Sex-Related Differences in Patients with Mitral Regurgitation Undergoing Mitral Valve Surgery: A Propensity Score-Matched Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Charicteristics
3.2. All-Cause Mortality
3.3. Length of Hospital Stay
3.4. Subgroup Analyses
3.5. Rignt Ventricular Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MR | Mitral regurgitation |
| MV | Mitral valve |
| TR | Tricuspid regurgitation |
| TV | Tricuspid valve |
| TVr | Tricuspid valve repair |
| LV | Left ventricle |
| BSA | Body surface area |
| PSM | Propensity score-matched/propensity score matching |
| HR | Hazard ratio |
| CI | Confidence interval |
| LOS | Post-operative length of hospital stay |
| NYHA | New York Heart Association |
| LVEF | Left ventricular ejection fraction |
| ROC | Receiver operating characteristic |
| AUC | Area under the receiver operating characteristic curve |
| LVESD | Left ventricular end-systolic diameter |
| LVESDi | Left ventricular end-systolic diameter indexed to body surface area |
| LVEDD | Left ventricular end-diastolic diameter |
| LVEDDi | Left ventricular end-diastolic diameter indexed to body surface area |
References
- Waldron, C.; Hundito, A.; Krane, M.; Geirsson, A.; Mori, M. Gender and Sex Differences in the Management, Intervention, and Outcomes of Patients With Severe Primary Mitral Regurgitation. J. Am. Heart Assoc. 2024, 13, e033635. [Google Scholar] [CrossRef]
- Avierinos, J.-F.; Tribouilloy, C.; Bursi, F.; Grigioni, F.; Vanoverschelde, J.-L.; Resseguier, N.; Théron, A.; Pasquet, A.; Pradier, J.; Biagini, E.; et al. Degenerative mitral regurgitation due to flail leaflet: Sex-related differences in presentation, management, and outcomes. Eur. Heart J. 2024, 45, 2306–2316. [Google Scholar] [CrossRef]
- Abadie, B.Q.; Cremer, P.C.; Vakamudi, S.; Gillinov, A.M.; Svensson, L.G.; Cho, L. Sex-Specific Prognosis of Left Ventricular Size and Function Following Repair of Degenerative Mitral Regurgitation. J. Am. Coll. Cardiol. 2024, 83, 303–312. [Google Scholar] [CrossRef]
- Wagner, C.M.; Fu, W.; Brescia, A.A.; Woodford, J.; Green, C.; Likosky, D.S.; Hawkins, R.B.; Romano, M.A.; Ailawadi, G.; Bolling, S.F. Sex-Based Differences in Concomitant Tricuspid Repair During Degenerative Mitral Surgery. Ann. Thorac. Surg. 2024, 118, 147–154. [Google Scholar] [CrossRef]
- McNeely, C.; Vassileva, C. Mitral Valve Surgery in Women: Another Target for Eradicating Sex Inequality. Circ. Cardiovasc. Qual. Outcomes 2016, 9 (Suppl. 1), S94–S96. [Google Scholar] [CrossRef]
- Van Kampen, A.; Butte, S.; Paneitz, D.C.; Nagata, Y.; Langer, N.B.; Borger, M.A.; D’Alessandro, D.A.; Sundt, T.M.; Melnitchouk, S. Presentation and outcomes of women and men undergoing surgery for degenerative mitral regurgitation. Eur. J. Cardiothorac. Surg. 2024, 66, ezae312. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- EL-Andari, R.; Bozso, S.J.; Fialka, N.M.; Kang, J.J.H.; Nagendran, J. Does sex impact outcomes after mitral valve surgery? A systematic review and meta-analysis. Scand. J. Surg. 2022, 111, 99–109. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Chehab, O.; Long, E.; Androshchuk, V.; Gill, H.; Avlonitis, V.; Bosco, P.; Lucchese, G.; Patterson, T.; Redwood, S.; Rajani, R. Right ventricular to pulmonary arterial coupling as a predictor of survival in patients undergoing mitral valve surgery for mitral regurgitation. Eur. J. Cardiothorac. Surg. 2024, 66, ezae421. [Google Scholar] [CrossRef]
- Seeburger, J.; Eifert, S.; Pfannmüller, B.; Garbade, J.; Vollroth, M.; Misfeld, M.; Borger, M.; Mohr, F. Gender Differences in Mitral Valve Surgery. Thorac. Cardiovasc. Surg. 2012, 61, 42–46. [Google Scholar] [CrossRef]
- Malik, M.I.; Nedadur, R.; Fox, S.; Hage, A.; Hage, F.; Tzemos, N.; Chu, M.W.A. Overcoming the Disparity in Mitral Valve Repair: A Sex-Based Analysis of Long-Term Outcomes. Ann. Thorac. Surg. 2024, S0003497524006945. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ma, W.-G.; Zeng, J.-W.; Han, Y.-F.; Sun, K.; Huang, W.-Q. Gender Differences in 381 Patients Undergoing Isolated Mitral Regurgitation Repair. Thorac. Cardiovasc. Surg. 2024. [Google Scholar] [CrossRef]
- Harbaum, L.; Hennigs, J.K.; Pott, J.; Ostermann, J.; Sinning, C.R.; Sau, A.; Sieliwonczyk, E.; Ng, F.S.; Rhodes, C.J.; Tello, K.; et al. Sex-Specific Genetic Determinants of Right Ventricular Structure and Function. Am. J. Respir. Crit. Care Med. 2025, 211, 113–123. [Google Scholar] [CrossRef]
- Namazi, F.; Van Der Bijl, P.; Vo, N.M.; Van Wijngaarden, S.E.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Sex differences in prognosis of significant secondary mitral regurgitation. ESC Heart Fail. 2021, 8, 3539–3546. [Google Scholar] [CrossRef]
- Mantovani, F.; Clavel, M.-A.; Michelena, H.I.; Suri, R.M.; Schaff, H.V.; Enriquez-Sarano, M. Comprehensive Imaging in Women With Organic Mitral Regurgitation. JACC Cardiovasc. Imaging 2016, 9, 388–396. [Google Scholar] [CrossRef]
- House, C.M.; Moriarty, K.A.; Nelson, W.B. Sex difference in mitral valve prolapse regurgitant volume is resolved by normalization of regurgitant volume to left ventricular end-diastolic volume. Int. J. Cardiovasc. Imaging 2024, 40, 2047–2055. [Google Scholar] [CrossRef]
- Berg-Hansen, C.E.; Sindre, R.B.; Grymyr, L.M.D.; Rogge, B.; Valeur, A.E.; Urheim, S.; Hung, J.; Cramariuc, D. Sex differences in left atrial volumes, mechanics, and stiffness in primary mitral regurgitation—A combined 2D and 3D echocardiographic study. Eur. Heart J.-Cardiovasc. Imaging 2024, 25, 1118–1126. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Impact of Chest Wall Conformation on the Outcome of Primary Mitral Regurgitation due to Mitral Valve Prolapse. J. Cardiovasc. Echogr. 2022, 32, 29–37. [Google Scholar] [CrossRef]
- Hohneck, A.; Ansari, U.; Natale, M.; Wittig, K.; Overhoff, D.; Riffel, P.; Boettcher, M.; Akin, I.; Duerschmied, D.; Papavassiliu, T. Description of a new clinical syndrome: Thoracic constriction without evidence of the typical funnel-shaped depression—The “invisible” pectus excavatum. Sci. Rep. 2023, 13, 12036. [Google Scholar] [CrossRef]


| Characteristic | Whole Cohort | PSM Cohort | ||||
|---|---|---|---|---|---|---|
| Male (n = 76) | Female (n = 67) | p-Value | Male (n = 38) | Female (n = 38) | p-Value | |
| Clinical Characteristics | ||||||
| Age (years) | 68 (59, 75) | 67 (54, 76) | 0.9 | 68 (60, 75) | 67 (56, 77) | >0.9 |
| BMI (kg/m2) | 26.4 (24.1, 29.2) | 26.1 (23.6, 29.1) | 0.5 | 25.5 (23.3, 26.9) | 25.6 (22.7, 29.6) | 0.9 |
| Diabetes | 6 (7.9%) | 5 (7.5%) | >0.9 | 2 (5.3%) | 3 (7.9%) | >0.9 |
| Hypertension | 36 (47%) | 38 (57%) | 0.3 | 21 (55%) | 22 (58%) | 0.8 |
| COPD | 6 (7.9%) | 4 (6.0%) | 0.8 | 4 (11%) | 3 (7.9%) | >0.9 |
| Atrial Fibrillation | 30 (39%) | 23 (36%) | 0.5 | 12 (32%) | 13 (34%) | 0.8 |
| Coronary Artery Disease | 11 (14%) | 6 (9.0%) | 0.3 | 5 (13%) | 4 (11%) | >0.9 |
| Prior MI | 3 (3.9%) | 3 (4.5%) | >0.9 | 2 (5.3%) | 2 (5.3%) | >0.9 |
| Prior CVA | 5 (6.6%) | 9 (13%) | 0.2 | 3 (7.9%) | 4 (11%) | >0.9 |
| Prior Cardiac Operation | 2 (2.6%) | 5 (7.5%) | 0.3 | 2 (5.3%) | 2 (5.3%) | >0.9 |
| eGFR (mL/min/1.73 m2) | 68 (57, 83) | 71 (57, 80) | 0.6 | 67 (57, 78) | 72 (53, 81) | >0.9 |
| NYHA ≥ 3 | 34 (45%) | 49 (73%) | <0.001 | 21 (55%) | 24 (63%) | 0.5 |
| Logistic EuroSCORE | 3.9 (2.2, 7.1) | 5.5 (3.2, 8.4) | 0.006 | 4.1 (1.9, 7.2) | 4.9 (3.1, 7.6) | 0.2 |
| Preprocedural Imaging | ||||||
| Ejection Fraction (%) | 60 (53, 65) | 60 (55, 65) | 0.5 | 60 (55, 65) | 60 (55, 64) | 0.5 |
| LVEDD (mm) | 59 (54, 62) | 51 (45, 57) | <0.001 | 60 (56, 62) | 53 (46, 58) | <0.001 |
| LVEDDi (mm/m2) | 29.0 (27.0, 32.0) | 30.0 (26.0, 33.0) | 0.7 | 29.5 (27.3, 32.8) | 30.0 (26.3, 33.0) | 0.9 |
| LVESD (mm) | 38 (34, 43) | 33 (28, 37) | <0.001 | 38 (36, 42) | 33 (28, 40) | 0.001 |
| LVESDi (mm/m2) | 19.0 (17.0, 21.0) | 19.0 (15.0, 21.0) | 0.9 | 19.5 (18.0, 21.0) | 18.5 (15.5, 21.0) | 0.4 |
| TR Grade ≥ 2+ | 23 (30%) | 23 (34%) | 0.6 | 9 (24%) | 12 (32%) | 0.4 |
| Operative Characteristics | ||||||
| Urgent Operation | 8 (11%) | 17 (25%) | 0.020 | 7 (18%) | 7 (18%) | >0.9 |
| MV Replacement (vs. Repair) | 8 (11%) | 19 (28%) | 0.007 | 8 (21%) | 8 (21%) | >0.9 |
| Concomitant TV Intervention | 20 (26%) | 23 (34%) | 0.3 | 7 (18%) | 11 (29%) | 0.3 |
| Secondary MR (vs. Primary) | 12 (16%) | 29 (43%) | <0.001 | 10 (26%) | 11 (29%) | 0.8 |
| Cardiopulmonary Bypass Time (mins) | 133 (111, 149) | 103 (83, 125) | <0.001 | 126 (108, 146) | 110 (88, 130) | 0.009 |
| Cross-Clamp Time (mins) | 91 (81, 112) | 75 (60, 90) | <0.001 | 87 (82, 111) | 82 (61, 96) | 0.010 |
| Outcomes | ||||||
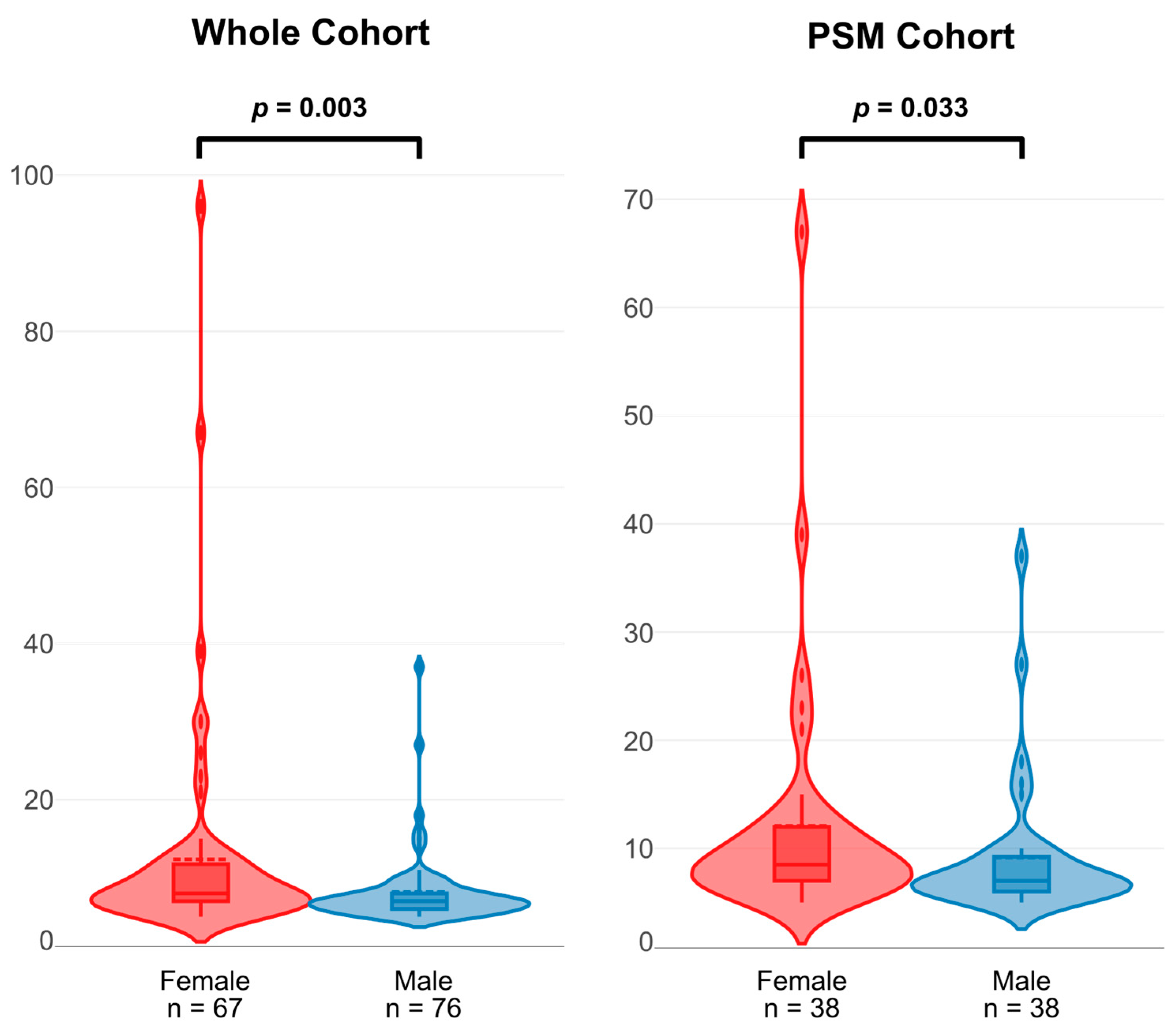
| Post-Operative LOS (days) * | 7 (6, 8) | 8 (7, 12) | 0.003 | 7 (6, 9) | 9 (7, 12) | 0.033 |
| All-Cause Mortality | 13 (17%) | 16 (24%) | 0.3 | 6 (16%) | 7 (18%) | 0.8 |
| Variable | AUC | Specificity | Sensitivity | False Positive Rate |
|---|---|---|---|---|
| LVESD > 40 mm | 0.545 | 26.7% | 69.2% | 73.3% |
| LVESDi > 19 mm/m2 | 0.565 | 46.0% | 69.4% | 54.0% |
| All-Cause Mortality | |||
|---|---|---|---|
| Subgroup | Sex | HR [95% CI] | p-Value |
| MV Replacement (vs. Repair) | Male | 0.96 [0.10–4.84] | 0.96 |
| Female | 0.78 [0.08–3.71] | 0.78 | |
| Concomitant TV Intervention | Male | 5.71 [1.20–27.2] | 0.03 |
| Female | 6.80 [1.63–37.92] | <0.01 | |
| Urgent Operation (vs. Elective) | Male | 2.49 [0.43–11.30] | 0.28 |
| Female | 4.85 [1.08–21.06] | 0.04 | |
| Length of Hospital Stay | |||
| Subgroup | Sex | β [95% CI] | p-Value |
| MV Replacement (vs. Repair) | Male | 5.6 [4.5 to 11] | 0.034 |
| Female | 4.7 [−4.5 to 14] | 0.31 | |
| Concomitant TV Intervention | Male | 5.0 [−0.62 to 11] | 0.080 |
| Female | 5.0 [−3.1 to 13] | 0.22 | |
| Urgent Operation (vs. Elective) | Male | 4.0 [−1.7 to 9.7] | 0.17 |
| Female | 5.2 [−4.4 to 15] | 0.28 | |
| Characteristic | Whole Cohort | PSM Cohort | ||||
|---|---|---|---|---|---|---|
| Male (n = 76) | Female (n = 67) | p-Value | Male (n = 38) | Female (n = 38) | p-Value | |
| TAPSE (mm) | 22 (19, 26) | 20 (18, 22) | 0.005 | 22 (19, 26) | 20 (18, 23) | 0.191 |
| PASP (mmHg) | 39 (30, 54) | 34 (30, 55) | 0.982 | 37 (30, 59) | 32 (30, 45) | 0.284 |
| TAPSE/PASP (mm/mmHg) | 0.57 (0.36, 0.86) | 0.51 (0.37, 0.67) | 0.183 | 0.51 (0.35, 0.86) | 0.62 (0.44, 0.68) | 0.888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, E.; Chehab, O.; Rajah, T.; Dunn, R.; Androshchuk, V.; Wilcox, J.; Gill, H.; Avlonitis, V.; Bosco, P.; Lucchese, G.; et al. Sex-Related Differences in Patients with Mitral Regurgitation Undergoing Mitral Valve Surgery: A Propensity Score-Matched Study. J. Clin. Med. 2025, 14, 3054. https://doi.org/10.3390/jcm14093054
Long E, Chehab O, Rajah T, Dunn R, Androshchuk V, Wilcox J, Gill H, Avlonitis V, Bosco P, Lucchese G, et al. Sex-Related Differences in Patients with Mitral Regurgitation Undergoing Mitral Valve Surgery: A Propensity Score-Matched Study. Journal of Clinical Medicine. 2025; 14(9):3054. https://doi.org/10.3390/jcm14093054
Chicago/Turabian StyleLong, Edouard, Omar Chehab, Tanisha Rajah, Roberta Dunn, Vitaliy Androshchuk, Joshua Wilcox, Harminder Gill, Vassilios Avlonitis, Paolo Bosco, Gianluca Lucchese, and et al. 2025. "Sex-Related Differences in Patients with Mitral Regurgitation Undergoing Mitral Valve Surgery: A Propensity Score-Matched Study" Journal of Clinical Medicine 14, no. 9: 3054. https://doi.org/10.3390/jcm14093054
APA StyleLong, E., Chehab, O., Rajah, T., Dunn, R., Androshchuk, V., Wilcox, J., Gill, H., Avlonitis, V., Bosco, P., Lucchese, G., Patterson, T., Redwood, S., & Rajani, R. (2025). Sex-Related Differences in Patients with Mitral Regurgitation Undergoing Mitral Valve Surgery: A Propensity Score-Matched Study. Journal of Clinical Medicine, 14(9), 3054. https://doi.org/10.3390/jcm14093054






