Cardiopulmonary Exercise Testing Correlates with Quantitative Left Ventricular [99mTc]-DPD Uptake in Transthyretin Amyloid Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Study Design
2.2. Study Population
2.3. Diagnosis of Cardiac Transthyretin Amyloidosis
2.4. Cardiopulmonary Exercise Testing
2.5. Imaging Assessments
2.5.1. DPD Scintigraphy and SPECT/CT Imaging
2.5.2. Cardiac Magnetic Resonance Imaging
2.5.3. Transthoracic Echocardiography
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Study Participants and Baseline Characteristics
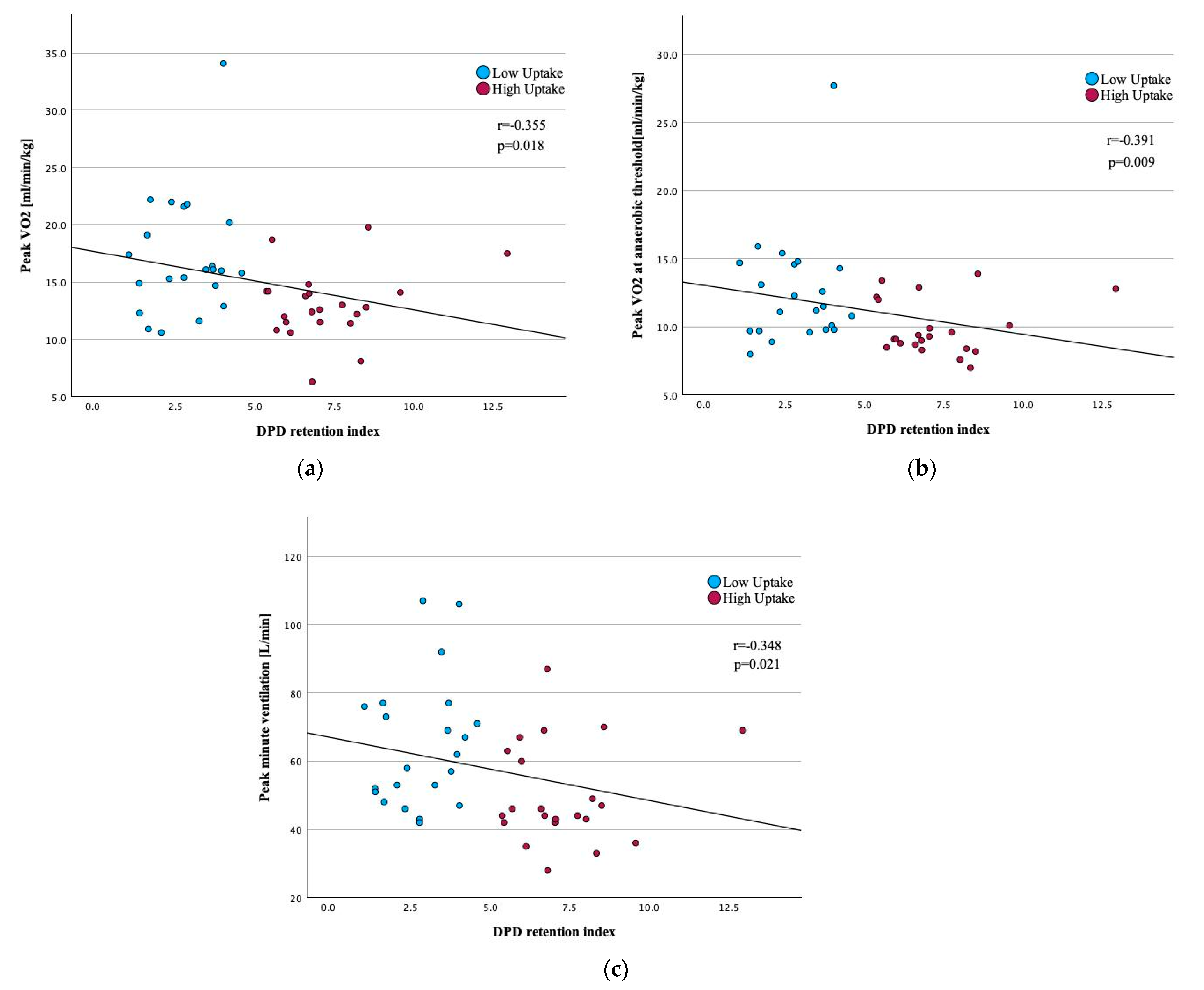
3.2. Correlation Between CPET Variables and Quantitative LV [99mTc]-DPD Uptake
3.3. Correlation Between CPET, LV [99mTc]-DPD Quantification and Key Clinical, Laboratory, and Imaging Parameters
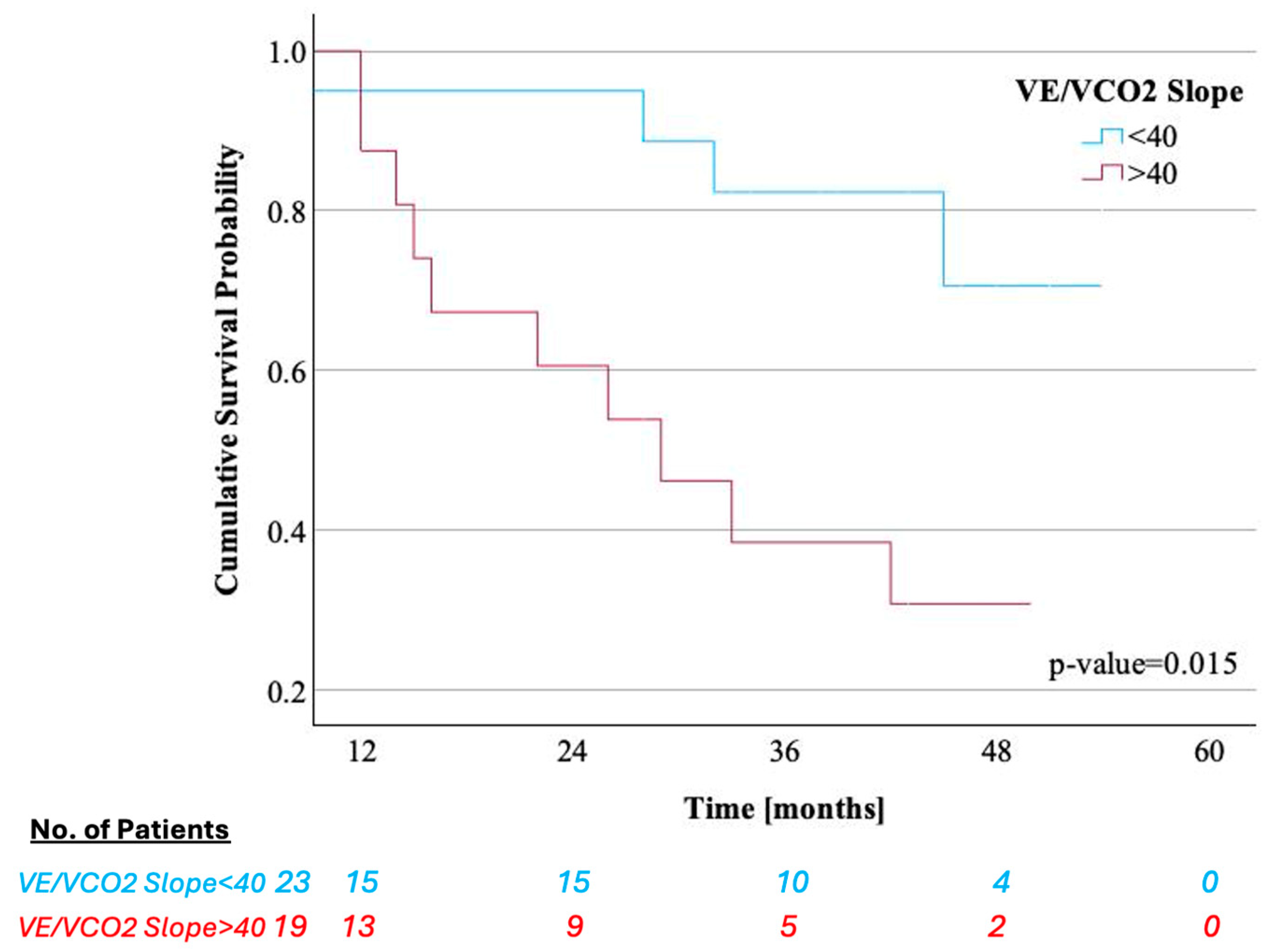
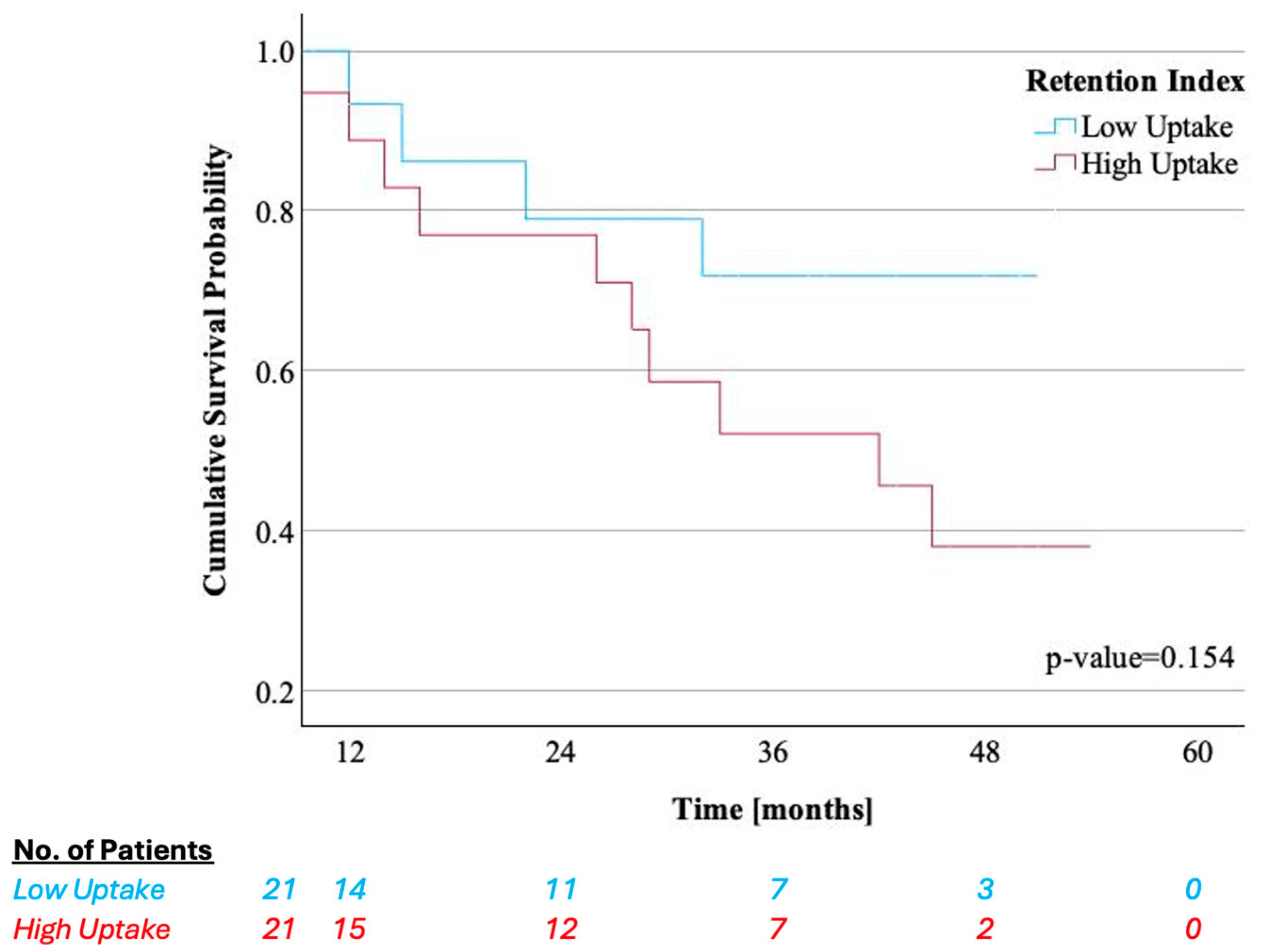
3.4. Association of Key CPET Variables and Quantitative LV [99mTc]-DPD Uptake with Survival and HF Hospitalizations
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWT | 6 min walk test |
| ATTR-CM | Transthyretin amyloid cardiomyopathy |
| AT | Anaerobic threshold |
| BMI | Body mass index |
| CMR | Cardiac magnetic resonance |
| CPET | Cardiopulmonary exercise testing |
| [99mTc]-DPD | [99mTc]-3,3-diphosphono-1,2-propanodicarboxylic acid |
| ECV | Extracellular volume |
| FC | Functional capacity |
| HF | Heart failure |
| LV | Left ventricle |
| NYHA | New York Heart Association |
| [99mTc]-PYP | [99mTc]-pyrophosphate |
| SPECT/CT | Single photon emission computed tomography/computed tomography |
| TTE | Transthoracic echocardiography |
| VCO2 | Carbon dioxide production |
| VE | Pulmonary ventilation |
| VE/VCO2 slope | Ventilation to carbon dioxide production |
| VO2 | Oxygen consumptions |
Appendix A
| Variables | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Univariable Regression | Multivariable Regression | |||
| Demographic and clinical parameters | ||||
| Age, years | 0.965 (0.908–1.026) | 0.252 | ||
| Sex, Male | 0.553 (0.173–1.768) | 0.318 | ||
| Body Mass Index, kg/m2 | 1.015 (0.923–1.116) | 0.762 | ||
| NYHA Functional Class ≥ III | 2.216 (0.740–6.636) | H00.155 | ||
| 6-min Walk Distance, m | 0.999 (0.995–1.003) | 0.694 | ||
| Concomitant medication | ||||
| Beta-blockers | 0.947 (0.316–2.842) | 0.923 | ||
| MRA | 2.849 (0.791–10.262) | 0.109 | ||
| Diuretics | 3.404 (0.444–26.077) | 0.238 | ||
| Laboratory parameters | ||||
| NT-proBNP, pg/mL | 1.000 (1.000–1.000) | 0.422 | ||
| Troponin T, ng/L | 1.022 (1.004–1.041) | 0.017 | 1.026 (0.985–1.069) | 0.213 |
| eGFR, mL/min/1.73 m2 | 0.967 (0.944–0.991) | 0.007 | 0.985 (0.927–1.045) | 0.612 |
| Cardiopulmonary Exercise Testing | ||||
| Peak VO2, mL/min/kg | 0.831 (0.712–0.970) | 0.019 | 0.681 (0.327–1.415) | 0.303 |
| VO2 at AT, mL/min/kg | 0.593 (0.387–0.908) | 0.016 | 1.016 (0.431–2.397) | 0.970 |
| Peak O2 Pulse, mL/beat | 0.860 (0.726–1.018) | 0.079 | ||
| VE/VCO2 slope | 1.040 (0.979–1.104) | 0.206 | ||
| Peak workload, Watt | 0.988 (0.968–1.008) | 0.230 | ||
| Peak VE, L/min | 0.981 (0.951–1.012) | 0.234 | ||
| Nuclear imaging parameters | ||||
| Perugini Grading Scale | 2.287 (0.637–8.210) | 0.205 | ||
| DPD retention index | 1.164 (0.951–1.423) | 0.141 | ||
| Transthoracic echocardiography parameters | ||||
| Interventricular septum, mm | 0.985 (0.874–1.110) | 0.802 | ||
| LV ejection fraction, % | 0.992 (0.939–1.048) | 0.776 | ||
| LV end-diastolic diameter, mm | 1.013 (0.932–1.101) | 0.760 | ||
| LV global longitudinal strain, -% | 1.058 (0.919–1.218) | 0.430 | ||
| RV end-diastolic diameter, mm | 1.016 (0.924–1.116) | 0.750 | ||
| RV global longitudinal strain, -% | 1.048 (0.947–1.159) | 0.366 | ||
| Cardiac magnetic resonance imaging parameters | n = 32 | n = 17 | n = 15 | |
| Interventricular septum, mm | 1.039 (0.943–1.146) | 0.438 | ||
| LV ejection fraction, % | 1.013 (0.956–1.074) | 0.653 | ||
| RV ejection fraction, % | 1.001 (0.947–1.057) | 0.979 | ||
| MOLLI-ECV, % | 1.043 (0.975–1.115) | 0.218 | ||
| Native T1 time, ms | 1.011 (1.004–1.019) | 0.004 | 1.017 (1.004–1.030) | 0.011 |
Appendix B

References
- Bloom, M.W.; Gorevic, P.D. Cardiac Amyloidosis. Ann. Intern. Med. 2023, 176, Itc33–Itc48. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient with Cardiac Amyloidosis: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef] [PubMed]
- Achten, A.; van Empel, V.P.M.; Weerts, J.; Mourmans, S.; Beckers-Wesche, F.; Spanjers, M.; Gingele, A.; Rocca, H.-P.B.-L.; Wijk, S.S.-V.; Knackstedt, C. Changes in the diagnostic trajectory of transthyretin cardiac amyloidosis over six years. Heart Vessel. 2024, 39, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Benson, M.D.; Dyck, P.J.; Grogan, M.; Coelho, T.; Cruz, M.; Berk, J.L.; Plante-Bordeneuve, V.; Schmidt, H.H.J.; Merlini, G. Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis. J. Am. Coll. Cardiol. 2015, 66, 2451–2466. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Khor, Y.M.; Cuddy, S.A.M.; Singh, V.; Falk, R.H.; Di Carli, M.F.; Dorbala, S. (99m)Tc Bone-Avid Tracer Cardiac Scintigraphy: Role in Noninvasive Diagnosis of Transthyretin Cardiac Amyloidosis. Radiology 2023, 306, e221082. [Google Scholar] [CrossRef]
- Mehta, P.; Chapel, D.B.; Goyal, N.; Yu, D.B.; Mor-Avi, V.; Narang, A.; Addetia, K.; Sarswat, N.; Lang, R.M.; Husain, A.N.; et al. A histopathologic schema to quantify the burden of cardiac amyloidosis: Relationship with survival and echocardiographic parameters. Echocardiography 2019, 36, 285–291. [Google Scholar] [CrossRef]
- Willixhofer, R.; Rettl, R.; Kronberger, C.; Ermolaev, N.; Gregshammer, B.; Duca, F.; Binder, C.; Kammerlander, A.; Alasti, F.; Kastner, J.; et al. Cardiopulmonary exercise testing in transthyretin amyloid cardiomyopathy patients: A long-term follow-up study. J. Cardiovasc. Med. 2024, 25, 704–712. [Google Scholar] [CrossRef]
- Badr Eslam, R.; Öztürk, B.; Rettl, R.; Capelle, C.D.J.; Qin, H.; Binder, C.; Dachs, T.-M.; Ligios, L.C.; Duca, F.; Dalos, D.; et al. Impact of Tafamidis and Optimal Background Treatment on Physical Performance in Patients with Transthyretin Amyloid Cardiomyopathy. Circ. Heart Fail. 2022, 15, e008381. [Google Scholar] [CrossRef]
- Willixhofer, R.; Ermolaev, N.; Kronberger, C.; Eslami, M.; Vilsmeier, J.; Rettl, R.; Nitsche, C.; Kammerlander, A.; Bergler-Klein, J.; Kastner, J.; et al. Prognostic Value of Submaximal Cardiopulmonary Exercise Testing in Patients with Cardiac Amyloidosis. Circ. Rep. 2025, 7, 76–85. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.; Deney, A.; Lairez, O.; Vergaro, G.; Emdin, M.; Carecci, A.; Inamo, J.; Montfort, A.; Neviere, R.; Damy, T.; et al. Prognostic value of cardiopulmonary exercise testing in cardiac amyloidosis. Eur. J. Heart Fail. 2021, 23, 231–239. [Google Scholar] [CrossRef]
- Hwang, J.W.; Lee, S.C.; Kim, D.; Kim, J.; Kim, E.K.; Chang, S.A.; Park, S.-J.; Kim, S.M.; Choe, Y.H.; Park, S.W. Role of cardiovascular magnetic resonance imaging and cardiopulmonary exercise test in predicting composite clinical outcomes in patients with hypertrophic cardiomyopathy. PLoS ONE 2023, 18, e0285887. [Google Scholar] [CrossRef] [PubMed]
- Carriere, C.; Corrà, U.; Piepoli, M.; Bonomi, A.; Merlo, M.; Barbieri, S.; Salvioni, E.; Binno, S.; Mapelli, M.; Righini, F.; et al. Anaerobic Threshold and Respiratory Compensation Point Identification During Cardiopulmonary Exercise Tests in Chronic Heart Failure. Chest 2019, 156, 338–347. [Google Scholar] [CrossRef]
- Tabet, J.Y.; Beauvais, F.; Thabut, G.; Tartière, J.M.; Logeart, D.; Cohen-Solal, A. A critical appraisal of the prognostic value of the VE/VCO2 slope in chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2003, 10, 267–272. [Google Scholar] [CrossRef]
- Ramsay, S.C.; Lindsay, K.; Fong, W.; Patford, S.; Younger, J.; Atherton, J. Tc-HDP quantitative SPECT/CT in transthyretin cardiac amyloid and the development of a reference interval for myocardial uptake in the non-affected population. Eur. J. Hybrid Imaging 2018, 2, 17. [Google Scholar] [CrossRef]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef]
- Rettl, R.; Duca, F.; Kronberger, C.; Binder, C.; Willixhofer, R.; Ermolaev, N.; Poledniczek, M.; Hofer, F.; Nitsche, C.; Hengstenberg, C.; et al. Prognostic implication of DPD quantification in transthyretin cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar]
- Patel, R.K.; Bandera, F.; Venneri, L.; Porcari, A.; Razvi, Y.; Ioannou, A.; Chacko, L.; Martinez-Naharro, A.; Rauf, M.U.; Knight, D.; et al. Cardiopulmonary Exercise Testing in Evaluating Transthyretin Amyloidosis. JAMA Cardiol. 2024, 9, 367–376. [Google Scholar] [CrossRef]
- Zhuang, C.; Luo, X.; Wang, Q.; Wang, W.; Sun, R.; Zhang, X.; Yu, J. The effect of exercise training and physiotherapy on diastolic function, exercise capacity and quality of life in patients with heart failure with preserved ejection fraction: A systematic review and meta-analysis. Kardiol. Pol. 2021, 79, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Pislaru, C.; Alashry, M.M.; Ionescu, F.; Petrescu, I.; Pellikka, P.A.; Grogan, M.; Dispenzieri, A.; Pislaru, S.V. Increased Myocardial Stiffness Detected by Intrinsic Cardiac Elastography in Patients with Amyloidosis: Impact on Outcomes. JACC Cardiovasc. Imaging 2019, 12, 375–377. [Google Scholar] [CrossRef]
- Duca, F.; Rettl, R.; Kronberger, C.; Binder, C.; Mann, C.; Dusik, F.; Schrutka, L.; Dalos, D.; Öztürk, B.; Dachs, T.M.; et al. Myocardial structural and functional changes in cardiac amyloidosis: Insights from a prospective observational patient registry. Eur. Heart J. Cardiovasc. Imaging 2023, 25, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Damy, T.; Fontana, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018, 39, 2799–2806. [Google Scholar] [CrossRef]
- Nies, R.J.; Ney, S.; Kindermann, I.; Bewarder, Y.; Zimmer, A.; Knebel, F.; Hahn, K.; Spethmann, S.; Luedike, P.; Michel, L.; et al. Real-world characteristics and treatment of cardiac transthyretin amyloidosis: A multicentre, observational study. ESC Heart Fail. 2024, 12, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Tana, M.; Tana, C.; Palmiero, G.; Mantini, C.; Coppola, M.G.; Limongelli, G.; Schiavone, C.; Porreca, E. Imaging findings of right cardiac amyloidosis: Impact on prognosis and clinical course. J. Ultrasound 2023, 26, 605–614. [Google Scholar] [CrossRef]
- Licordari, R.; Minutoli, F.; Recupero, A.; Campisi, M.; Donato, R.; Mazzeo, A.; Dattilo, G.; Baldari, S.; Vita, G.; Zito, C.; et al. Early Impairment of Right Ventricular Morphology and Function in Transthyretin-Related Cardiac Amyloidosis. J. Cardiovasc. Echogr. 2021, 31, 17–22. [Google Scholar] [CrossRef]
- Cantone, A.; Serenelli, M.; Sanguettoli, F.; Maio, D.; Fabbri, G.; Dal Passo, B.; Agostoni, P.; Grazzi, G.; Campo, G.; Rapezzi, C. Cardiopulmonary exercise testing predicts prognosis in amyloid cardiomyopathy: A systematic review and meta-analysis. ESC Heart Fail. 2023, 10, 2740–2744. [Google Scholar] [CrossRef]
- Dalia, T.; Acharya, P.; Chan, W.-C.; Sauer, A.J.; Weidling, R.; Fritzlen, J.; Goyal, A.; Miller, D.; Knipper, E.; Porter, C.B.; et al. Prognostic Role of Cardiopulmonary Exercise Testing in Wild-Type Transthyretin Amyloid Cardiomyopathy Patients Treated with Tafamidis. J. Card. Fail. 2021, 27, 1285–1289. [Google Scholar] [CrossRef]
- Bartolini, S.; Baldasseroni, S.; Fattirolli, F.; Silverii, M.V.; Piccioli, L.; Perfetto, F.; Marchionni, N.; Di Mario, C.; Martone, R.; Taborchi, G.; et al. Poor right ventricular function is associated with impaired exercise capacity and ventilatory efficiency in transthyretin cardiac amyloid patients. Intern. Emerg. Med. 2021, 16, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Yunis, A.; Doros, G.; Luptak, I.; Connors, L.H.; Sam, F. Use of Ventilatory Efficiency Slope as a Marker for Increased Mortality in Wild-Type Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2019, 124, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Pugliatti, P.; Trimarchi, G.; Barocelli, F.; Pizzino, F.; Di Spigno, F.; Tedeschi, A.; Piccione, M.C.; Irrera, P.; Aschieri, D.; Niccoli, G.; et al. Advancing Cardiac Amyloidosis Care Through Insights from Cardiopulmonary Exercise Testing. J. Clin. Med. 2024, 13, 7285. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.; Costa, P.F.; Kersting, D.; Jentzen, W.; Weber, M.; Lüdike, P.; Carpinteiro, A.; Oubari, S.; Hagenacker, T.; Thimm, A.; et al. Quantitative (99m)Tc-DPD-SPECT/CT assessment of cardiac amyloidosis. J. Nucl. Cardiol. 2023, 30, 101–111. [Google Scholar] [CrossRef]

| Variables | All (n = 44) | Low [99mTc]-DPD Uptake (n = 22) | High [99mTc]-DPD Uptake (n = 22) | p-Value |
|---|---|---|---|---|
| Demographic and clinical parameters | ||||
| Age, years | 78.0 (73.0–78.0) | 79.0 (72.0–80.0) | 75.0 (71.0–79.0) | 0.689 |
| Sex, Male | 36 (82.0) | 20 (91.0) | 16 (73.0) | 0.240 |
| Body Mass Index, kg/m2 | 27.0 (25.1–28.7) | 24.5 (24.0–27.3) | 28.0 (25.1–31.8) | 0.124 |
| NYHA Functional Class | 0.025 | |||
| Class I | 6 (14.0) | 5 (23.0) | 1 (4.0) | |
| Class II | 22 (50.0) | 13 (59.0) | 9 (41.0) | |
| Class III | 16 (36.0) | 4 (18.0) | 12 (55.0) | |
| 6 min Walk Distance, m | 422.0 (366.1–458.3) | 458.0 (347.0–496.1) | 405.0 (341.2–462.1) | 0.222 |
| ATTRv | 5 (11.0) | 2 (9.0) | 3 (14.0) | 1.000 |
| Comorbidities | ||||
| Arterial hypertension | 24 (54.0) | 10 (45.0) | 14 (64.0) | 0.364 |
| Atrial fibrillation or flutter | 26 (59.0) | 13 (59.0) | 13 (59.0) | 1.000 |
| Coronary artery disease | 13 (29.0) | 10 (45.0) | 3 (14.0) | 0.045 |
| Carpal tunnel syndrome | 25 (57.0) | 13 (59.0) | 12 (54.0) | 1.000 |
| Polyneuropathy | 30 (68.0) | 14 (64.0) | 16 (73.0) | 0.747 |
| Concomitant medication | ||||
| Beta-blockers | 18 (41.0) | 9 (41.0) | 9 (41.0) | 1.000 |
| MRA | 23 (52.0) | 9 (41.0) | 14 (64.0) | 0.227 |
| Diuretics | 30 (68.0) | 13 (59.0) | 17 (77.0) | 0.332 |
| Anticoagulants | 26 (59.0) | 13 (59.0) | 13 (59.0) | 1.000 |
| Statins | 21 (48.0) | 11 (50.0) | 10 (45.5) | 1.000 |
| Laboratory parameters | ||||
| NT-proBNP, pg/mL | 1876.0 (1611.0–2595.3) | 1364.0 (1051.0–2063.0) | 2341.0 (1844.0–3506.4) | 0.029 |
| Troponin T, ng/L | 41.0 (39.2–57.3) | 39.0 (33.7–62.0) | 46.0 (36.1–61.2) | 0.916 |
| eGFR, mL/min/1.73 m2 | 68.1 (59.1–75.4) | 79.0 (64.0–85.0) | 58.0 (47.0–72.2) | 0.126 |
| Cardiopulmonary Exercise Testing | ||||
| Peak VO2, mL/min/kg | 15.0 (15.0–18.0) | 16.1 (15.0–19.4) | 12.7 (11.7–14.4) | 0.002 |
| VO2 at AT, mL/min/kg | 10.0 (10.2–12.3) | 11.4 (10.7–14.3) | 9.2 (9.0–10.8) | 0.003 |
| Peak O2 Pulse, mL/beat | 11.4 (10.4–12.4) | 11.4 (10.2–13.6) | 11.4 (9.3–12.3) | 0.379 |
| VE/VCO2 slope | 39.4 (36.9–41.6) | 39.3 (35.1–43.0) | 39.7 (36.5–42.5) | 0.606 |
| Peak workload, Watt | 80.0 (68.2–88.2) | 90.0 (77.3–108.1) | 56.0 (53.0–73.4) | 0.003 |
| Peak VE, L/min | 52.5 (52.0–63.2) | 60.0 (57.0–73.2) | 45.0 (43.8–57.0) | 0.004 |
| Nuclear imaging parameters | ||||
| Perugini Grading Scale | 0.760 | |||
| 2 | 18 (41.0) | 8 (36.0) | 10 (45.0) | |
| 3 | 26 (59.0) | 14 (64.0) | 12 (54.0) | |
| [99mTc]-DPD retention index | 5.0 (4.3–6.0) | 2.8 (2.4–3.3) | 6.8 (6.5–8.0) | <0.001 |
| Transthoracic echocardiography parameters | ||||
| Interventricular septum, mm | 18.0 (17.4–20.2) | 19.0 (17.1–21.3) | 17.0 (16.4–20.5) | 0.540 |
| LV ejection fraction, % | 51.0 (47.1–53.0) | 52.0 (47.3–54.4) | 50.0 (44.4–53.4) | 0.518 |
| LV end-diastolic diameter, mm | 43.0 (42.0–45.5) | 43.0 (40.5–46.0) | 44.0 (41.0–47.2) | 0.556 |
| LV global longitudinal strain, -% | 12.0 (13.5–11.4) | 13.2 (15.0–11.5) | 12.0 (13.0–10.4) | 0.162 |
| RV end-diastolic diameter, mm | 34.0 (32.3–36.0) | 34.0 (31.2–35.5) | 34.0 (32.0–37.0) | 0.787 |
| RV global longitudinal strain, -% | 15.0 (16.1–13.0) | 16.3 (18.1–13.4) | 12.0 (15.3–11.2) | 0.166 |
| Cardiac magnetic resonance imaging parameters (n = 32) | ||||
| Interventricular septum, mm | 18.3 (16.4–21.1) | 19.0 (15.2–21.0) | 17.6 (15.1–24.4) | 0.737 |
| LV ejection fraction, % | 47.1 (43.4–52.0) | 46.0 (40.3–52.0) | 49.0 (42.2–56.0) | 0.766 |
| RV ejection fraction, % | 47.5 (42.0–51.0) | 45.3 (42.0–53.0) | 50.0 (36.4–54.0) | 0.518 |
| MOLLI-ECV, % | 45.0 (41.0–51.0) | 43.0 (35.3–51.0) | 46.0 (42.1–56.0) | 0.450 |
| T1-Mapping, ms | 1112.0 (1089.4–1158.3) | 1113.0 (1069.1–1166.0) | 1110.0 (1069.1–1166.0) | 0.734 |
| CPET Variable | Correlation Coefficient | p-Value |
|---|---|---|
| Peak VO2, mL/min/kg | −0.355 | 0.018 |
| VO2 at AT, mL/min/kg | −0.391 | 0.009 |
| Peak O2 Pulse, mL/beat | 0.075 | 0.629 |
| VE/VCO2 slope | 0.125 | 0.419 |
| Peak workload, Watt | 0.262 | 0.082 |
| Peak VE, L/min | −0.348 | 0.021 |
| Variables | Peak VO2, mL/min/kg | VE/VCO2 Slope | [99mTc]-DPD Retention Index |
|---|---|---|---|
| Clinical parameters | Correlation coefficient | Correlation coefficient | Correlation coefficient |
| Body Mass Index, kg/m2 | −0.197 | −0.085 | 0.299 * |
| NYHA Functional Class ≥ III | −0.549 ** | 0.324 * | 0.324 * |
| 6-min Walk Distance, m | 0.384 * | −0.451 ** | 0.012 |
| Laboratory parameters | |||
| NT-proBNP, pg/mL | −0.530 ** | 0.348 * | 0.261 |
| Troponin T, ng/L | −0.431 ** | 0.587 ** | 0.028 |
| eGFR, mL/min/1.73 m2 | 0.402 ** | −0.390 ** | −0.138 |
| Transthoracic echocardiography parameters | |||
| Interventricular septum, mm | −0.041 | 0.312 * | 0.119 |
| LV ejection fraction, % | 0.283 | 0.033 | 0.062 |
| LV end-diastolic diameter, mm | −0.102 | 0.299 * | −0.001 |
| LV global longitudinal strain, -% | −0.282 | 0.236 | 0.283 |
| RV end-diastolic diameter, mm | −0.254 | 0.032 | −0.031 |
| RV global longitudinal strain, -% | −0.350 * | 0.289 | 0.291 |
| Cardiac magnetic resonance imaging parameters | n = 32 | n = 17 | n = 15 |
| Interventricular septum, mm | −0.103 | 0.214 | 0.250 |
| LV ejection fraction, % | 0.211 | −0.016 | 0.048 |
| RV ejection fraction, % | 0.374 * | −0.104 | −0.003 |
| MOLLI-ECV, % | −0.149 | 0.302 | 0.315 |
| Native T1 time, ms | −0.276 | 0.464 ** | 0.231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermolaev, N.; Rettl, R.; Willixhofer, R.; Kronberger, C.; Poledniczek, M.; Schmid, L.M.; Duca, F.; Binder, C.; Eslami, M.; Beitzke, D.; et al. Cardiopulmonary Exercise Testing Correlates with Quantitative Left Ventricular [99mTc]-DPD Uptake in Transthyretin Amyloid Cardiomyopathy. J. Clin. Med. 2025, 14, 2999. https://doi.org/10.3390/jcm14092999
Ermolaev N, Rettl R, Willixhofer R, Kronberger C, Poledniczek M, Schmid LM, Duca F, Binder C, Eslami M, Beitzke D, et al. Cardiopulmonary Exercise Testing Correlates with Quantitative Left Ventricular [99mTc]-DPD Uptake in Transthyretin Amyloid Cardiomyopathy. Journal of Clinical Medicine. 2025; 14(9):2999. https://doi.org/10.3390/jcm14092999
Chicago/Turabian StyleErmolaev, Nikita, René Rettl, Robin Willixhofer, Christina Kronberger, Michael Poledniczek, Lena Marie Schmid, Franz Duca, Christina Binder, Mahshid Eslami, Dietrich Beitzke, and et al. 2025. "Cardiopulmonary Exercise Testing Correlates with Quantitative Left Ventricular [99mTc]-DPD Uptake in Transthyretin Amyloid Cardiomyopathy" Journal of Clinical Medicine 14, no. 9: 2999. https://doi.org/10.3390/jcm14092999
APA StyleErmolaev, N., Rettl, R., Willixhofer, R., Kronberger, C., Poledniczek, M., Schmid, L. M., Duca, F., Binder, C., Eslami, M., Beitzke, D., Loewe, C., Hacker, M., Kammerlander, A., Kastner, J., Bergler-Klein, J., Calabretta, R., & Badr Eslam, R. (2025). Cardiopulmonary Exercise Testing Correlates with Quantitative Left Ventricular [99mTc]-DPD Uptake in Transthyretin Amyloid Cardiomyopathy. Journal of Clinical Medicine, 14(9), 2999. https://doi.org/10.3390/jcm14092999








