Continuous Intracranial Pressure Monitoring in Children with ‘Benign’ External Hydrocephalus
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Statement
2.2. Patient Selection
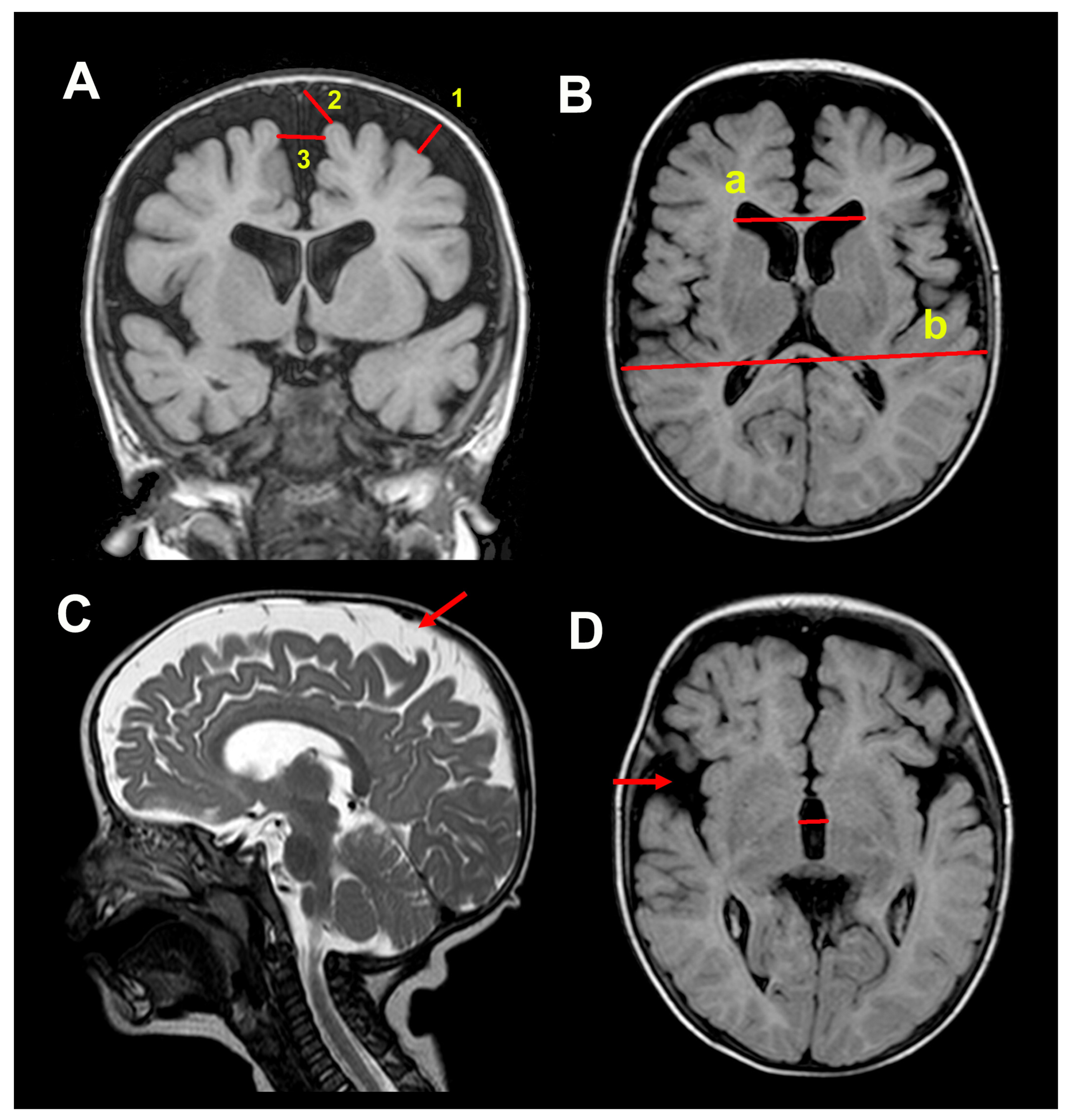
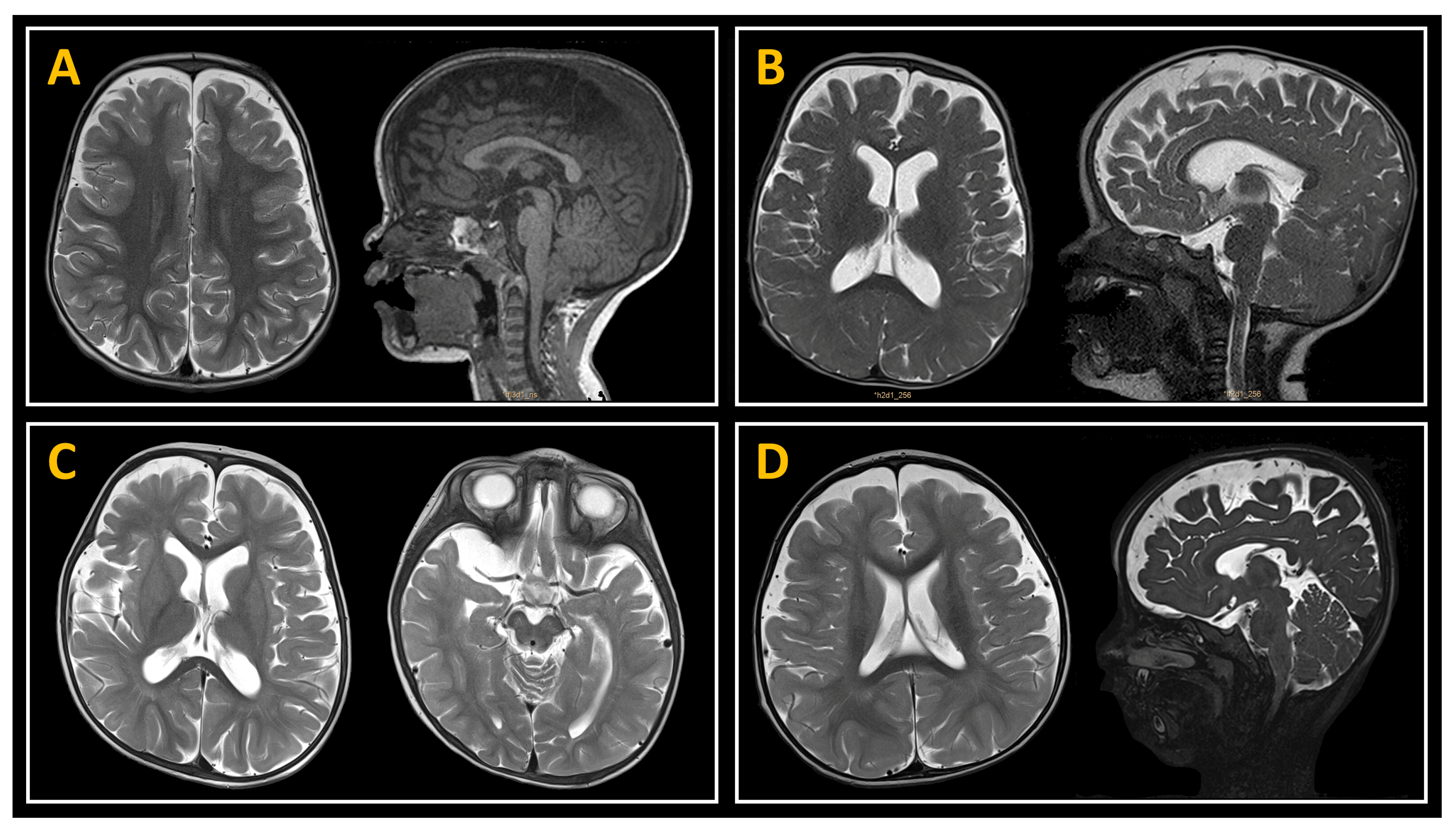
2.3. Neuroimaging Studies
2.4. Psychomotor Development Assessment
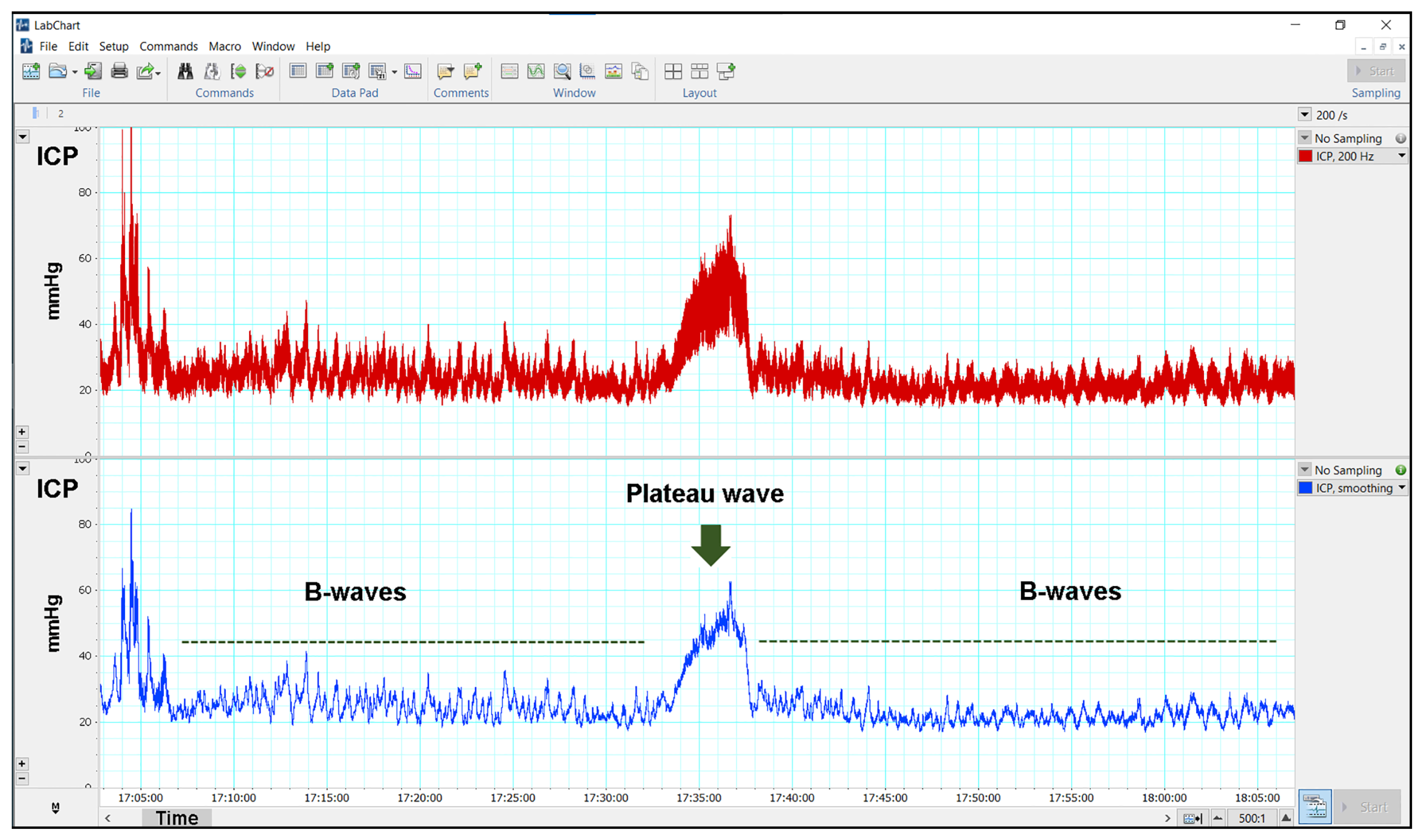
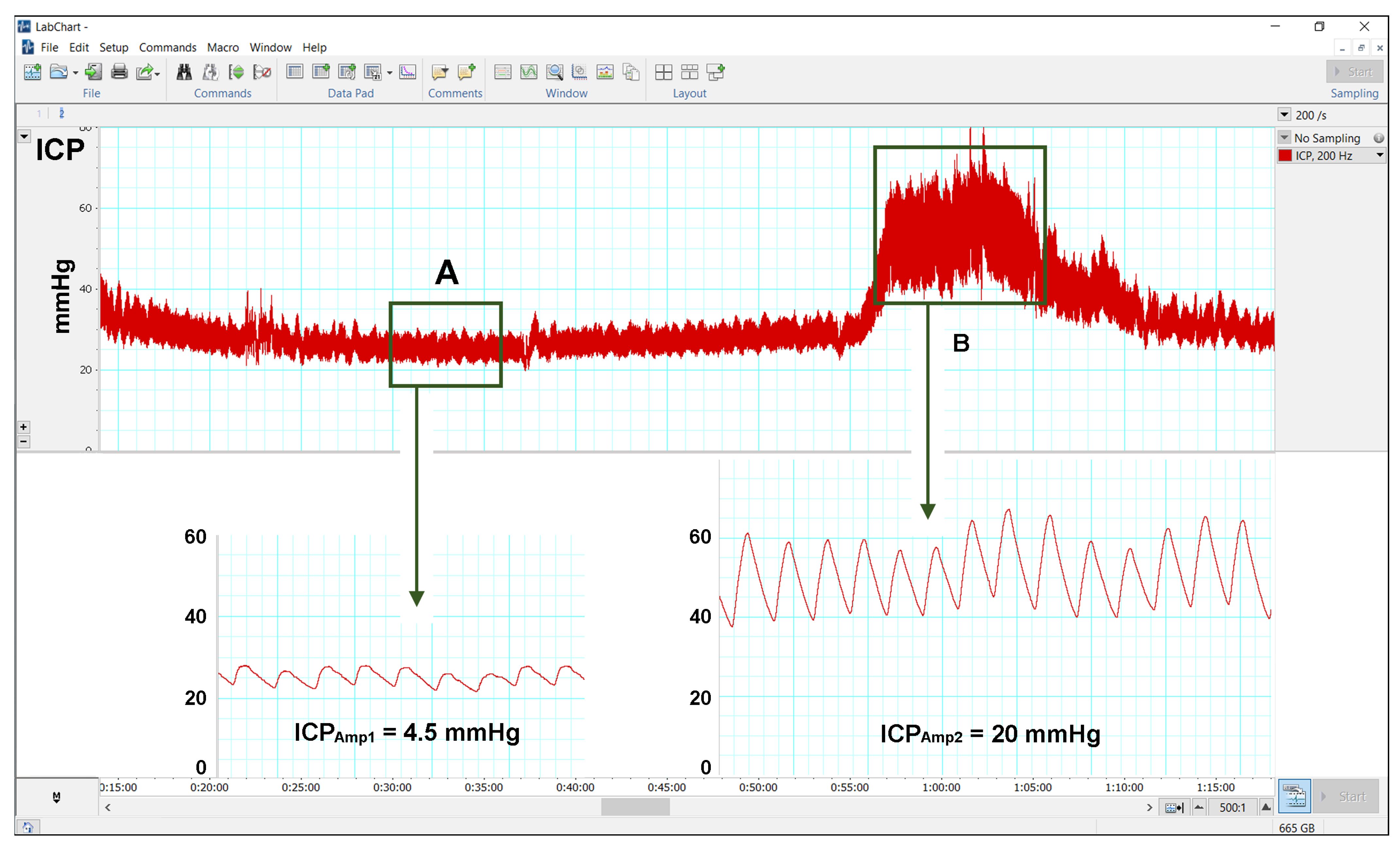
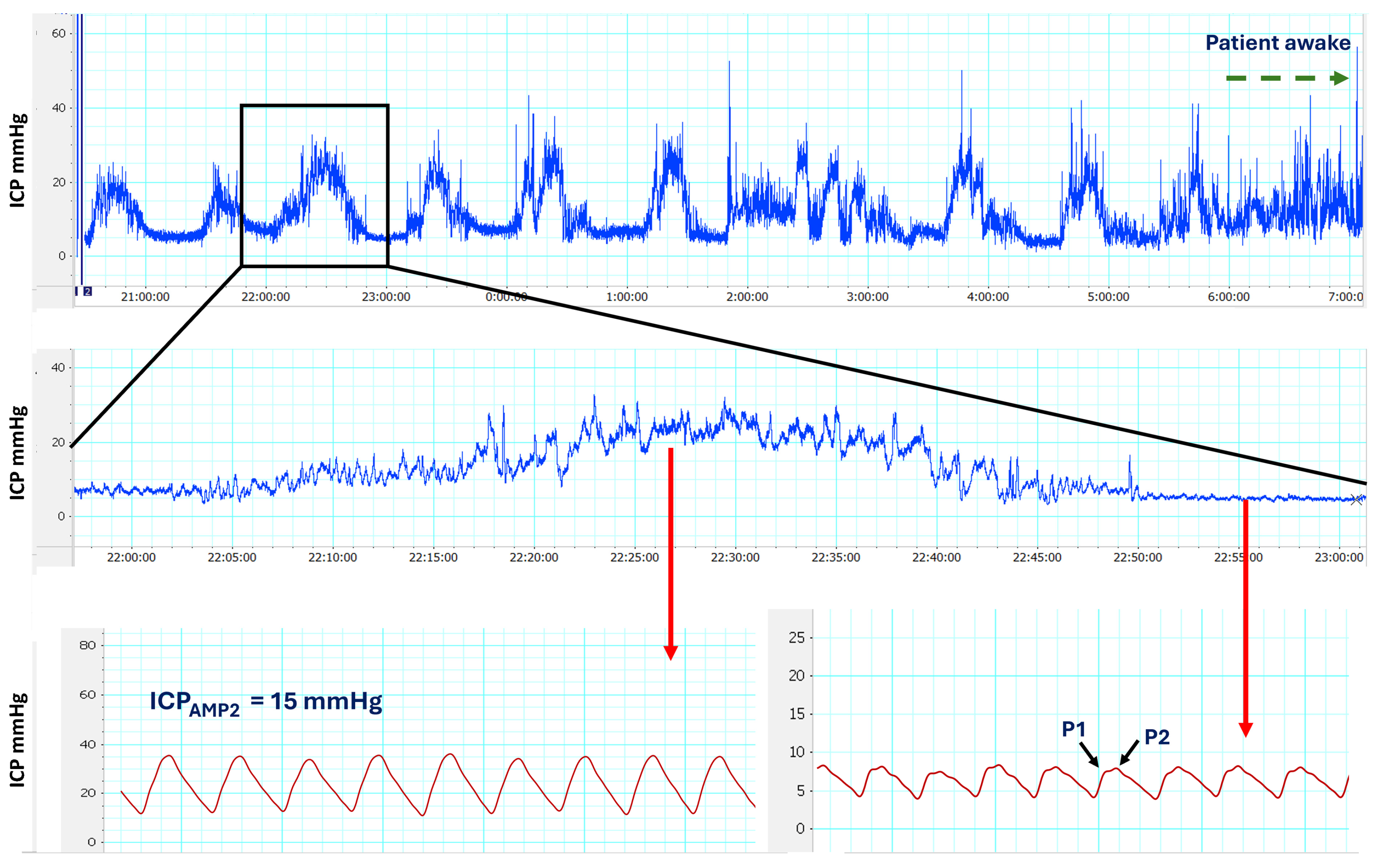
2.5. Methodology for ICP Monitoring
2.6. Criteria for Shunting and Postoperative Adverse Events
2.7. Statistical Analysis
3. Results
3.1. Patient Population and Symptoms
3.2. Neuroimaging Data
3.3. Results of Continuous ICP Monitoring
3.4. Treatment and Complications
4. Discussion
4.1. Pathophysiology of BEH: Unresolved Questions and Controversies
4.2. Benign External Hydrocephalus: A Misleading Label
4.3. ICP Abnormalities in Children with BEH
5. Conclusions
6. Limitations of the Study
7. Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| BEH | Benign external hydrocephalus |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| EI | Evans’ index |
| HC | Head circumference |
| ICP | Intracranial pressure |
| ICPAMP1 | Pulsatility of the cardiac ICP wave on the regular ICP recording |
| ICPAMP2 | Pulsatility of the cardiac ICP wave on top of the B-waves or A-waves |
| IQ | Intelligence quotient |
| FA | Fractional anisotropy |
| Max | Maximum |
| MRI | Magnetic Resonance Imaging |
| Min | Minimum |
| ON | Optic nerve |
| REM | Rapid eye movement |
| Rout | Resistance to CSF outflow |
| SAS | Subarachnoid space |
| SD | Standard deviation |
| WM | White matter |
References
- Ment, L.R.; Duncan, C.C.; Geehr, R. Benign enlargement of the subarachnoid spaces in the infant. J. Neurosurg. 1981, 54, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Prassopoulos, P.; Cavouras, D.; Golfinopoulos, S.; Nezi, M. The size of the intra- and extraventricular cerebrospinal fluid compartments in children with idiopathic benign widening of the frontal subarachnoid space. Neuroradiology 1995, 37, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, N.; Nikkhah, A. Benign Enlargement of Subarachnoid Space in Infancy: “A Review with Emphasis on Diagnostic Work-Up”. Iran. J. Child. Neurol. 2018, 12, 7–15. [Google Scholar] [PubMed]
- Marino, M.A.; Morabito, R.; Vinci, S.; Germano, A.; Briguglio, M.; Alafaci, C.; Mormina, E.; Longo, M.; Granata, F. Benign external hydrocephalus in infants. A single centre experience and literature review. Neuroradiol. J. 2014, 27, 245–250. [Google Scholar] [CrossRef]
- Maruccia, F.; Gomariz, L.; Rosas, K.; Durduran, T.; Paredes-Carmona, F.; Sahuquillo, J.; Poca, M.A. Neurodevelopmental profile in children with benign external hydrocephalus syndrome. A pilot cohort study. Childs Nerv. Syst. 2021, 37, 2799–2806. [Google Scholar] [CrossRef]
- Mikkelsen, R.; Rodevand, L.N.; Wiig, U.S.; Zahl, S.M.; Berntsen, T.; Skarbo, A.B.; Egge, A.; Helseth, E.; Andersson, S.; Wester, K. Neurocognitive and psychosocial function in children with benign external hydrocephalus (BEH)-a long-term follow-up study. Childs Nerv. Syst. 2017, 33, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zahl, S.M.; Egge, A.; Helseth, E.; Wester, K. Benign external hydrocephalus: A review, with emphasis on management. Neurosurg. Rev. 2011, 34, 417–432. [Google Scholar] [CrossRef]
- Shukla, D. Benign external hydrocephalus. J. Pediatr. Neurosci. 2014, 9, 293–294. [Google Scholar] [CrossRef]
- Paciorkowski, A.R.; Greenstein, R.M. When is enlargement of the subarachnoid spaces not benign? A genetic perspective. Pediatr. Neurol. 2007, 37, 1–7. [Google Scholar] [CrossRef]
- Wiig, U.S.; Zahl, S.M.; Egge, A.; Helseth, E.; Wester, K. Epidemiology of Benign External Hydrocephalus in Norway-A Population-Based Study. Pediatr. Neurol. 2017, 73, 36–41. [Google Scholar] [CrossRef]
- Ravid, S.; Maytal, J. External hydrocephalus: A probable cause for subdural hematoma in infancy. Pediatr. Neurol. 2003, 28, 139–141. [Google Scholar] [CrossRef] [PubMed]
- McNeely, P.D.; Atkinson, J.D.; Saigal, G.; O’Gorman, A.M.; Farmer, J.P. Subdural hematomas in infants with benign enlargement of the subarachnoid spaces are not pathognomonic for child abuse. AJNR Am. J. Neuroradiol. 2006, 27, 1725–1728. [Google Scholar] [PubMed]
- Pascual-Castroviejo, I.; Pascual-Pascual, S.I.; Velazquez-Fragua, R. A study and follow-up of ten cases of benign enlargement of the subarachnoid spaces. Rev. Neurol. 2004, 39, 701–706. [Google Scholar]
- Nickel, R.E.; Gallenstein, J.S. Developmental prognosis for infants with benign enlargement of the subarachnoid spaces. Dev. Med. Child. Neurol. 1987, 29, 181–186. [Google Scholar] [CrossRef]
- Hellbusch, L.C. Benign extracerebral fluid collections in infancy: Clinical presentation and long-term follow-up. J. Neurosurg. 2007, 107, 119–125. [Google Scholar] [CrossRef]
- Bateman, G.A.; Napier, B.D. External hydrocephalus in infants: Six cases with MR venogram and flow quantification correlation. Childs Nerv. Syst. 2011, 27, 2087–2096. [Google Scholar] [CrossRef]
- Bateman, G.A.; Alber, M.; Schuhmann, M.U. An association between external hydrocephalus in infants and reversible collapse of the venous sinuses. Neuropediatrics 2014, 45, 183–187. [Google Scholar] [CrossRef]
- Schulz, M.; Ahmadi, S.A.; Spors, B.; Thomale, U.W. Intracranial pressure measurement in infants presenting with progressive macrocephaly and enlarged subarachnoid spaces. Acta Neurochir. Suppl. 2012, 114, 261–266. [Google Scholar] [CrossRef]
- Shinnar, S.; Gammon, K.; Bergman, E.W., Jr.; Epstein, M.; Freeman, J.M. Management of hydrocephalus in infancy: Use of acetazolamide and furosemide to avoid cerebrospinal fluid shunts. J. Pediatr. 1985, 107, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Eidlitz-Markus, T.; Shuper, A.; Constantini, S. Short-term subarachnoid space drainage: A potential treatment for extraventricular hydrocephalus. Childs Nerv. Syst. 2003, 19, 367–370. [Google Scholar] [CrossRef]
- Tsubokawa, T.; Nakamura, S.; Satoh, K. Effect of temporary subdural-peritoneal shunt on subdural effusion with subarachnoid effusion. Childs Brain 1984, 11, 47–59. [Google Scholar] [CrossRef]
- World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997, 277, 925–926. [Google Scholar] [CrossRef]
- Nellhaus, G. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics 1968, 41, 106–114. [Google Scholar] [CrossRef]
- Poca, M.A.; Martinez-Ricarte, F.R.; Portabella, M.; Torne, R.; Fuertes, M.L.; Gonzalez-Tartiere, P.; Sahuquillo, J. Head circumference: The forgotten tool for hydrocephalus management. A reference interval study in the Spanish population. Clin. Neurol. Neurosurg. 2013, 115, 2382–2387. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant and Toddler Development—Third Edition: Administration Manual, 3rd ed.; Harcourt Assessment: San Antonio, TX, USA, 2006; Volume a. [Google Scholar]
- Bayley, N. Bayley Scales of Infant and Toddler Development—Third Edition: Technical Manual, 3rd ed.; Harcourt Assessment: San Antonio, TX, USA, 2006; Volume b. [Google Scholar]
- Bayley, N. Spanish Adaptation of the Bayley Scales of Infant and Toddler Development; Pearson Educación SA: Madrid, Spain, 2015. [Google Scholar]
- Halevy, A.; Cohen, R.; Viner, I.; Diamond, G.; Shuper, A. Development of Infants With Idiopathic External Hydrocephalus. J. Child. Neurol. 2015, 30, 1044–1047. [Google Scholar] [CrossRef]
- Evans, W.A.J. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch. Neurol. Psychiat 1942, 42, 931–937. [Google Scholar] [CrossRef]
- Sari, E.; Sari, S.; Akgun, V.; Ozcan, E.; Ince, S.; Babacan, O.; Saldir, M.; Acikel, C.; Basbozkurt, G.; Yesilkaya, S.; et al. Measures of ventricles and evans’ index: From neonate to adolescent. Pediatr. Neurosurg. 2015, 50, 12–17. [Google Scholar] [CrossRef]
- Johnson, S.; Moore, T.; Marlow, N. Using the Bayley-III to assess neurodevelopmental delay: Which cut-off should be used? Pediatr. Res. 2014, 75, 670–674. [Google Scholar] [CrossRef]
- Poca, M.A.; Martinez-Ricarte, F.; Sahuquillo, J.; Lastra, R.; Torne, R.; Armengol, M.S. Intracranial pressure monitoring with the Neurodur-P epidural sensor: A prospective study in patients with adult hydrocephalus or idiopathic intracranial hypertension. J. Neurosurg. 2008, 108, 934–942. [Google Scholar] [CrossRef]
- Dai, H.; Jia, X.; Pahren, L.; Lee, J.; Foreman, B. Intracranial Pressure Monitoring Signals After Traumatic Brain Injury: A Narrative Overview and Conceptual Data Science Framework. Front. Neurol. 2020, 11, 959. [Google Scholar] [CrossRef]
- Riedel, C.S.; Martinez-Tejada, I.; Andresen, M.; Wilhjelm, J.E.; Jennum, P.; Juhler, M. Transient intracranial pressure elevations (B waves) are associated with sleep apnea. Fluids Barriers CNS 2023, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- ADInstruments: What are the advantages and disadvantages to the various smoothing functions available in LabChart? Available online: https://www.adinstruments.com/support/knowledge-base/what-are-advantages-and-disadvantages-various-smoothing-functions-available (accessed on 22 April 2025).
- Lundberg, N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr. Scand. 1960, 36, 1–193. [Google Scholar] [CrossRef]
- Martinez-Tejada, I.; Arum, A.; Wilhjelm, J.E.; Juhler, M.; Andresen, M. B waves: A systematic review of terminology, characteristics, and analysis methods. Fluids Barriers CNS 2019, 16, 33. [Google Scholar] [CrossRef]
- Raftopoulos, C.; Chaskis, C.; Delecluse, F.; Cantraine, F.; Bidaut, L.; Brotchi, J. Morphological quantitative analysis of intracranial pressure waves in normal pressure hydrocephalus. Neurol. Res. 1992, 14, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Eide, P.K.; Brean, A. Intracranial pulse pressure amplitude levels determined during preoperative assessment of subjects with possible idiopathic normal pressure hydrocephalus. Acta Neurochir. 2006, 148, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Eide, P.K.; Sorteberg, W. Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: A 6-year review of 214 patients. Neurosurgery 2010, 66, 80–91. [Google Scholar] [CrossRef]
- Eide, P.K. Abnormal Intracranial Pulse Pressure Amplitude Despite Normalized Static Intracranial Pressure in Idiopathic Intracranial Hypertension Refractory to Conservative Medical Therapy. Life 2021, 11, 537. [Google Scholar] [CrossRef]
- Eide, P.K.; Brean, A. Cerebrospinal fluid pulse pressure amplitude during lumbar infusion in idiopathic normal pressure hydrocephalus can predict response to shunting. Cerebrospinal Fluid. Res. 2010, 7, 5. [Google Scholar] [CrossRef]
- Poca, M.A.; Sahuquillo, J.; Topczewski, T.; Peñarrubia, M.J.; Muns, A. Is intracranial pressure monitoring in the epidural space reliable? Fact and fiction. J. Neurosurg. 2007, 106, 548–556. [Google Scholar] [CrossRef]
- Dao Trong, P.; Olivares, A.; El Damaty, A.; Unterberg, A. Adverse events in neurosurgery: A comprehensive single-center analysis of a prospectively compiled database. Acta Neurochir. 2023, 165, 585–593. [Google Scholar] [CrossRef]
- Fandak, J.; Markart, S.; Willems, E.P.; Wildermuth, S.; Frauenfelder, T.; Fischer, T.; Dietrich, T.J.; Waelti, S.L. Prospective measurement of the width of cerebrospinal fluid spaces by cranial ultrasound in neurologically healthy children aged 0-19 months. BMC Pediatr. 2024, 24, 315. [Google Scholar] [CrossRef]
- Barlow, C.F. CSF dynamics in hydrocephalus—With special attention to external hydrocephalus. Brain Dev. 1984, 6, 119–127. [Google Scholar] [CrossRef]
- Bateman, G.A.; Siddique, S.H. Cerebrospinal fluid absorption block at the vertex in chronic hydrocephalus: Obstructed arachnoid granulations or elevated venous pressure? Fluids Barriers CNS 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Sainz, L.V.; Zipfel, J.; Kerscher, S.R.; Weichselbaum, A.; Bevot, A.; Schuhmann, M.U. Cerebro-venous hypertension: A frequent cause of so-called “external hydrocephalus” in infants. Childs Nerv. Syst. 2019, 35, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Sainz, L.V.; Schuhmann, M.U. Subarachnomegaly-venous congestion of infancy. Childs Nerv. Syst. 2021, 37, 3455–3463. [Google Scholar] [CrossRef]
- Cinalli, G.; Di Martino, G.; Russo, C.; Cristofano, A.; Picariello, S.; Cinalli, M.A.; Mirone, G.; Mazio, F.; Quarantelli, M.; Spennato, P.; et al. Jugular foramen stenosis in external hydrocephalus in infants. Childs Nerv. Syst. 2024, 40, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Barrit, S.; El Hadwe, S.; Lubicz, B.; De Witte, O. External hydrocephalus associated with dural sigmoid sinus arteriovenous fistula: A case report. Br. J. Neurosurg. 2024, 38, 1167–1169. [Google Scholar] [CrossRef]
- Muenchberger, H.; Assaad, N.; Joy, P.; Brunsdon, R.; Shores, E.A. Idiopathic macrocephaly in the infant: Long-term neurological and neuropsychological outcome. Childs Nerv. Syst. 2006, 22, 1242–1248. [Google Scholar] [CrossRef]
- Alvarez, L.A.; Maytal, J.; Shinnar, S. Idiopathic external hydrocephalus: Natural history and relationship to benign familial macrocephaly. Pediatrics 1986, 77, 901–907. [Google Scholar] [CrossRef]
- Laubscher, B.; Deonna, T.; Uske, A.; van Melle, G. Primitive megalencephaly in children: Natural history, medium term prognosis with special reference to external hydrocephalus. Eur. J. Pediatr. 1990, 149, 502–507. [Google Scholar] [CrossRef]
- Yew, A.Y.; Maher, C.O.; Muraszko, K.M.; Garton, H.J. Long-term health status in benign external hydrocephalus. Pediatr. Neurosurg. 2011, 47, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.D.; Nordahl, C.W.; Young, G.S.; Wootton-Gorges, S.L.; Lee, A.; Liston, S.E.; Harrington, K.R.; Ozonoff, S.; Amaral, D.G. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 2013, 136, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Zahl, S.M.; Egge, A.; Helseth, E.; Skarbo, A.B.; Wester, K. Quality of life and physician-reported developmental, cognitive, and social problems in children with benign external hydrocephalus-long-term follow-up. Childs Nerv. Syst. 2019, 35, 245–250. [Google Scholar] [CrossRef]
- Nasiri, J.; Madihi, Y.; Mirzadeh, A.S.; Mohammadzadeh, M. Neurodevelopmental Outcomes of Infants with Benign Enlargement of the Subarachnoid Space. Iran. J. Child. Neurol. 2021, 15, 33–40. [Google Scholar] [CrossRef]
- Serlin, Y.; Ben-Arie, G.; Lublinsky, S.; Flusser, H.; Friedman, A.; Shelef, I. Distorted Optic Nerve Portends Neurological Complications in Infants With External Hydrocephalus. Front. Neurol. 2021, 12, 596294. [Google Scholar] [CrossRef]
- Nogueira, G.J.; Zaglul, H.F. Hypodense extracerebral images on computed tomography in children. “External hydrocephalus”: A misnomer? Childs Nerv. Syst. 1991, 7, 336–341. [Google Scholar] [CrossRef]
- Massager, N.; Wayenberg, J.L.; Raftopoulos, C.; Christophe, C.; Vermeylen, D.; Franco, P. Anterior fontanelle pressure monitoring for the evaluation of asymptomatic infants with increased head growth rate. Childs. Nerv. Syst. 1996, 12, 38–42. [Google Scholar] [CrossRef]
- Fric, R.; Langvatn, E.A.; Due-Tonnessen, B.J.; Eide, P.K. The role of pulsatile and static intracranial pressure measurements in the management of children with craniosynostosis-an institutional experience from 49 patients. Acta Neurochir. 2021, 163, 2015–2023. [Google Scholar] [CrossRef]
- Hanlo, P.W.; Gooskens, R.J.H.M.; vanSchooneveld, M.; Tulleken, C.A.F.; vanderKnaap, M.S.; Faber, J.A.J.; Willemse, J. The effect of intracranial pressure on myelination and the relationship with neurodevelopment in infantile hydrocephalus. Dev. Med. Child. Neurol. 1997, 39, 286–291. [Google Scholar] [CrossRef]
- Cascio, C.J.; Gerig, G.; Piven, J. Diffusion tensor imaging: Application to the study of the developing brain. J. Am. Acad. Child. Adolesc. Psychiatry 2007, 46, 213–223. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, W.; Hertzler, D.A.; Cancelliere, A.; Altaye, M.; Mangano, F.T. Diffusion tensor imaging findings in young children with benign external hydrocephalus differ from the normal population. Childs Nerv. Syst. 2012, 28, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Coroneos, N.J.; McDowall, D.G.; Gibson, R.M.; Pickerodt, V.; Keaney, N.P. A comparison of extradural pressure with cerebrospinal fluid pressure. Eur. Neurol. 1972, 8, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Coroneos, N.J.; McDowall, D.G.; Pickerodt, V.W.; Keaney, N.P.; Gibson, R.M. A comparison of intracranial extradural pressure with subarachnoid pressure. Br. J. Anaesth. 1971, 43, 1198. [Google Scholar]
- Coroneos, N.J.; McDowall, D.G.; Gibson, R.M.; Pickerodt, V.W.; Keaney, N.P. Measurement of extradural pressure and its relationship to other intracranial pressures. An experimental and clinical study. J. Neurol. Neurosurg. Psychiatry 1973, 36, 514–522. [Google Scholar] [CrossRef]
- Perez-Marquez, M.; Marruecos, L.; Betbes, A.J.; Ballus, J.; Bak, E.; Molet, J.; Net, A. Valoración de dos métodos para la medición de la presión intracranial [Evaluation of two methods for intracranial pressure measure]. Neurocirugia 1997, 8, 205–210. [Google Scholar] [CrossRef]
- Eide, P.K.; Sorteberg, W. Simultaneous measurements of intracranial pressure parameters in the epidural space and in brain parenchyma in patients with hydrocephalus. J. Neurosurg. 2010, 113, 1317–1325. [Google Scholar] [CrossRef]
- Chinta, S.; Walker, K.; Halliday, R.; Loughran-Fowlds, A.; Badawi, N. A comparison of the performance of healthy Australian 3-year-olds with the standardised norms of the Bayley Scales of Infant and Toddler Development (version-III). Arch. Dis. Child. 2014, 99, 621–624. [Google Scholar] [CrossRef]
- Allen, L.; O’Connell, A.; Kiermer, V. How can we ensure visibility and diversity in research contributions? How the Contributor Role Taxonomy (CRediT) is helping the shift from authorship to contributorship. Learn. Publ. 2019, 32, 71–74. [Google Scholar] [CrossRef]
| Pre-Term | Full-Term | Total Cohort | p * | |
|---|---|---|---|---|
| n | 16 | 20 | 36 | |
| Birth events | ||||
| 9/7 | 13/7 | 22/14 | 0.593 |
| 32.5 (25–36) | 39 (37–41) | 37 (25–41) | <0.001 |
| 9 (3–10) | 9 (6–10) | 9 (3–10) | 0.137 |
| 9.5 (5–10) | 10 (9–10) | 10 (5 –10) | 0.028 |
| 2 (0.57–3.04) | 3.3 (2–4.5) | 2.65 (0.57–4.5) | <0.001 |
| 32.5 (24.5–41) | 35.5 (32–38) | 34.3 (24.5–41) | 0.007 |
| 5 | 0 | 5 | |
| 4 | 6 | 10 | |
| Findings at the time of ICP monitoring | ||||
| 23.9 ± 9.34 | 23.4 ± 16.1 | 23.6 ± 13.3 | 0.524 |
| 12 | 19 | 31 | 0.149 |
| 8 | 12 | 20 | 0.549 |
- Irritability | 0 | 3 | 3 | |
- Hypotonia | 7 | 5 | 12 | |
- Headaches | 1 | 2 | 3 | |
- Wide anterior fontanel | 0 | 2 | 2 | |
- Dilated scalp veins | 0 | 2 | 2 | |
- Delayed motor development *** | 9 | 9 | 18 | 0.502 |
| 9 | 11 | 20 | 0.922 |
| 2 | 6 | 8 | 0.257 |
| Neuroimaging data | ||||
| 0.29 (0.27–0.32) | 0.3 (0.23–0.34) | 0.29 (0.23–0.34) | 0.654 |
| 7.8 (3.2–11) | 9.8 (3–14) | 8.3 (3–14) | 0.130 |
| 10.7 (7–18) | 10.9 (7.1–17) | 10.8 (7–18) | 0.949 |
| 12 (4.6–20.7) | 11 (5–15.8) | 11.5 (4.6–20.7) | 0.166 |
| 12.2 (6–24.7) | 10.5 (6.3–22.5) | 11.4 (6–24.7) | 0.535 |
| 12 | 13 | 25 | 0.718 |
| 10 | 15 | 25 | 0.483 |
| 2 | 1 | 3 | 0.574 |
| Isolated BEH | BEH Associated with Other Conditions | Total Cohort | p * | |
|---|---|---|---|---|
| n | 23 | 13 | 36 | |
| Birth events | ||||
| 13/10 | 9/4 | 22/14 | 0.501 |
| 36 (29–40) | 38 (25–41) | 37 (25–41) | 0.136 |
| 9 (3–10) | 9 (6–10) | 9 (3–10) | 0.729 |
| 10 (5–10) | 10 (9–10) | 10 (5 –10) | 0.674 |
| 2.31 (0.57–4.5) | 3.3 (1.04–3.8) | 2.65 (0.57–4.5) | 0.043 |
| 34 (24.5–41) | 36 (27–38) | 34.3 (24.5–41) | 0.092 |
| Findings at the time of ICP monitoring | ||||
| 22 (6–65) | 19 (8–33) | 22 (6–65) | 0.269 |
| 20 | 11 | 31 | 1 |
| 8 | 12 | 20 | 0.001 |
- Irritability | 0 | 3 | 3 | |
- Hypotonia | 6 | 6 | 12 | |
- Headaches | 1 | 2 | 3 | |
- Wide anterior fontanel | 1 | 1 | 2 | |
- Dilated scalp veins | 2 | 0 | 2 | |
| 10 | 8 | 18 | 0.489 |
| 13 | 7 | 20 | 1 |
| 3 | 5 | 8 | 0.107 |
| Neuroimaging data | ||||
| 0.30 (0.24–0.34) | 0.26 (0.23–0.34) | 0.29 (0.23–0.34) | 0.097 |
| 8.2 (3.2–14) | 8.4 (3–12.7) | 8.3 (3–14) | 0.767 |
| 10.5 (7–18) | 12 (7.1–17) | 10.8 (7–18) | 0.468 |
| 12 (4.6–20.7) | 11.5 (5–14) | 11.5 (4.6–20.7) | 0.365 |
| 11 (6–24.7) | 13 (6.3–16.5) | 11.4 (6–24.7) | 0.315 |
| 15 | 10 | 25 | 0.708 |
| 16 | 9 | 25 | 1 |
| 3 | 0 | 3 | 0.288 |
| Pre-Term | Full-Term | Total Cohort | p * | |
|---|---|---|---|---|
| n | 16 | 20 | 36 | |
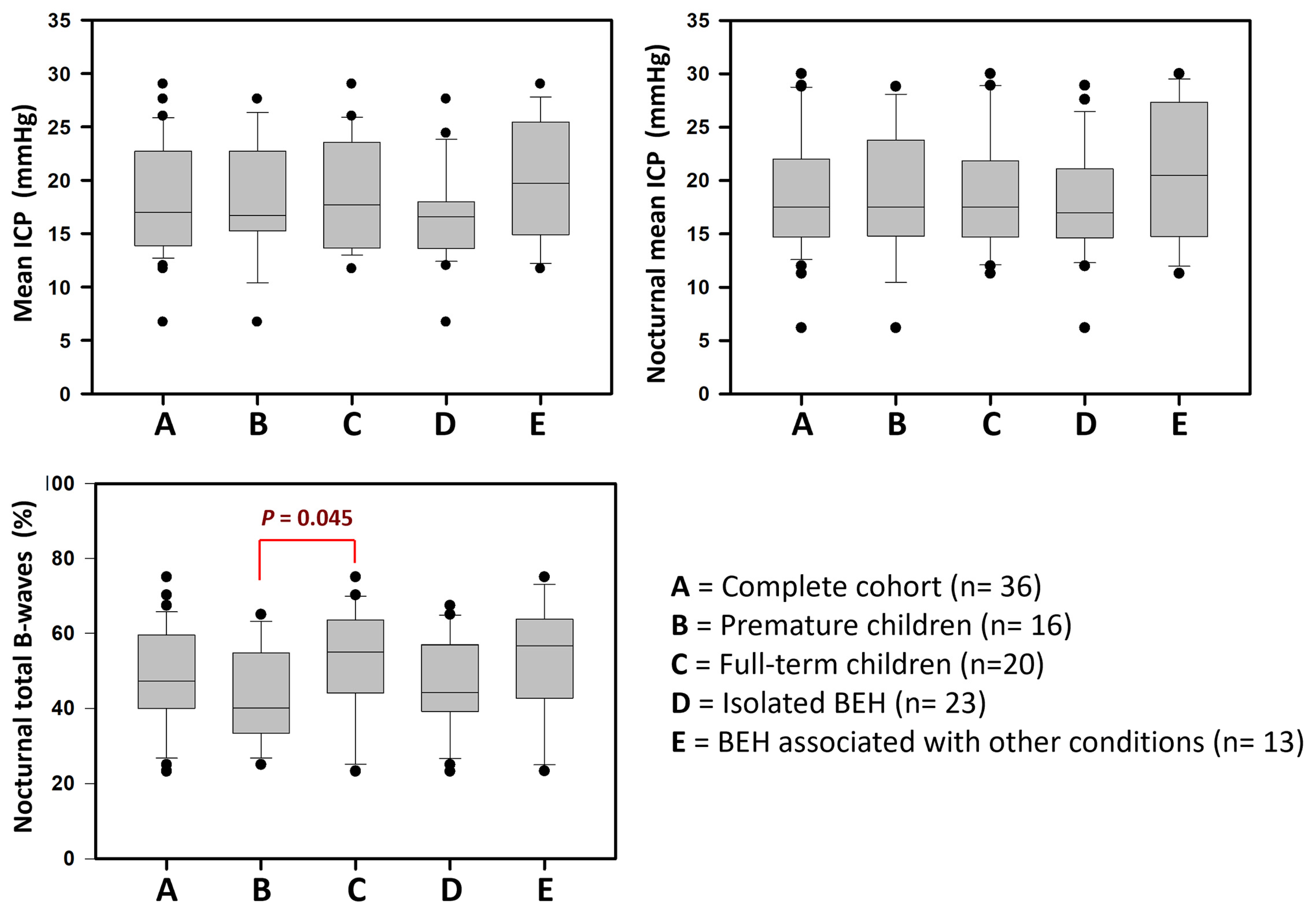
| Mean ICP (mmHg) | 16.7 (6.7–27.6) | 17.7 (11.7–29) | 17 (6.7–29) | 0.811 |
| Diurnal mean ICP (mmHg) | 17.7 (7–27) | 16.7 (9.5–31) | 17.6 (7–31) | 0.828 |
| Nocturnal mean ICP (mmHg) | 17.5 (6.2–28.8) | 17.5 (11.3–30) | 17.5 (6.2–30) | 0.973 |
| Slow ICP waves (%) | ||||
| Nocturnal low-amplitude B-waves | 26.7 (2.5–43) | 34.9 (6.5–58) | 29.4 (2.5–58) | 0.042 |
| Nocturnal high-amplitude B-waves | 17.9 (8.6–37.6) | 18.4 (0–50) | 17.9 (0–50) | 0.811 |
| Nocturnal total B-waves | 40 (25–65) | 55 (23.2–75) | 47.4 (23.2–75) | 0.045 |
| Presence of plateau waves (yes) | 1 | 2 | 3 | |
| Cardiac ICP waves | ||||
| n | 14 | 17 | 31 | |
| Amplitude | ||||
| 4 (3–9) | 4 (2.8–5.5) | 4 (2.8–9) | 0.516 |
| 12.5 (8–21) | 10 (5.5–18) | 11 (5.5–21) | 0.033 |
| Morphology of cardiac ICP waves | ||||
| 5 | 2 | 8 | |
| 14 | 17 | 31 | |
| Isolated BEH | BEH Associated with Other Conditions | Total Cohort | p * | |
|---|---|---|---|---|
| n | 23 | 13 | 36 | |
| Mean ICP (mmHg) | 16.6 (6.7–27.6) | 19.7 (11.7–29) | 17 (6.7–29) | 0.093 |
| Diurnal mean ICP (mmHg) | 14.2 (7–25.8) | 22.3 (12.2–31) | 17.6 (7–31) | 0.013 |
| Nocturnal mean ICP (mmHg) | 17 (6.2–28.9) | 20.5 (11.3–30) | 17.5 (6.2–30) | 0.212 |
| Slow ICP waves (%) | ||||
| Nocturnal low-amplitude B-waves | 29.4 (2.5–44.2) | 27.4 (7.5–58) | 29.4 (2.5–58) | 0.645 |
| Nocturnal high-amplitude B-waves | 16.7 (6.7–38) | 20 (0–50) | 17.9 (0–50) | 0.458 |
| Nocturnal total B-waves | 44.2 (23.2–67.4) | 56.7 (23.3–75) | 47.4 (23.2–75) | 0.166 |
| Presence of plateau waves (yes) | 2 | 1 | 3 | |
| Cardiac ICP waves | ||||
| n | 20 | 11 | 31 | |
| Amplitude | ||||
| 4 (2.8–5) | 4 (3–9) | 4 (2.8–9) | 0.169 |
| 11 (5.5–16) | 11 (6–21) | 11 (5.5–21) | 0.468 |
| Morphology of cardiac ICP waves | ||||
| 4 | 4 | 8 | |
| 20 | 11 | 31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poca, M.A.; Lopez-Bermeo, D.; Cano, P.; Maruccia, F.; Fajardo, C.; Delgado, I.; Munar, F.; Garcia-Merino, A.; Sahuquillo, J. Continuous Intracranial Pressure Monitoring in Children with ‘Benign’ External Hydrocephalus. J. Clin. Med. 2025, 14, 3042. https://doi.org/10.3390/jcm14093042
Poca MA, Lopez-Bermeo D, Cano P, Maruccia F, Fajardo C, Delgado I, Munar F, Garcia-Merino A, Sahuquillo J. Continuous Intracranial Pressure Monitoring in Children with ‘Benign’ External Hydrocephalus. Journal of Clinical Medicine. 2025; 14(9):3042. https://doi.org/10.3390/jcm14093042
Chicago/Turabian StylePoca, Maria A., Diego Lopez-Bermeo, Paola Cano, Federica Maruccia, Carolina Fajardo, Ignacio Delgado, Francisca Munar, Anna Garcia-Merino, and Juan Sahuquillo. 2025. "Continuous Intracranial Pressure Monitoring in Children with ‘Benign’ External Hydrocephalus" Journal of Clinical Medicine 14, no. 9: 3042. https://doi.org/10.3390/jcm14093042
APA StylePoca, M. A., Lopez-Bermeo, D., Cano, P., Maruccia, F., Fajardo, C., Delgado, I., Munar, F., Garcia-Merino, A., & Sahuquillo, J. (2025). Continuous Intracranial Pressure Monitoring in Children with ‘Benign’ External Hydrocephalus. Journal of Clinical Medicine, 14(9), 3042. https://doi.org/10.3390/jcm14093042









