain Stimul. 2013, 6, 545–553. [Google Scholar] [CrossRef]
- Meyer, A.M.; Getz, H.R.; Brennan, D.M.; Hu, T.M.; Friedman, R.B. Telerehabilitation of anomia in primary progressive aphasia. Aphasiology 2016, 30, 483–507. [Google Scholar] [CrossRef]
- Shah-Basak, P.; Fernandez, A.; Armstrong, S.E.M.; Hodzic-Santor, B.H.; Lavoie, M.; Jokel, R.; Meltzer, J.A. Behavioural and neurophysiological responses to written naming treatment and high definition tDCS: A case study in advanced primary progressive aphasia. Aphasiology 2022, 36, 1182–1205. [Google Scholar] [CrossRef]
- Hung, J.; Bauer, A.; Grossman, M.; Hamilton, R.H.; Coslett, H.B.; Reilly, J. Semantic feature training in combination with transcranial direct current stimulation (tDCS) for progressive anomia. Front. Hum. Neurosci. 2017, 11, 223285. [Google Scholar] [CrossRef]
- Themistocleous, C.; Webster, K.; Tsapkini, K. Effects of tDCS on sound duration in patients with apraxia of speech in primary progressive aphasia. Brain Sci. 2021, 11, 335. [Google Scholar] [CrossRef]
- Jafari, S.; Khatoonabadi, A.R.; Noroozian, M.; Mehri, A.; Ashayeri, H.; Nickels, L. The effect of word retrieval therapy in primary progressive aphasia: A single-case study. Arch. Neurosci. 2018, 5, e67577. [Google Scholar] [CrossRef]
- de Aguiar, V.; Rofes, A.; Wendt, H.; Ficek, B.N.; Webster, K.; Tsapkini, K. Treating lexical retrieval using letter fluency and tDCS in primary progressive aphasia: A single-case study. Aphasiology 2022, 36, 353–379. [Google Scholar] [CrossRef]
- Lavoie, M.; Bier, N.; Laforce, R.; Macoir, J. Improvement in functional vocabulary and generalization to conversation following a self-administered treatment using a smart tablet in primary progressive aphasia. Neuropsychol. Rehabil. 2020, 30, 1224–1254. [Google Scholar] [CrossRef] [PubMed]
- Croot, K.; Taylor, C.; Abel, S.; Jones, K.; Krein, L.; Hameister, I.; Ruggero, L.; Nickels, L. Measuring gains in connected speech following treatment for word retrieval: A study with two participants with primary progressive aphasia. Aphasiology 2015, 29, 1265–1288. [Google Scholar] [CrossRef]
- Tsapkini, K.; Frangakis, C.; Gomez, Y.; Davis, C.; Hillis, A.E. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology 2014, 28, 1112–1130. [Google Scholar] [CrossRef]
- Fenner, A.S.; Webster, K.T.; Ficek, B.N.; Frangakis, C.E.; Tsapkini, K. Written verb naming improves after tDCS over the left IFG in primary progressive aphasia. Front. Psychol. 2019, 10, 1396. [Google Scholar] [CrossRef]
- Henry, M.L.; Hubbard, H.I.; Grasso, S.M.; Dial, H.R.; Beeson, P.M.; Miller, B.L.; Gorno-Tempini, M.L. Treatment for word retrieval in semantic and logopenic variants of primary progressive aphasia: Immediate and long-term outcomes. J. Speech Lang. Hear. Res. 2019, 62, 2723–2749. [Google Scholar] [CrossRef]
- Henry, M.L.; Rising, K.; DeMarco, A.T.; Miller, B.; Gorno-Tempini, M.; Beeson, P. Examining the value of lexical retrieval treatment in PPA: Two positive cases. Brain Lang. 2013, 127, 145–156. [Google Scholar] [CrossRef]
- Kim, M. Effect of lexical retrieval cascade treatment on naming and discourse of individuals with logopenic variant of PPA. Clin. Arch. Commun. Disord. 2017, 2, 197. [Google Scholar] [CrossRef]
- Dressel, K.; Huber, W.; Frings, L.; Kümmerer, D.; Saur, D.; Mader, I.; Hüll, M.; Weiller, C.; Abel, S. Model-oriented naming therapy in semantic dementia: A single-case fMRI study. Aphasiology 2010, 24, 1537–1558. [Google Scholar] [CrossRef]
- Jokel, R.; Rochon, E.; Anderson, N.D. Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychol. Rehabil. 2010, 20, 16–41. [Google Scholar] [CrossRef]
- Senaha, M.L.H.; Brucki, S.M.D.; Nitrini, R. Rehabilitation in semantic dementia: Study of the effectiveness of lexical reacquisition in three patients. Dement. Neuropsychol. 2010, 4, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Beeson, P.M.; King, R.M.; Bonakdarpour, B.; Henry, M.L.; Cho, H.; Rapcsak, S.Z. Positive effects of language treatment for the logopenic variant of primary progressive aphasia. J. Mol. Neurosci. 2011, 45, 724–736. [Google Scholar] [CrossRef]
- Mayberry, E.J.; Sage, K.; Ehsan, S.; Ralph, M.A.L. Relearning in semantic dementia reflects contributions from both medial temporal lobe episodic and degraded neocortical semantic systems. Neuropsychologia 2011, 49, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Jokel, R.; Anderson, N.D. Quest for the best: Effects of errorless and active encoding on word re-learning in semantic dementia. Neuropsychol. Rehabil. 2012, 22, 187–214. [Google Scholar] [CrossRef]
- Henry, M.L.; Meese, M.V.; Truong, S.; Babiak, M.C.; Miller, B.L.; Gorno-Tempini, M.L. Treatment for apraxia of speech in nonfluent variant primary progressive aphasia. Behav. Neurol. 2013, 26, 77–88. [Google Scholar] [CrossRef]
- Hoffman, P.; Clarke, N.; Jones, R.W.; Noonan, K.A. Vocabulary relearning in semantic dementia: Positive and negative consequences of increasing variability in the learning experience. Neuropsychologia 2015, 76, 240–253. [Google Scholar] [CrossRef]
- Suárez-González, A.; Heredia, C.G.; Savage, S.A.; Gil-Néciga, E.; García-Casares, N.; Franco-Macías, E.; Berthier, M.L.; Caine, D. Restoration of conceptual knowledge in a case of semantic dementia. Neurocase 2015, 21, 309–321. [Google Scholar] [CrossRef]
- Roncero, C.; Kniefel, H.; Service, E.; Thiel, A.; Probst, S.; Chertkow, H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 247–253. [Google Scholar] [CrossRef]
- Ficek, B.N.; Wang, Z.; Zhao, Y.; Webster, K.T.; Desmond, J.E.; Hillis, A.E.; Frangakis, C.; Faria, A.V.; Caffo, B.; Tsapkini, K. The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage Clin. 2018, 19, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.M.; Shuster, K.M.; Henry, M.L. Comparing the effects of clinician and caregiver-administered lexical retrieval training for progressive anomia. Neuropsychol. Rehabil. 2019, 29, 866–895. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Faria, A.V.; Tippett, D.C.; Hillis, A.E.; Friedman, R.B. The relationship between baseline volume in temporal areas and post-treatment naming accuracy in primary progressive aphasia. Aphasiology 2017, 31, 1059–1077. [Google Scholar] [CrossRef]
- Suárez-González, A.; Savage, S.A.; Caine, D. Successful short-term re-learning and generalisation of concepts in semantic dementia. Neuropsychol. Rehabil. 2018, 28, 1095–1109. [Google Scholar] [CrossRef]
- Croot, K.; Raiser, T.; Taylor-Rubin, C.; Ruggero, L.; Ackl, N.; Wlasich, E.; Danek, A.; Scharfenberg, A.; Foxe, D.; Hodges, J.R.; et al. Lexical retrieval treatment in primary progressive aphasia: An investigation of treatment duration in a heterogeneous case series. Cortex 2019, 115, 133–158. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Paternicò, D.; Cosseddu, M.; Brambilla, M.; Petesi, M.; Premi, E.; Gasparotti, R.; Zanetti, O.; Padovani, A.; et al. Grey matter density predicts the improvement of naming abilities after tDCS intervention in agrammatic variant of primary progressive aphasia. Brain Topogr. 2016, 29, 738–751. [Google Scholar] [CrossRef]
- Sayadnasiri, M.; Dieji, B.; Noroozi, M.; Rezaei, O. The effect of zolpidem on language function of patients with nonfluent variant of frontotemporal dementia: A pilot study. Clin. Neuropharmacol. 2021, 44, 81–84. [Google Scholar] [CrossRef]
- Wang, Z.; Ficek, B.N.; Webster, K.T.; Herrmann, O.; Frangakis, C.E.; Desmond, J.E.; Onyike, C.U.; Caffo, B.; Hillis, A.E.; Tsapkini, K. Specificity in generalization effects of transcranial direct current stimulation over the left inferior frontal gyrus in primary progressive aphasia. Neuromodul. Technol. Neural Interface 2023, 26, 850–860. [Google Scholar] [CrossRef]
- Lerman, A.; Mais, D.; Nissani, Y.; Malcolm, T. Preserving lexical retrieval skills across languages in a bilingual person with logopenic primary progressive aphasia. Aphasiology 2023, 37, 432–455. [Google Scholar] [CrossRef]
- Watanabe, M.; Cartwright, J.; Pierce, J.E. Positive effects of speech and language therapy group interventions in primary progressive aphasia: A systematic review. Int. J. Lang. Commun. Disord. 2024, 59, 1832–1849. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Ferrari, C.; Gobbi, E.; Macis, A.; Cappa, S.F. Effectiveness of language training and non-invasive brain stimulation on oral and written naming performance in primary progressive aphasia: A meta-analysis and systematic review. Neurosci. Biobehav. Rev. 2020, 108, 498–525. [Google Scholar] [CrossRef]
- Nissim, N.R.; Moberg, P.J.; Hamilton, R.H. Efficacy of noninvasive brain stimulation (tDCS or TMS) paired with language therapy in the treatment of primary progressive aphasia: An exploratory meta-analysis. Brain Sci. 2020, 10, 597. [Google Scholar] [CrossRef]
- Lomi, F.; Simonelli, I.; Cappa, S.; Pasqualetti, P.; Rossi, S. Noninvasive brain stimulation in primary progressive aphasia with and without concomitant speech and language therapy: Systematic review and meta-analysis. Neuropsychol. Rev. 2025, 1–27. [Google Scholar] [CrossRef]
- Tee, B.L.; Gorno-Tempini, M.L. Primary progressive aphasia: A model for neurodegenerative disease. Curr. Opin. Neurol. 2019, 32, 255–265. [Google Scholar] [CrossRef]
- Grossman, M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012, 11, 545–555. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Coventry, C.; Bigio, E.H.; Geula, C.; Thompson, C.; Bonakdarpour, B.; Gefen, T.; Rogalski, E.J.; Weintraub, S. Nosology of primary progressive aphasia and the neuropathology of language. Adv. Exp. Med. Biol. 2021, 1281, 33–49. [Google Scholar] [CrossRef]
- Goldberg, Z.-L.; El-Omar, H.; Foxe, D.; Leyton, C.E.; Ahmed, R.M.; Piguet, O.; Irish, M. Cognitive and neural mechanisms of social communication dysfunction in primary progressive aphasia. Brain Sci. 2021, 11, 1600. [Google Scholar] [CrossRef]
- Europa, E.; Iaccarino, L.; Perry, D.C.; Weis, E.; Welch, A.E.; Rabinovici, G.D.; Miller, B.L.; Gorno-Tempini, M.L.; Henry, M.L. Diagnostic assessment in primary progressive aphasia: An illustrative case example. Am. J. Speech-Lang. Pathol. 2020, 29, 1833–1849. [Google Scholar] [CrossRef]
- Mazzeo, S.; Morinelli, C.; Polito, C.; Giacomucci, G.; Moschini, V.; Ingannato, A.; Balestrini, J.; Frigerio, D.; Emiliani, F.; Galdo, G.; et al. Data-driven subtypes of mixed semantic-logopenic PPA: Linguistic features, biomarker profiles and brain metabolic patterns. J. Neurol. Sci. 2024, 460, 122998. [Google Scholar] [CrossRef]
- Mesulam, M. Primary progressive aphasia: A dementia of the language network. Dement. Neuropsychol. 2013, 7, 2–9. [Google Scholar] [CrossRef]
- Carlos, A.F.; Josephs, K.A. Frontotemporal lobar degeneration with TAR DNA-binding protein 43 (TDP-43): Its journey of more than 100 years. J. Neurol. 2022, 269, 4030–4054. [Google Scholar] [CrossRef] [PubMed]
- Sitek, E.J.; Narożańska, E.; Brockhuis, B.; Muraszko-Klaudel, A.; Lass, P.; Harciarek, M.; Sławek, J. Neuroimaging in the differential diagnosis of primary progressive aphasia–Illustrative case series in the light of new diagnostic criteria. Pol. J. Radiol. 2014, 79, 251. [Google Scholar] [CrossRef]
- Roytman, M.; Chiang, G.; Gordon, M.; Franceschi, A. Multimodality imaging in primary progressive aphasia. Am. J. Neuroradiol. 2022, 43, 1230–1243. [Google Scholar] [CrossRef]
- Caso, F.; Mandelli, M.L.; Henry, M.; Gesierich, B.; Bettcher, B.M.; Ogar, J.; Filippi, M.; Comi, G.; Magnani, G.; Sidhu, M.; et al. In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology 2014, 82, 239–247. [Google Scholar] [CrossRef]
- Mendez, M.F.; Nasir, I. Distinguishing semantic variant primary progressive aphasia from Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2023, 7, 227–234. [Google Scholar] [CrossRef]
- Momota, Y.; Konishi, M.; Takahata, K.; Kishimoto, T.; Tezuka, T.; Bun, S.; Tabuchi, H.; Ito, D.; Mimura, M. Case report: Non-Alzheimer’s disease tauopathy with logopenic variant primary progressive aphasia diagnosed using amyloid and tau PET. Front. Neurol. 2022, 13, 1049113. [Google Scholar] [CrossRef]
- Henry, M.L.; Gorno-Tempini, M.L. The logopenic variant of primary progressive aphasia. Curr. Opin. Neurol. 2010, 23, 633–637. [Google Scholar] [CrossRef]
- Gkintoni, E.; Michou, E. Advancing neuropsychological rehabilitation in primary progressive aphasia based on principles of cognitive neuroscience: A scoping review and systematic analysis of the data. Brain Sci. 2024, 14, 1234. [Google Scholar] [CrossRef]
- Schaeverbeke, J.; Evenepoel, C.; Bruffaerts, R.; Van Laere, K.; Bormans, G.; Dries, E.; Tousseyn, T.; Nelissen, N.; Peeters, R.; Vandenbulcke, M.; et al. Cholinergic depletion and basal forebrain volume in primary progressive aphasia. NeuroImage Clin. 2017, 13, 271–279. [Google Scholar] [CrossRef]
- Members of the Task Force on Cognitive Rehabilitation; Cappa, S.F.; Benke, T.; Clarke, S.; Rossi, B.; Stemmer, B.; van Heugten, C.M. EFNS guidelines on cognitive rehabilitation: Report of an EFNS task force. Eur. J. Neurol. 2005, 12, 665–680. [Google Scholar] [CrossRef]
- Gallée, J.; Volkmer, A. Role of the speech-language therapist/pathologist in primary progressive aphasia. Neurol. Clin. Pract. 2023, 13, e200178. [Google Scholar] [CrossRef]
- Elsahar, Y.; Hu, S.; Bouazza-Marouf, K.; Kerr, D.; Mansor, A. Augmentative and alternative communication (AAC) advances: A review of configurations for individuals with a speech disability. Sensors 2019, 19, 1911. [Google Scholar] [CrossRef]
- Robinaugh, G.; Henry, M.L. Behavioral interventions for primary progressive aphasia. Handb. Clin. Neurol. 2022, 185, 221–240. [Google Scholar] [CrossRef]
| Study | Publication Year | Country | Study Design | Population | PPA Subtype | Therapy Used | Treatment Duration | Age, Mean (SD) or Range | Gender (M/F) |
|---|---|---|---|---|---|---|---|---|---|
| Tsapkinia [28] | 2018 | USA | Randomized controlled trial | 36 | nfvPPA: 14, svPPA: 10, lvPPA: 12 | tDCS and language/spelling therapy | 6 w | N/A | 20/16 |
| Rogalski [29] | 2022 | USA | Prospective experimental study | 49 | nfvPPA: 15, svPPA: 4, lvPPA: 18, other variants: 12 | Speech-language therapy | N/A | 67.1 (7.3) | 25/24 |
| Joubert [30] | 2023 | Canada | Single case experimental study | 1 | svPPA: 1 | Smartphone-based cognitive and picture naming therapy | 5 W | 66 | 0/1 |
| Meyer [31] | 2023 | USA | Prospective experimental study | 37 | N/A | Phonological and orthographic treatment | N/A | 70.61 (7.49) | N/A |
| Aguiar [32] | 2020 | USA | Randomized controlled trial | 40 | nfvPPA: 15, lvPPA: 17, svPPA: 8 | Picture naming, spelling and tDCS | 3 W | 67.68 (6.76) | 22/18 |
| Nissim [33] | 2022 | USA | Prospective experimental study | 12 | lvPPA: 8, svPPA: 2, nfvPPA: 2 | HD-tDCS and CILT | 4 W | 66.92 (6.37) | 8/4 |
| Henry [34] | 2018 | USA | Prospective experimental study | 10 | nfvPPA: 10 | VISTA | N/A | 67.70 (5.5) | 4/6 |
| Pytel [35] | 2021 | Spain | Randomized controlled trial | 20 | nfvPPA: 16, svPPA: 4 | rTMS | 10 w | N/A | 8/12 |
| Montagut [36] | 2021 | Spain | Prospective experimental study | 8 | svPPA: 8 | Errorless learning therapy | 8 w | 64 (10.5) | 4/4 |
| Huang [37] | 2023 | China | Randomized controlled trial | 40 | nfvPPA: 16, svPPA: 12, lvPPA: 12 (20 patients in control, and 20 patients in intervention) | rTMS | 4 w | 65.2 (6.6) | 19/21 |
| Borrego-Ecija [38] | 2023 | Spain | Randomized controlled trial | 15 | svPPA: 4, lvPPA: 5, nfvPPA: 6 | tDCS and speech therapy | 4 w | 63 (8.4) | 5/10 |
| Schaffer [39] | 2022 | USA | Single case experimental study | 1 | nfvPPA: 1 | VISTA | 6 w | 72 | 1/0 |
| Schaffer [40] | 2021 | USA | Single case experimental study | 1 | nfvPPA: 1 | Spontaneous speech and fluency therapy | 6 w | 78 | 0/1 |
| Meyer [41] | 2018 | USA | Prospective experimental study | 14 | svPPA: 5, lvPPA: 9 | Phonological and orthographic treatment | 24 w | 65.6 (5.3) | 5/9 |
| Meyer [42] | 2019 | USA | Prospective experimental study | 26 | svPPA: 5, lvPPA: 9, nfvPPA: 12 | Phonological and orthographic treatment | 24 w | 69.2 (8.1) | 12/14 |
| Farrajota [43] | 2012 | Portugal | Prospective experimental study | 20 | Intervention: nfvPPA: 2, svPPA: 2, lvPPA: 6 Control: nfvPPA: 0, svPPA: 6, lvPPA: 4 | SLT | N/A | 68 (7.8) | 14/6 |
| Cotelli [44] | 2014 | Italy | Randomized controlled trial | 16 | lvPPA: 16 | tDCS and ICAT | 2 w | 66.9 (8.2) | 6/10 |
| Dial [45] | 2019 | USA | Non-randomized trial | 31 | nfvPPA: 10, svPPA: 10, lvPPA: 11 | LRT and VISTA | N/A | 67.6 (3.9) | 13/18 |
| Krajenbrink [46] | 2020 | Australia | Single case experimental study | 1 | svPPA: 1 | RRIPP and COEN | 6 w | 60 | 1/0 |
| Tylor-Rubin [47] | 2022 | Australia | Case series | 4 | svPPA: 3, lvPPA: 1 | RRIPP | 6 w | 69.25 (5.2) | 0/4 |
| Gervits [48] | 2016 | USA | Prospective experimental study | 6 | lvPPA: 4, nfvPPA: 2 | tDCS | 2 w | 66.2 (5.7) | 1/5 |
| Rogalski [17] | 2016 | USA | Prospective experimental study | 31 | N/A | SLT | 8 w | 67.2 (6.9) | 13/18 |
| Jokel [49] | 2016 | Canada | Case Series | 4 | svPPA: 4 | Errorless learning therapy | 10 w | 61.25 (7.75) | N/A |
| Meyer [50] | 2015 | USA | Single case experimental study | 1 | lvPPA: 1 | Phonological and orthographic treatment | 48 w | 69 | 0/1 |
| Trebbastoni [51] | 2013 | Italy | Single case experimental study | 1 | lvPPA: 1 | hf-rTMS | 20 s | 50 | 1/0 |
| Meyer [52] | 2016 | USA | Case series | 3 | nfvPPA: 1, svPPA: 1, lvPPA: 1 | Phonological and orthographic treatment | 24 w | 61.6 | 1/2 |
| Shah-Basak [53] | 2022 | Canada | Single case experimental study | 1 | nfvPPA: 1 | Written naming and HD-tDCS | 2 w | 67 | 1/0 |
| Hung [54] | 2017 | USA | Prospective experimental study | 4 | svPPA: 3, lvPPA: 1 | Semantic feature training and tDCS | 2 w | 66.6 (8.56) | 3/2 |
| Themistocleous [55] | 2021 | USA | Prospective experimental study | 8 | nfvPPA with AOS: 8 | tDCS and speech therapy | 6 w | 66 (8.3) | 4/4 |
| Jafari [56] | 2018 | Iran | Single case experimental study | 1 | nfvPPA with AOS: 1 | Cueing hierarchy and story-retelling therapy | 8 w | 56 | 0/1 |
| Aguiar [56] | 2022 | USA | Single case experimental study | 1 | lvPPA: 1 | LeFT and anodal tDCS | 12 w | 72 | 1/0 |
| Lavoie [58] | 2020 | Canada | Case series | 5 | svPPA: 2, lvPPA: 3 | Tablet-based SFA | 4 w | 72 | 2/3 |
| Croot [59] | 2015 | Australia | Case series | 2 | lvPPA: 2 | RRIPP | 2 w | 67 (13) | 1/1 |
| Tsapkini [60] | 2014 | USA | Crossover trial | 6 | nfvPPA: 2, lvPPA: 4 | tDCS | N/A | N/A | 3/3 |
| Fenner [61] | 2019 | USA | Randomized controlled trial | 11 | nfvPPA: 6, lvPPA: 5 | tDCS and verb naming and spelling therapy | N/A | 67.6 (7.7) | 5/6 |
| Henry [62] | 2019 | USA | Prospective experimental study | 18 | svPPA: 9, lvPPA: 9 | LRT | 12 w | 65.28 (8.32) | 7/11 |
| Henry [63] | 2013 | USA | Case series | 2 | svPPA: 1, lvPPA: 1 | Structured oral reading therapy | 12 w | 57 (3) | 0/1 |
| Kim [64] | 2017 | USA | Case series | 2 | lvPPA: 2 | LRC | N/A | 68.5 (5.5) | N/A |
| Dressel [65] | 2010 | Germany | Single case experimental study | 1 | svPPA: 1 | Naming therapy and cueing hierarchies | 4 w | 48 | 1/0 |
| Jokel [66] | 2010 | Canada | Single case experimental study | 1 | svPPA: 1 | Errorless learning therapy | 12 w | N/A | 1/0 |
| Senaha [67] | 2010 | Brazil | Single case experimental study | 3 | svPPA: 3 | Lexical reacquisition (errorless learning) | 24–72 w | 62.66 (10.14) | 2/1 |
| Beeson [68] | 2011 | USA | Single case experimental study | 1 | lvPPA: 1 | Semantic retrieval therapy | 2 w | 77 | 1/0 |
| Mayberry [69] | 2011 | UK | Prospective experimental study | 2 | svPPA: 2 | Relearning therapy | 3 w | 58.5 (6.5) | 1/1 |
| Jokel [70] | 2012 | Canada | Prospective experimental study | 7 | svPPA: 7 | Errorless learning therapy | 8–12 w | 68.28 (9.96) | 3/4 |
| Henry [71] | 2013 | USA | Single case experimental study | 1 | lvPPA: 1 | LRC | 4 w | 73 | 2/0 |
| Hoffman [72] | 2015 | UK | Case series | 3 | svPPA: 3 | Vocabulary relearning | 3 w | 59.57 (2.87) | 0/3 |
| Suárez-González [73] | 2015 | Spain, UK, Australia | Single case experimental study | 1 | svPPA: 1 | COEN and naming Therapy | 12 w | 57 | 0/1 |
| Roncero [74] | 2017 | Canada | Randomized controlled trial | 10 | nfvPPA: 6, svPPA: 2, lvPPA: 2 | tDCS and object naming | 3 w | 67.4 (5.9) | 7/3 |
| Ficek [75] | 2018 | USA | Randomized controlled trial | 36 | Intervention: nfvPPA: 5, lvPPA: 9, svPPA: 3, Control: nfvPPA: 8, lvPPA: 6, svPPA: 5 | tDCS and language therapy | 6 w | 67.2 (6.5) | 13/11 |
| Grasso [76] | 2019 | USA | Single case experimental study | 2 | Mixed PPA: 1, Mild cognitive Impairment: 1 | LRT | N/A | 72.5 (6.5) | 1/1 |
| Meyer [77] | 2017 | USA | Case series | 21 | lvPPA: 9, svPPA: 5, nfvPPA: 7 | Orthographic treatment | 24 w | 69.1 (2.3) | 10/11 |
| Suárez-González [78] | 2011 | UK | Single case experimental study | 1 | svPPA: 1 | COEN and naming therapy | 1 w | 62 | 0/1 |
| Croot [79] | 2019 | Australia | Case series | 8 | nfvPPA: 3, lvPPA: 2, svPPA: 2, Mixed PPA: 1 | LRT | 2–4 w | 64.87 (5.5) | 3/5 |
| Cotelli [80] | 2016 | Italy | Case series | 18 | nfvPPA: 18 | tDCS | N/A | 66.5 (9.5) | 9/9 |
| Sayadnasiri [81] | 2021 | Iran | Single case experimental study | 13 | nfvPPA: 13 | Zolpidem | 6 w | 58.5 (4.5) | 8/5 |
| Wang [82] | 2023 | USA | Randomized controlled trial | 36 | lvPPA: 14, nfvPPA: 13, svPPA: 9 | tDCS and lexical/semantic therapy | 8 w | 50–80 | 19/17 |
| Lerman [83] | 2023 | Israel | Single case experimental study | 1 | lvPPA: 1 | VNeST | 10 w | 70 | 0/1 |
| First Author | Year | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Overall Risk |
|---|---|---|---|---|---|---|---|
| Tsapkinia [28] | 2018 | Low | Some Concerns | Low | Low | Low | Low |
| Aguiar [32] | 2020 | Low | Low | Low | Some Concerns | Low | Some Concerns |
| Cotelli [44] | 2014 | Low | Low | Some Concerns | Low | Low | Low |
| Huang [37] | 2023 | Low | Low | Low | Low | Some Concerns | Low |
| Borrego-Ecija [38] | 2023 | Some Concerns | Some Concerns | Low | Low | Low | Some Concerns |
| Pytel [37] | 2021 | Low | Some Concerns | Low | Some Concerns | Low | Some Concerns |
| Roncero [74] | 2017 | Low | Low | Low | Low | Low | Low |
| Ficek [75] | 2018 | Low | Low | Low | Low | Some Concerns | Low |
| Wang [82] | 2023 | Low | Some Concerns | Low | Low | Low | Some Concerns |
| First Author | Year | Bias Due to Confounding | Selection Bias | Classification Bias | Deviations from Intended Interventions | Missing Data | Measurement Bias | Reporting Bias | Overall Risk |
|---|---|---|---|---|---|---|---|---|---|
| Rogalski [29] | 2022 | Moderate | Moderate | Low | Some Concerns | Low | Low | Some Concerns | Moderate |
| Meyer [31] | 2023 | Moderate | Low | Some Concerns | Some Concerns | Low | Low | Some Concerns | Moderate |
| Nissim [33] | 2022 | Moderate | Some Concerns | Low | Low | Some Concerns | Low | Some Concerns | Moderate |
| Henry [34] | 2018 | Moderate | Low | Low | Some Concerns | Low | Low | Some Concerns | Moderate |
| Montagut [36] | 2021 | High | Some Concerns | Low | Some Concerns | Some Concerns | Low | High | High |
| Henry [62] | 2019 | Moderate | Low | Some Concerns | Some Concerns | Low | Low | Some Concerns | Moderate |
| Themistocleous [55] | 2021 | Moderate | Low | Low | Some Concerns | Low | Low | Low | Moderate |
| Sayadnasiri [81] | 2021 | High | Some Concerns | Low | Some Concerns | Low | Low | High | High |
| Lerman [83] | 2023 | Moderate | Low | Some Concerns | Low | Low | Low | Some Concerns | Moderate |
| First Author | Year | Patient Selection | Intervention Clearly Described | Outcome Measures Reliable | Follow-Up Adequate | Ethical Considerations | Overall Quality |
|---|---|---|---|---|---|---|---|
| Joubert [30] | 2023 | Yes | Yes | Yes | No | Yes | Moderate |
| Schaffer [39] | 2022 | Yes | Yes | No | No | Yes | Low |
| Schaffer [40] | 2021 | Yes | Yes | Yes | No | Yes | Moderate |
| Meyer [41] | 2018 | Yes | Yes | Yes | No | Yes | Moderate |
| Meyer [42] | 2019 | Yes | Yes | Yes | No | Yes | Moderate |
| Farrajota [43] | 2012 | No | Yes | Yes | No | Yes | Low |
| Dial [45] | 2019 | Yes | Yes | Yes | No | Yes | Moderate |
| Krajenbrink [46] | 2020 | No | Yes | No | No | Yes | Low |
| Taylor-Rubin [47] | 2022 | Yes | Yes | Yes | No | Yes | Moderate |
| Gervits [48] | 2016 | No | Yes | No | No | Yes | Low |
| Rogalski [17] | 2016 | No | Yes | No | No | Yes | Low |
| Jokel [49] | 2016 | Yes | Yes | Yes | No | Yes | Moderate |
| Meyer [50] | 2015 | No | Yes | No | No | Yes | Low |
| Trebbastoni [51] | 2013 | No | Yes | No | No | Yes | Low |
| Meyer [52] | 2016 | No | Yes | No | No | Yes | Low |
| Shah-Basak [53] | 2022 | Yes | Yes | Yes | No | Yes | Moderate |
| Hung [54] | 2017 | Yes | Yes | Yes | No | Yes | Moderate |
| Beeson [68] | 2011 | Yes | Yes | Yes | No | Yes | Moderate |
| Henry [71] | 2013 | No | Yes | No | No | Yes | Low |
| Hoffman [72] | 2015 | No | Yes | No | No | Yes | Low |
| Suárez-González [73] | 2015 | No | Yes | No | No | Yes | Low |
| Roncero [74] | 2017 | Yes | Yes | Yes | No | Yes | Moderate |
| Outcomes | Therapy Used | Authors | PPA Subtype | Follow-Up | Results (Intervention vs. Control) |
|---|---|---|---|---|---|
| Letter (naming) accuracy during spelling of trained words | tDCS and language/spelling therapy | Tsapkinia 2018 [28] | nfvPPA: 14, svPPA: 10, lvPPA: 12 | Post-Treatment | Intervention group language gains were improved and maintained significantly |
| 2 weeks | |||||
| 2 months | |||||
| Ficek 2018 [75] | Intervention: nfvPPA: 5, lvPPA: 9, svPPA: 3 Control: nfvPPA: 8, lvPPA: 6, svPPA: 5 | Post-Treatment | |||
| tDCS and picture naming and spelling therapy | Aguiar 2022 [57] | lvPPA: 1 | Post-Treatment | ||
| 2-weeks | |||||
| 2-months | |||||
| tDCS and lexical/semantic therapy | Wang 2023 [82] | lvPPA: 14, nfvPPA: 13, svPPA: 9 | Post-treatment | ||
| 2 weeks | |||||
| 2 months | |||||
| Phonological and orthographic treatment | Meyer 2018 [41] | svPPA: 5, lvPPA: 9 | Post-Treatment | ||
| Errorless learning therapy | Jokel 2012 [70] | svPPA: 7 | Post-Treatment | ||
| Letter (naming) accuracy during spelling of untrained words | tDCS and language/spelling therapy | Tsapkinia 2018 [28] | nfvPPA: 14, svPPA: 10, lvPPA: 12 | Post-Treatment | |
| 2 weeks | |||||
| 2 Months | |||||
| tDCS and picture naming and spelling | Aguiar 2022 [57] | lvPPA: 1 | Post-Treatment | ||
| 2-weeks | |||||
| 2-months | |||||
| Reading accuracy | rTMS | Pytel 2021 [35] | nfvPPA: 16, svPPA: 4 | Post-Treatment | |
| tDCS and speech therapy | Borrego-Ecija 2023 [38] | svPPA: 4, lvPPA: 5, nfvPPA: 6 | Post-Treatment | ||
| Structured oral reading therapy | Henry 2013 [71] | lvPPA: 1 | Post-Treatment | Reading accuracy improved after therapy implementation | |
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Production of correct, intelligible scripted words (Speech) | VISTA | Henry 2018 [34] | nfvPPA: 10 | Post-Treatment | Substantial improvement and maintenance in production of scripted word |
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| VISTA | Schaffer 2022 [39] | nfvPPA: 1 | Post-Treatment | ||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Spontaneous speech and fluency | Smartphone-based cognitive and picture naming | Joubert 2023 [30] | svPPA: 1 | Post-Treatment | Intervention group showed positive reinforcement of spontaneous speech and fluency with different generalization and maintenance reinforcements |
| 6-months | |||||
| HD-tDCS and CILT | Nissim 2022 [33] | lvPPA: 8, svPPA: 2, nfvPPA: 2 | Post-Treatment | ||
| 6-weeks | |||||
| tDCS and speech therapy | Borrego-Ecija 2023 [38] | svPPA: 4, lvPPA: 5, nfvPPA: 6 | Post-Treatment | ||
| Semantic feature training and tDCS | Hung 2017 [54] | svPPA: 3, lvPPA: 1 | Post-Treatment | ||
| 6-months | |||||
| tDCS and speech therapy | Themistocleous 2021 [55] | nfvPPA with AOS: 8 | Post-Treatment | ||
| 2-months | |||||
| tDCS | Gervits 2016 [48] | lvPPA: 4, nfvPPA: 2 | 2-weeks | ||
| 6-weeks | |||||
| 12-weeks | |||||
| LeFT and anodal tDCS | Aguiar 2022 [57] | lvPPA: 1 | Post-Treatment | ||
| 2-weeks | |||||
| 2-months | |||||
| tDCS | Tsapkini 2014 [60] | nfvPPA: 2, lvPPA: 4 | Post-Treatment | ||
| 2-weeks | |||||
| 2-months | |||||
| rTMS | Pytel 2021 [36] | nfvPPA: 16, svPPA: 4 | Post-Treatment | ||
| hf-rTMS | Trebbastoni 2013 [51] | lvPPA: 1 | Post-Treatment | ||
| VISTA | Henry 2018 [34] | nfvPPA: 10 | 1-year | ||
| VISTA | Schaffer 2022 [39] | nfvPPA: 1 | Post-Treatment | ||
| Spontaneous speech and fluency | Schaffer 2021 [40] | nfvPPA: 1 | Post-Treatment | ||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Errorless learning therapy | Jokel 2016 [49] | svPPA: 4 | Post-Treatment | ||
| Phonological and orthographic treatment | Meyer 2016 [41] | svPPA: 5, lvPPA: 9 | 1-month | ||
| Cueing hierarchy and story-retelling therapy | Jafari 2018 [56] | nfvPPA with AOS: 1 | Post-Treatment | ||
| RRIPP | Croot 2015 [59] | lvPPA: 2 | Patient 1: Post-Treatment, 1-month | ||
| Patient 2: Post-Treatment, 9-months | |||||
| Structured oral reading therapy | Henry 2013 [71] | lvPPA: 1 | Post-Treatment | ||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| LRC | Kim 2017 [64] | lvPPA: 2 | Post-Treatment | ||
| 2-months | |||||
| AOS | VISTA | Henry 2018 [34] | nfvPPA: 10 | Post-Treatment | Intervention group showed significant improvement in speech accuracy that was maintained post-treatment |
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Schaffer 2022 [39] | nfvPPA: 1 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Dial 2019 [45] | nfvPPA: 10, svPPA: 10, lvPPA: 11 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Structured oral reading therapy | Henry 2013 [71] | lvPPA: 1 | Post-Treatment | ||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Meyer 2016 [52] | nfvPPA: 1, svPPA: 1, lvPPA: 1 | 1-month | |||
| LeFT and tDCS | Aguiar 2022 [57] | lvPPA: 1 | Post-Treatment | ||
| 2-weeks | |||||
| 2-months | |||||
| tDCS+ speech therapy | Themistocleous 2021 [55] | nfvPPA with AOS: 8 | Post-Treatment | ||
| 2-months | |||||
| rTMS | Pytal 2021 [35] | nfvPPA: 16, svPPA 4 | Post-Treatment | ||
| HD-tDCS and story retelling with cueing hierarchy | Jafari 2018 [56] | nfvPPA with AOS: 1 | Post-Treatment | ||
| Shah-Basak 2022 [53] | nfvPPA: 1 | 5-days | |||
| 3-months | |||||
| Word retrieval | tDCS and language therapy | Tsapkinia 2018 [28] | nfvPPA: 14, svPPA: 10, lvPPA: 12 | Post-Treatment | Intervention group showed significant improvement in word retrieval potential that was maintained post-treatment |
| 2-weeks | |||||
| 2-months | |||||
| Cotelli 2014 [44] | lvPPA: 16 | 2-weeks | |||
| 12-weeks | |||||
| Roncero 2017 [74] | nfvPPA: 6, svPPA: 2, lvPPA: 2 | 2-weeks | |||
| Ficek 2018 [75] | Intervention:nfvPPA: 5, lvPPA: 9, svPPA: 3 Control: nfvPPA: 8, lvPPA: 6, svPPA: 5 | Post-Treatment | |||
| Huang 2023 [37] | nfvPPA: 16, svPPA: 12, lvPPA: 12 (20 patients in control, and 20 patients in intervention) | 1-month | |||
| 3-months | |||||
| 6-months | |||||
| Borrego-Ecija 2023 [38] | svPPA: 4, lvPPA: 5, nfvPPA: 6 | Post-Treatment | |||
| Tsapkini 2014 [60] | nfvPPA: 2, lvPPA: 4 | Post-Treatment | |||
| 2-weeks | |||||
| 2-months | |||||
| Gervits 2016 [47] | lvPPA: 4, nfvPPA: 2 | 2-weeks | |||
| 6-weeks | |||||
| 12-weeks | |||||
| Lerman 2023 [83] | lvPPA: 1 | Post-Treatment | |||
| Wang 2023 [82] | lvPPA: 14, nfvPPA: 13, svPPA: 9 | Post-Treatment | |||
| 2-weeks | |||||
| 2-months | |||||
| SLT | Rogalski 2022 [30] | nfvPPA: 15, svPPA: 4, lvPPA: 18, other variants: 12 | 2-months | ||
| 6-months | |||||
| Farrajota 2012 [43] | Intervention: nfvPPA: 2, svPPA: 2, lvPPA: 6 Control: nfvPPA: 0, svPPA: 6, lvPPA: 4 | Post-Treatment | |||
| Henry 2019 [62] | svPPA: 9, lvPPA: 9 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Jokel 2016 [49] | svPPA: 4 | Post-Treatment | |||
| Meyer 2017 [77] | lvPPA: 9, svPPA: 5, nfvPPA: 7 | 1-month | |||
| tDCS and spelling/picture naming | Aguiar 2020 [32] | nfvPPA: 15, lvPPA: 17, svPPA: 8 | Post-Treatment | ||
| 2-weeks | |||||
| 2-months | |||||
| Croot 2019 [79] | nfvPPA: 3, lvPPA: 2, svPPA: 2, Mixed PPA: 1 | Post-Treatment | |||
| Borrego-Ecija 2023 [38] | svPPA: 4, lvPPA: 5, nfvPPA: 6 | Post-Treatment | |||
| Phonological and orthographic treatment | Meyer 2018 [41] | svPPA: 5, lvPPA: 9 | Post-Treatment | ||
| Meyer 2019 [42] | svPPA: 5, lvPPA: 9, nfvPPA: 12 | 1-month | |||
| Meyer 2015 [50] | lvPPA: 1 | 1-week | |||
| 8-months | |||||
| 1-year | |||||
| 20-months | |||||
| 3-years | |||||
| Huang 2023 [37] | nfvPPA: 16, svPPA: 12, lvPPA: 12 (20 patients in control, and 20 patients in intervention) | 1-month | |||
| 3-months | |||||
| 6-months | |||||
| Errorless learning therapy | Jokel 2010 [66] | svPPA: 1 | Post-Treatment | ||
| Montagut 2021 [36] | svPPA: 8 | Post-Treatment | |||
| 1-month | |||||
| 3-months | |||||
| 6-months | |||||
| Gervits 2016 [48] | lvPPA: 4, nfvPPA: 2 | 2-weeks | |||
| 6-weeks | |||||
| 12-weeks | |||||
| LRC | Beeson 2011 [68] | lvPPA: 1 | Post-Treatment | ||
| 3-weeks | |||||
| 4-months | |||||
| 6-months | |||||
| Henry 2013 [63] | lvPPA: 1, svPPA: 1 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Meyer 2015 [50] | lvPPA: 1 | 1-week | |||
| 8-months | |||||
| 1-year | |||||
| 20-months | |||||
| 3-years | |||||
| Grasso 2019 [70] | svPPA: 7 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| Henry 2018 [34] | nfvPPA: 10 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| tDCS and CILT | Nissim 2022 [33] | lvPPA: 8, svPPA: 2, nfvPPA: 2 | Post-Treatment | ||
| 6-weeks | |||||
| Semantic feature training and tDCS | Hung 2017 [54] | svPPA: 3, lvPPA: 1 | Post-Treatment | ||
| 6-months | |||||
| Lavoie 2020 [58] | svPPA: 2, lvPPA: 3 | Post-Treatment | |||
| RRIPP and COEN | Krajenbrink 2020 [46] | svPPA: 1 | Post-Treatment | ||
| 2-weeks | |||||
| Tylor-Rubin 2022 [47] | svPPA: 3, lvPPA: 1 | Post-Treatment | |||
| VISTA | Henry 2018 [34] | nfvPPA: 10 | Post-Treatment | ||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Henry 2019 [66] | svPPA: 9, lvPPA: 9 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Schaffer 2022 [39] | nfvPPA: 1 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Smartphone-based cognitive and picture naming | Joubert 2023 [30] | svPPA: 1 | Post-Treatment | ||
| 6-months | |||||
| rTMS | Pytel 2021 [36] | nfvPPA: 16, svPPA: 4 | Post-Treatment | ||
| Reading abilities | Phonological and orthographic treatment | Meyer 2019 [42] | svPPA: 5, lvPPA: 9, nfvPPA: 12 | 1-month | Intervention group showed significant improvement in reading accuracy that was maintained post-treatment at various intervals |
| Meyer 2018 [41] | svPPA: 5, lvPPA: 9 | Post-Treatment | |||
| Henry 2013 [63] | svPPA: 1, lvPPA: 1 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Errorless learning therapy | Jokel 2010 [66] | svPPA: 1 | Post-Treatment | ||
| Senaha 2010 [67] | svPPA: 3 | Post-Treatment | |||
| Montagut 2021 [36] | svPPA: 8 | Post-Treatment | |||
| 1-month | |||||
| 3-months | |||||
| 6-months | |||||
| rTMS | Pytel 2021 [35] | nfvPPA: 16, svPPA: 4 | Post-Treatment | ||
| Henry 2019 [62] | svPPA: 9, lvPPA: 9 | Post-Treatment | |||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| Repetition abilities | tDCS and language therapy | Tsapkinia 2018 [28] | nfvPPA: 14, svPPA: 10, lvPPA: 12 | Post-Treatment | Intervention group showed improvement in reading accuracy that was maintained post-treatment at various intervals, some studies reported mixed results |
| 2-weeks | |||||
| 2-months | |||||
| Cotelli 2016 [80] | Post-Treatment | ||||
| 3-months | |||||
| Ficek 2018 [75] | Intervention: nfvPPA: 5, lvPPA: 9, svPPA: 3 Control: nfvPPA: 8, lvPPA: 6, svPPA: 5 | Post-Treatment | |||
| Gervits 2016 [48] | lvPPA: 4, nfvPPA: 2 | 2-weeks | |||
| 6-weeks | |||||
| 12-weeks | |||||
| Roncero 2017 [74] | nfvPPA: 6, svPPA: 2, lvPPA: 2 | 2-weeks | |||
| Fenner 2019 [61] | nfvPPA: 6, lvPPA: 5 | Post-Treatment | |||
| 2-weeks | |||||
| 2-months | |||||
| SLT | Farrajota 2012 [43] | Intervention: nfvPPA: 2, svPPA: 2, lvPPA: 6 Control: nfvPPA: 0, svPPA: 6, lvPPA: 4 | Post-Treatment | ||
| Meyer 2019 [42] | svPPA: 5, lvPPA: 9, nfvPPA: 12 | 1-month | |||
| Rogalski 2022 [29] | nfvPPA: 15, svPPA: 4, lvPPA: 18, other variants: 12 | 2-months | |||
| 6-months | |||||
| Errorless learning therapy | Jokel 2010 [66] | svPPA: 1 | Post-Treatment | ||
| Jokel 2016 [49] | svPPA: 4 | Post-Treatment | |||
| Themistocleous 2021 [55] | nfvPPA with AOS: 8 | Post-Treatment | |||
| 2-months | |||||
| Cognitive and picture naming therapy | Joubert 2023 [30] | svPPA: 1 | Post-Treatment | ||
| 6-months | |||||
| Phonological and orthographic therapy | Meyer 2015 [50] | lvPPA: 1 | 1-week | ||
| 8-months | |||||
| 1-year | |||||
| 20-months | |||||
| 3-years | |||||
| Meyer 2016 [41] | svPPA: 5, lvPPA: 9 | 1-month | |||
| Meyer 2019 [42] | svPPA: 5, lvPPA: 9, nfvPPA: 12 | 1-month | |||
| tDCS and cognitive therapy (ICAT) | Cotelli 2014 [44] | lvPPA: 16 | 2-weeks | ||
| 12-weeks | |||||
| Tsapkini 2014 [60] | nfvPPA: 2, lvPPA: 4 | Post-Treatment | |||
| 2-weeks | |||||
| 2-months | |||||
| rTMS and language therapy | Pytel 2021 [35] | nfvPPA: 16, svPPA: 4 | Post-Treatment | ||
| VISTA | Henry 2018 [34] | nfvPPA: 10 | Post-treatment | ||
| 3-months | |||||
| 6-months | |||||
| 1-year | |||||
| LRC | Grasso 2019 [76] | Mixed PPA: 1, Mild cognitive Impairment: 1 | Post-Treatment | ||
| 3-months | |||||
| 6-months | |||||
| Suárez-González 2015 [73] | svPPA: 1 | Post-Treatment | |||
| Croot 2019 [79] | nfvPPA: 3, lvPPA: 2, svPPA: 2, Mixed PPA: 1 | Post-Treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrasheed, A.S.; Alshamrani, R.A.; Al Ameer, A.A.; Alkahtani, R.M.; AlMohish, N.M.; AlQarni, M.A.; Alabdali, M.M. Safety and Efficacy of Different Therapeutic Interventions for Primary Progressive Aphasia: A Systematic Review. J. Clin. Med. 2025, 14, 3063. https://doi.org/10.3390/jcm14093063
Alrasheed AS, Alshamrani RA, Al Ameer AA, Alkahtani RM, AlMohish NM, AlQarni MA, Alabdali MM. Safety and Efficacy of Different Therapeutic Interventions for Primary Progressive Aphasia: A Systematic Review. Journal of Clinical Medicine. 2025; 14(9):3063. https://doi.org/10.3390/jcm14093063
Chicago/Turabian StyleAlrasheed, Abdulrahim Saleh, Reem Ali Alshamrani, Abdullah Ali Al Ameer, Reham Mohammed Alkahtani, Noor Mohammad AlMohish, Mustafa Ahmed AlQarni, and Majed Mohammad Alabdali. 2025. "Safety and Efficacy of Different Therapeutic Interventions for Primary Progressive Aphasia: A Systematic Review" Journal of Clinical Medicine 14, no. 9: 3063. https://doi.org/10.3390/jcm14093063
APA StyleAlrasheed, A. S., Alshamrani, R. A., Al Ameer, A. A., Alkahtani, R. M., AlMohish, N. M., AlQarni, M. A., & Alabdali, M. M. (2025). Safety and Efficacy of Different Therapeutic Interventions for Primary Progressive Aphasia: A Systematic Review. Journal of Clinical Medicine, 14(9), 3063. https://doi.org/10.3390/jcm14093063






