Rare Case of Grade 3 Undifferentiated Pleomorphic Sarcoma in Left Atrium, Mitral Valve, and Papillary Muscle
Abstract
1. Introduction
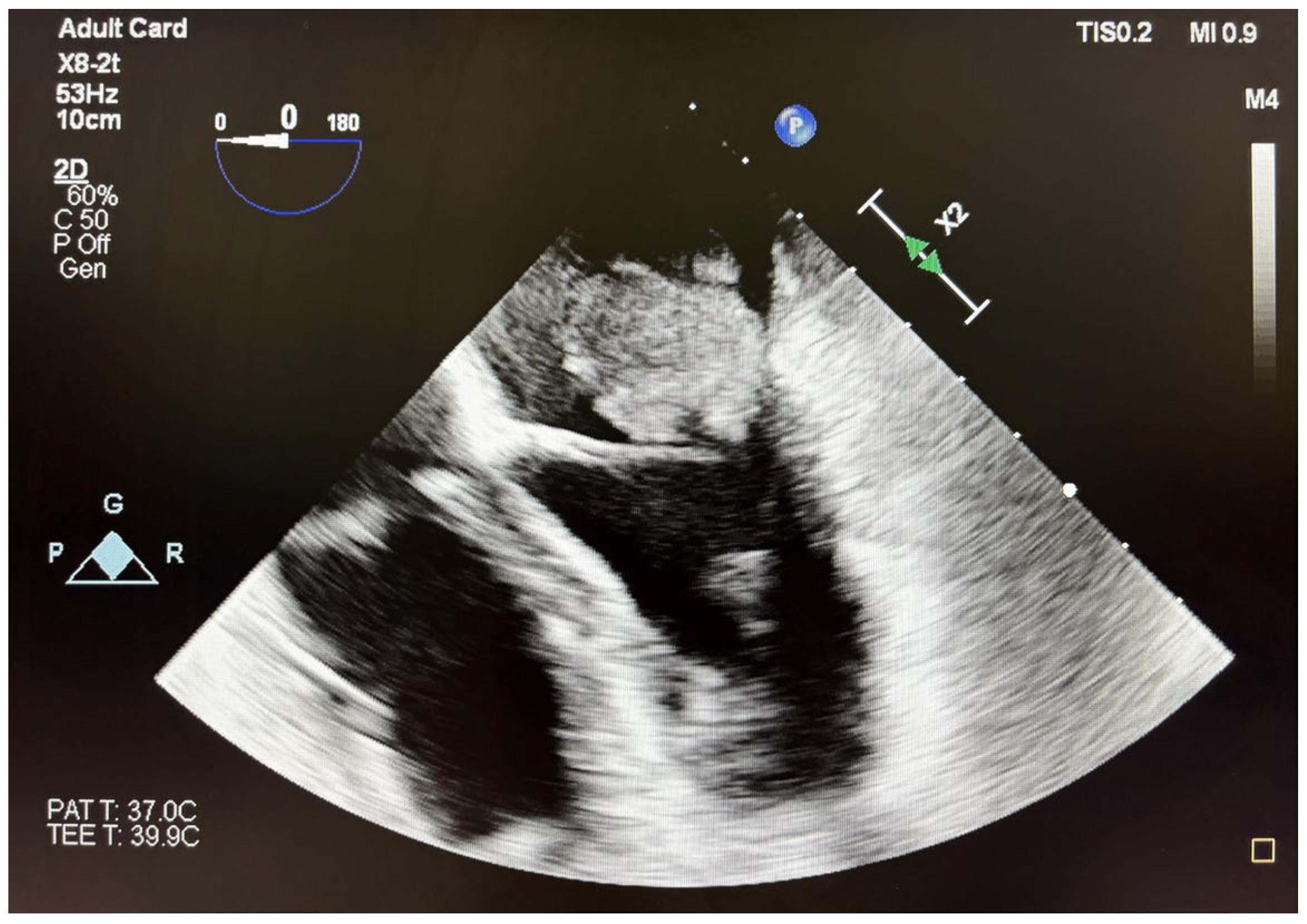
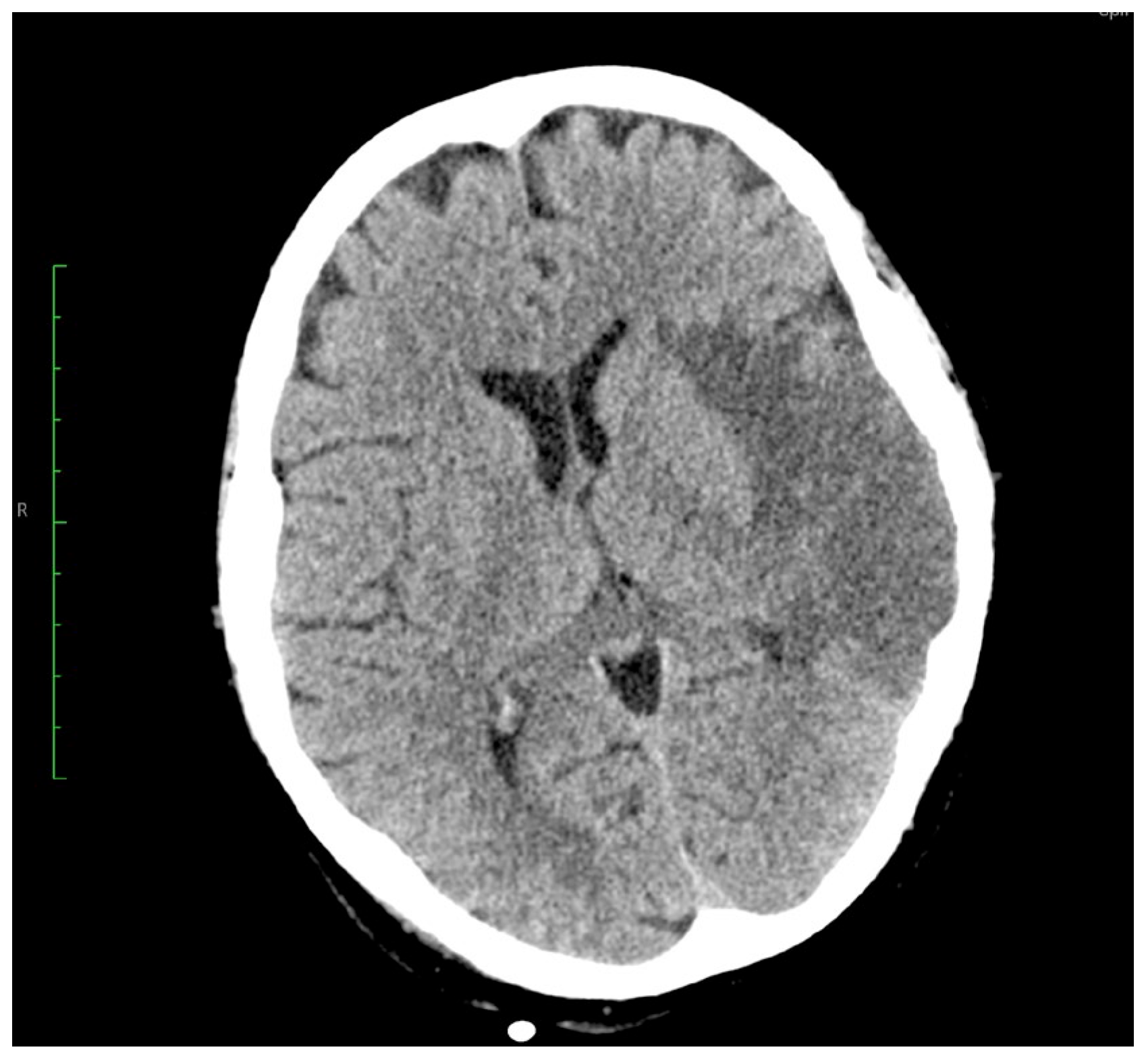
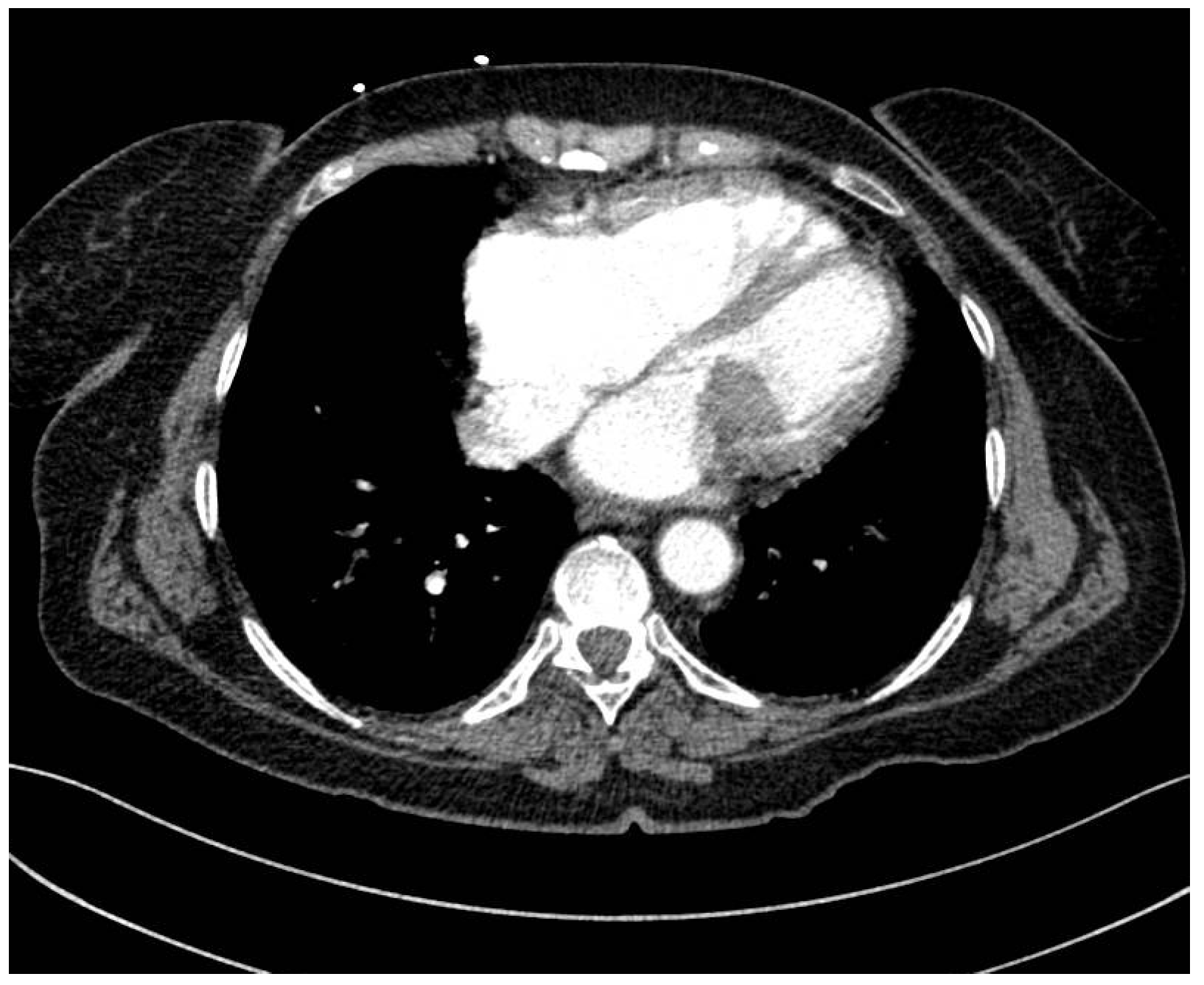
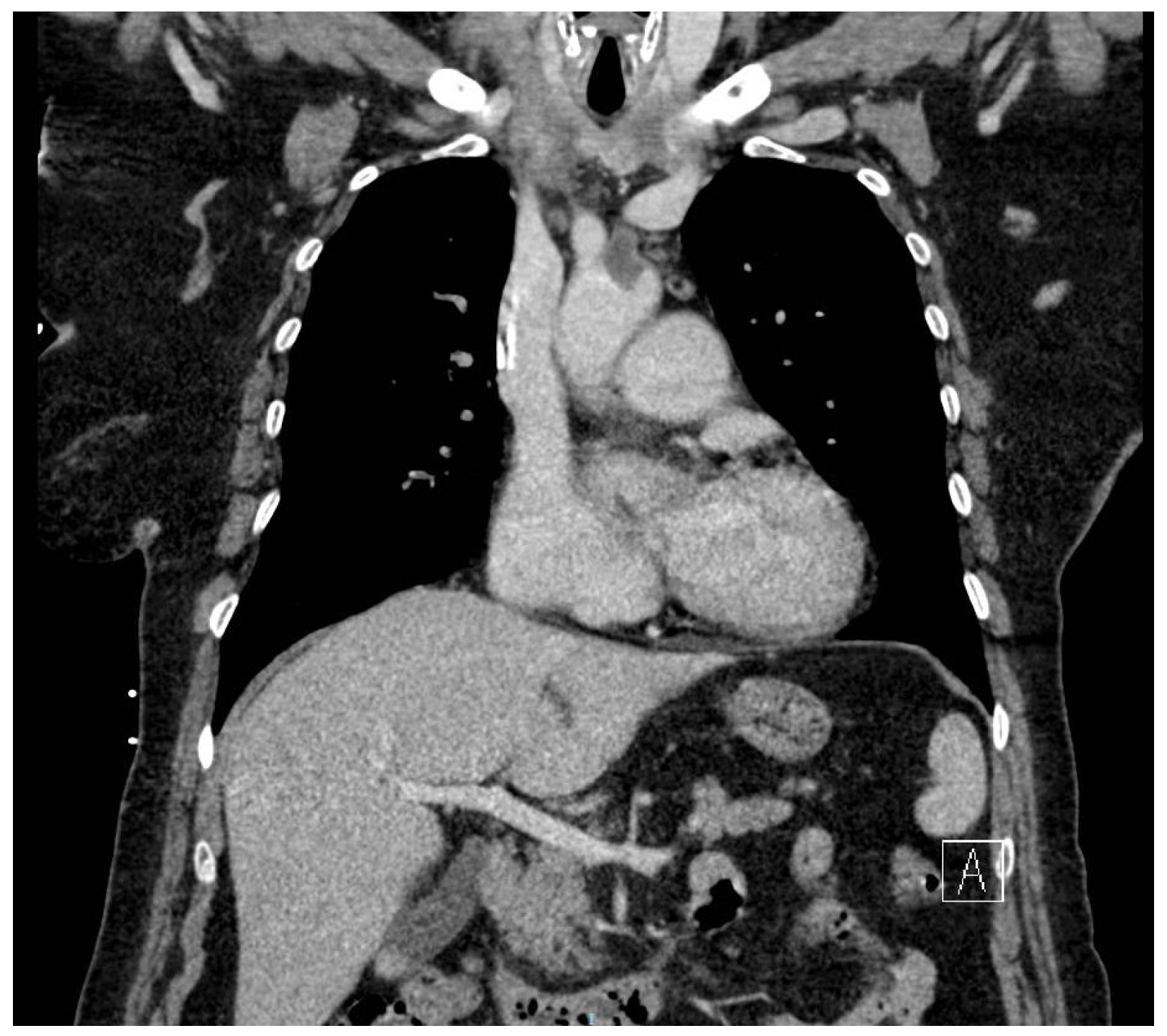
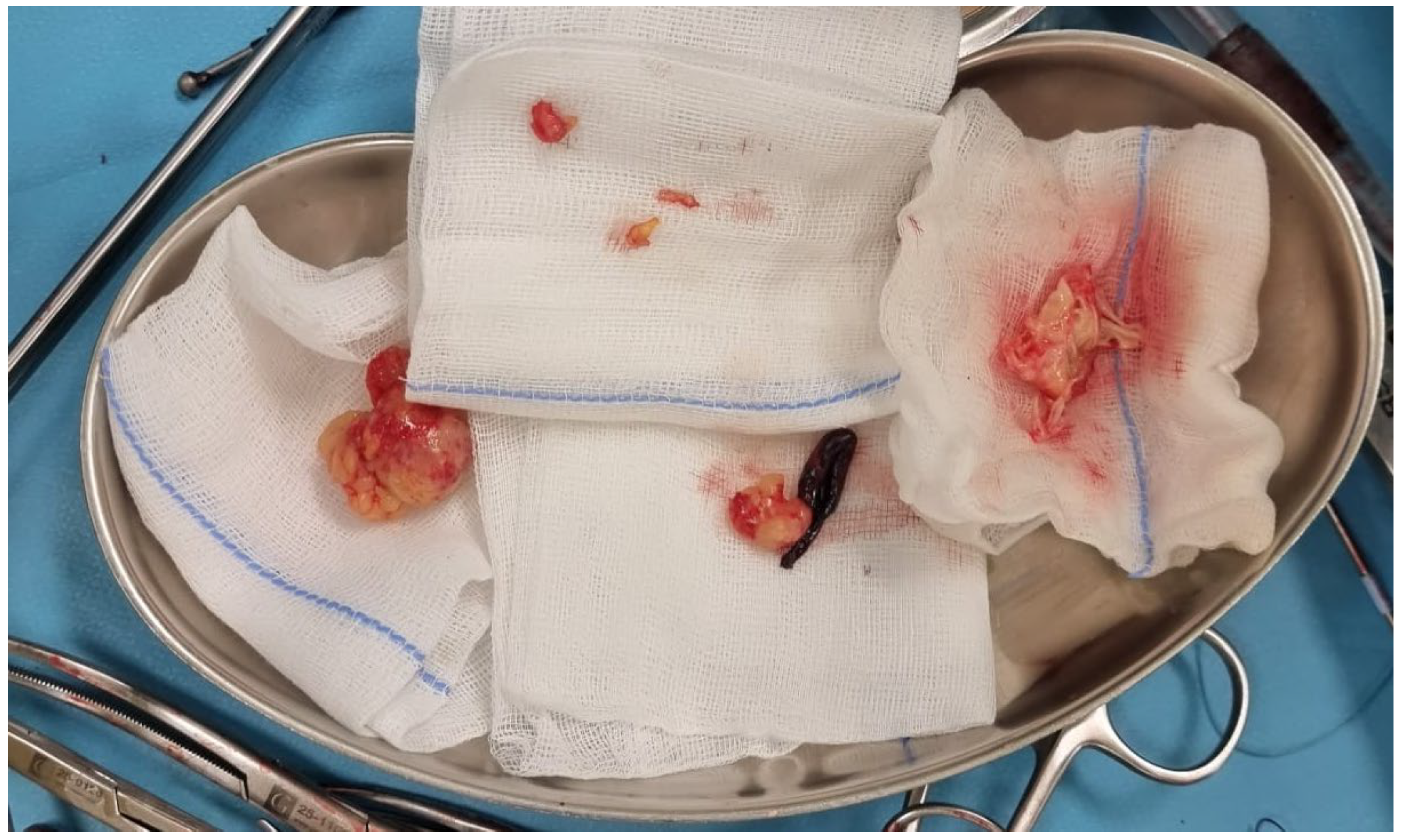
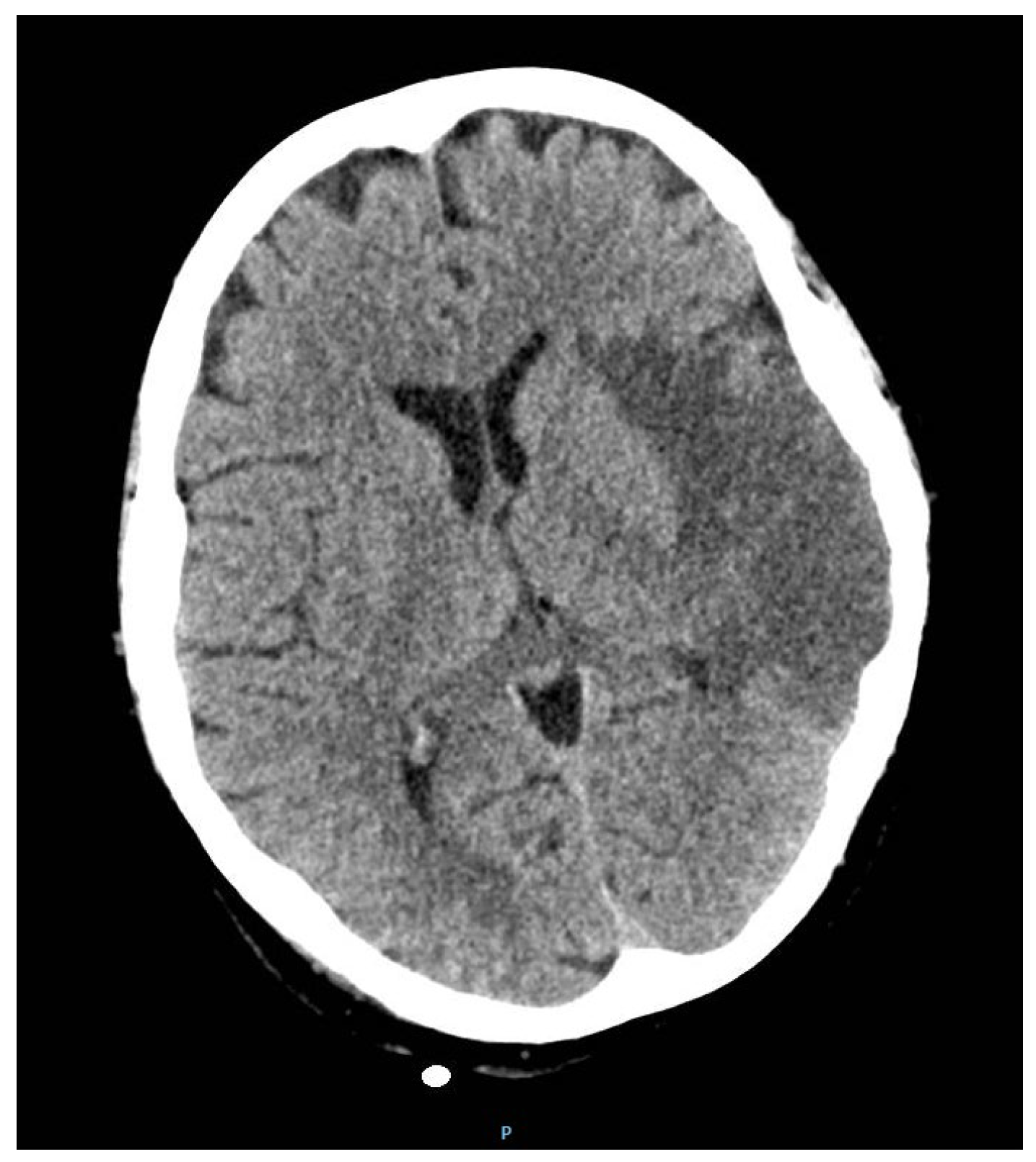
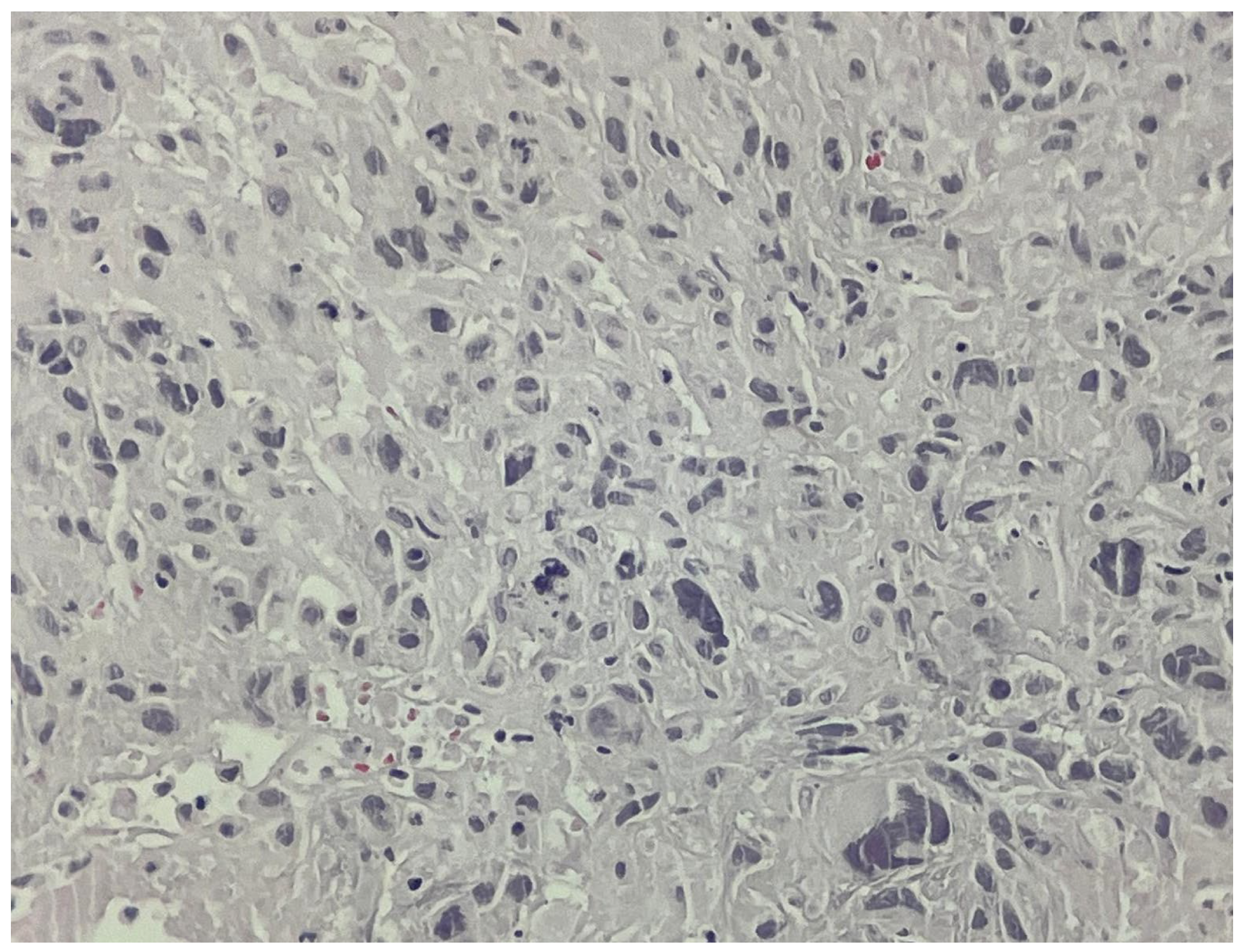
2. Case Report
3. Outcome and Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac tumors: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Cresti, A.; Chiavarelli, M.; Glauber, M.; Tanganelli, P.; Scalese, M.; Cesareo, F.; Guerrini, F.; Capati, E.; Focardi, M.; Severi, S. Incidence rate of primary cardiac tumors: A 14-year population study. J. Cardiovasc. Med. 2016, 17, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Dickens, P.; Chan, A.C. Tumors of the heart: A 20-year experience with a review of 12,485 consecutive autopsies. Arch. Pathol. Lab. Med. 1993, 117, 1027–1031. [Google Scholar] [PubMed]
- Butany, J.; Leong, S.W.; Carmichael, K.; Komeda, M. A 30-year analysis of cardiac neoplasms at autopsy. Cardiovasc. Pathol. 2005, 21, 675–680. [Google Scholar] [CrossRef]
- Oliveira, G.H.; Al-Kindi, S.G.; Hoimes, C.; Park, S.J. Characteristics and survival of malignant cardiac tumors: A 40-year analysis of >500 patients. J. Am. Coll. Cardiol. 2015, 132, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, A.M.; Popescu, D.; Buliga, T.; Filip, A.; Antoniac, I.; Gheorghiţǎ, D.; Molnar, A. Gastric Adenocarcinoma Associated with Acute Endocarditis of the Aortic Valve and Coronary Artery Disease in a 61-Year-Old Male with Multiple Comorbidities: Combined Surgical Management-Case Report. Medicina 2019, 55, 242. [Google Scholar] [CrossRef] [PubMed]
- Prol, T.; Petro, J.; Jain, H.; Raja, S.; Rachofsky, E.; Koulogiannis, K.P.; Horgan, S. Primary Cardiac Sarcoma Involving the Mitral Valve, an Insidious Form of Heart Failure. CASE 2020, 5, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lo Piccolo, F.; Santer, D.; Schulze, E.; Haaf, P. Undifferentiated Cardiac Sarcoma of the Mitral Valve: Multimodal Imaging Assessment. Radiol. Cardiothorac. Imaging. 2023, 5, e220291. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Chen, Z.; Xu, Z.; Chen, Y. Undifferentiated Cardiac Sarcoma on the Mitral Valve Mimicking Myxoma. Circ. J. 2022, 86, 335. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.P.; Mahenthiran, A.K.; Ranginani, A.K.; Mahenthiran, J. High-Grade Spindle Cell Sarcoma of the Heart: A Rare Cause of Mitral Valve Disease. JACC Case Rep. 2019, 1, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Coloma, C.; Saigí, M.; Díaz-Beveridge, R.; Penín, R.M.; Pané-Foix, M.; Mayordomo, E.; Melián, M.; Schuler, M.; García Del Muro, X.; Font de Mora, J. Identification of Actionable genetic Targets in Primary Cardiac Sarcomas. Onco Targets Ther. 2019, 12, 9265–9275. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; King, S.; Shi, B.; Sasse, A. Undifferentiated Pleomorphic Cardiac sarcoma: A Rare and Often Fatal Diagnosis. JACC Case Rep. 2024, 29, 102844. [Google Scholar] [CrossRef] [PubMed]
- Yager, K.; Fan, J.; Sanford, C.B.; Pourfarrokh, N.; Nguyen, V. Among the masses: Multiple Left Atrial Undifferentiated Pleomorphic Sarcomas. Ochsner J. 2024, 24, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.H.; Tanaka, A.; Chehadi, M.; Sandhu, H.K.; Miller, C.C., III; Safi, H.J.; Estrera, A.L. Rapid cooling is a safe technique in patients undergoing circulatory arrest for aortic repair. JTCVS Tech. 2022, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, H.; Antoniac, I.; Gheorghiță, D.; Safta, M.S.; Preda, S.; Broască, M.; Badilă, E.; Fronea, O.; Scafa-Udrişte, A.; Cacoveanu, M.; et al. Biomaterials as Haemostatic Agents in Cardiovascular Surgery: Review of Current Situation and Future Trends. Polymers 2022, 14, 1189. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preda, S.; Gangangari, K.K.; Tiganasu, R.; Liciu, A.; Nica, C.; Voicu, A.; Ichim, V.; Moldovan, H. Rare Case of Grade 3 Undifferentiated Pleomorphic Sarcoma in Left Atrium, Mitral Valve, and Papillary Muscle. J. Clin. Med. 2025, 14, 3053. https://doi.org/10.3390/jcm14093053
Preda S, Gangangari KK, Tiganasu R, Liciu A, Nica C, Voicu A, Ichim V, Moldovan H. Rare Case of Grade 3 Undifferentiated Pleomorphic Sarcoma in Left Atrium, Mitral Valve, and Papillary Muscle. Journal of Clinical Medicine. 2025; 14(9):3053. https://doi.org/10.3390/jcm14093053
Chicago/Turabian StylePreda, Silvia, Kishore K. Gangangari, Robert Tiganasu, Andreea Liciu, Claudia Nica, Alexandra Voicu, Vlad Ichim, and Horatiu Moldovan. 2025. "Rare Case of Grade 3 Undifferentiated Pleomorphic Sarcoma in Left Atrium, Mitral Valve, and Papillary Muscle" Journal of Clinical Medicine 14, no. 9: 3053. https://doi.org/10.3390/jcm14093053
APA StylePreda, S., Gangangari, K. K., Tiganasu, R., Liciu, A., Nica, C., Voicu, A., Ichim, V., & Moldovan, H. (2025). Rare Case of Grade 3 Undifferentiated Pleomorphic Sarcoma in Left Atrium, Mitral Valve, and Papillary Muscle. Journal of Clinical Medicine, 14(9), 3053. https://doi.org/10.3390/jcm14093053




