The Prevalence of Reduced Bone Mineral Density and the Impact of Specific Auxological Factors and Hormones on Bone Mass in Children with Endocrine Disorders
Abstract
1. Introduction
2. Materials and Methods
3. Results
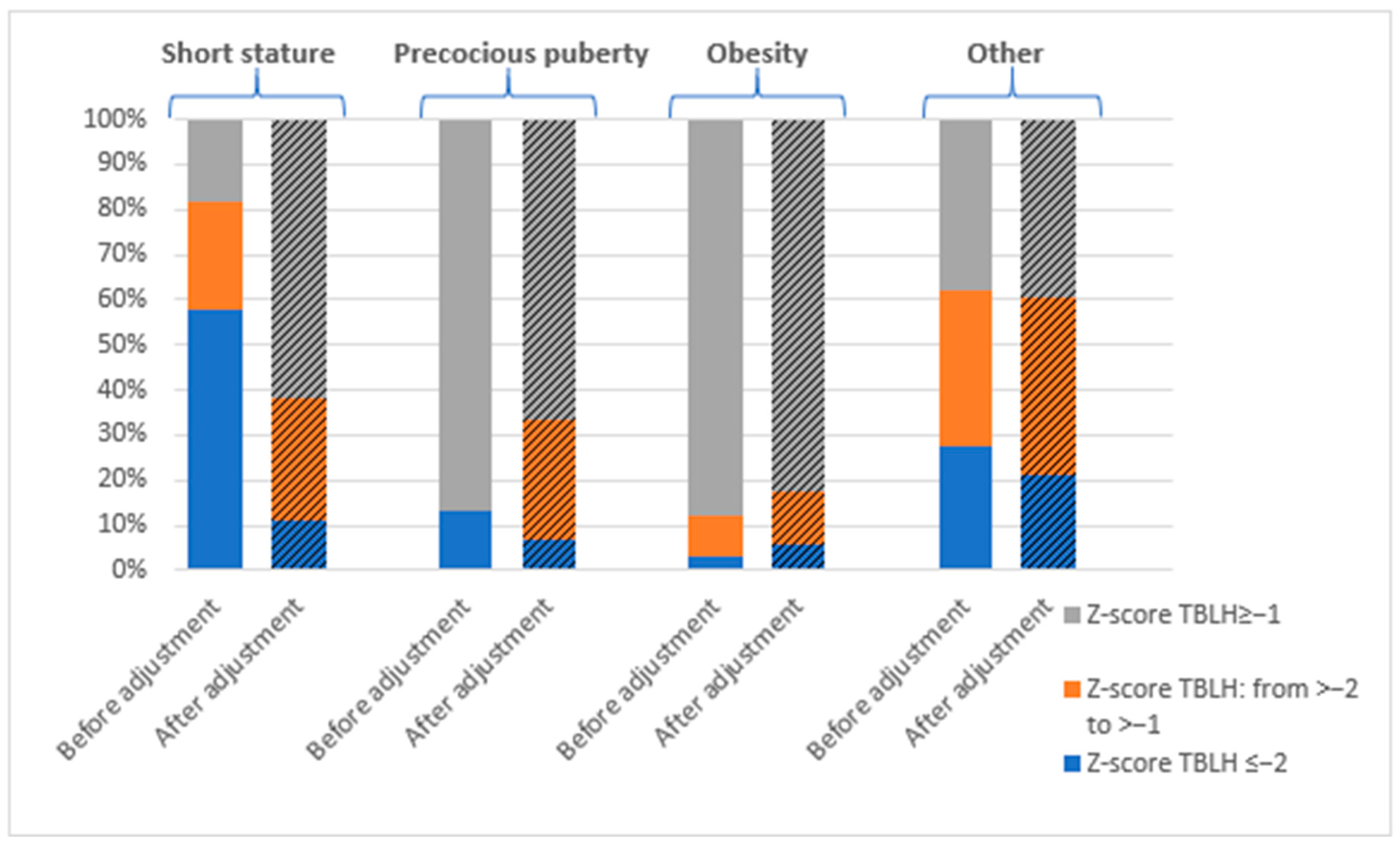
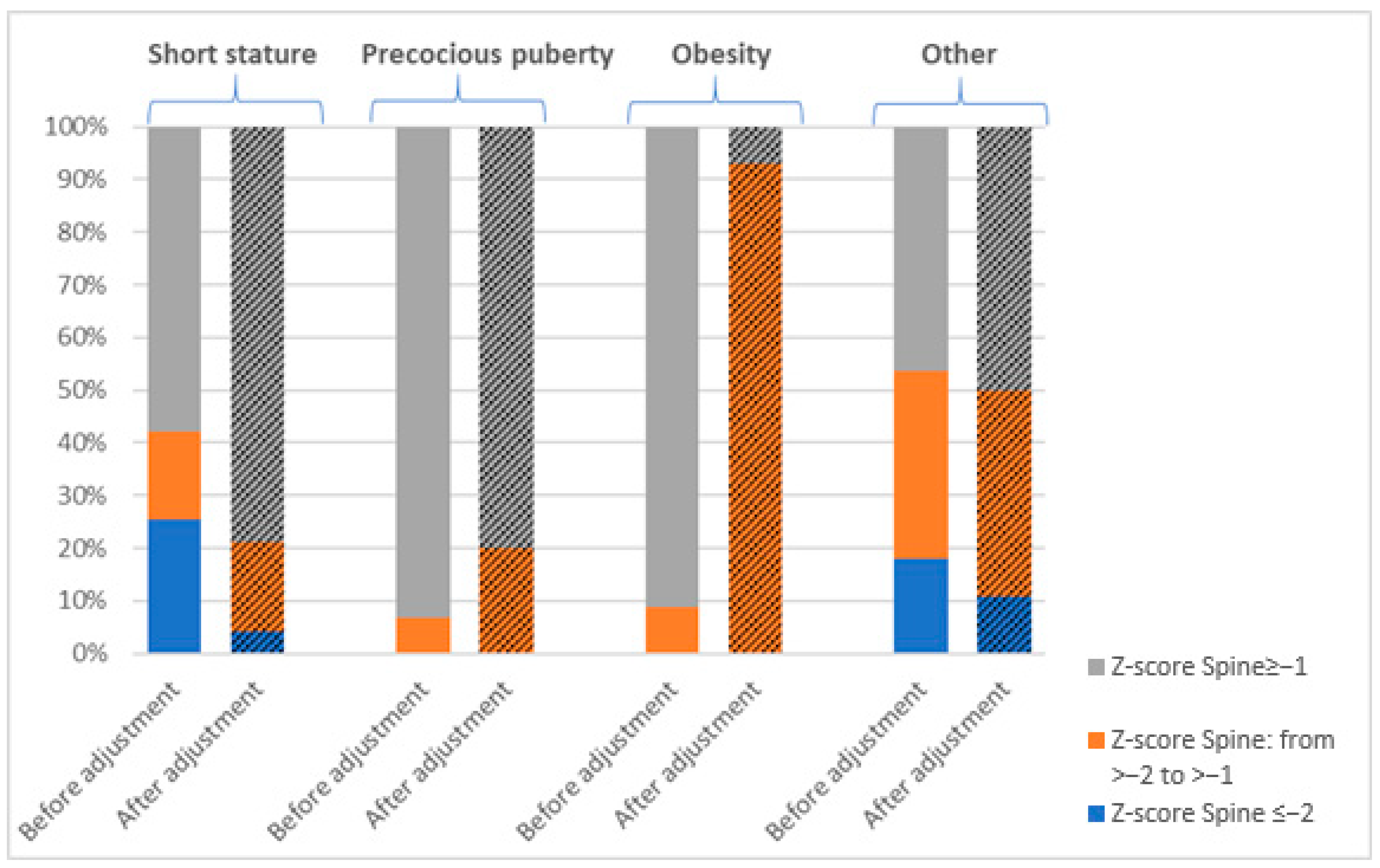
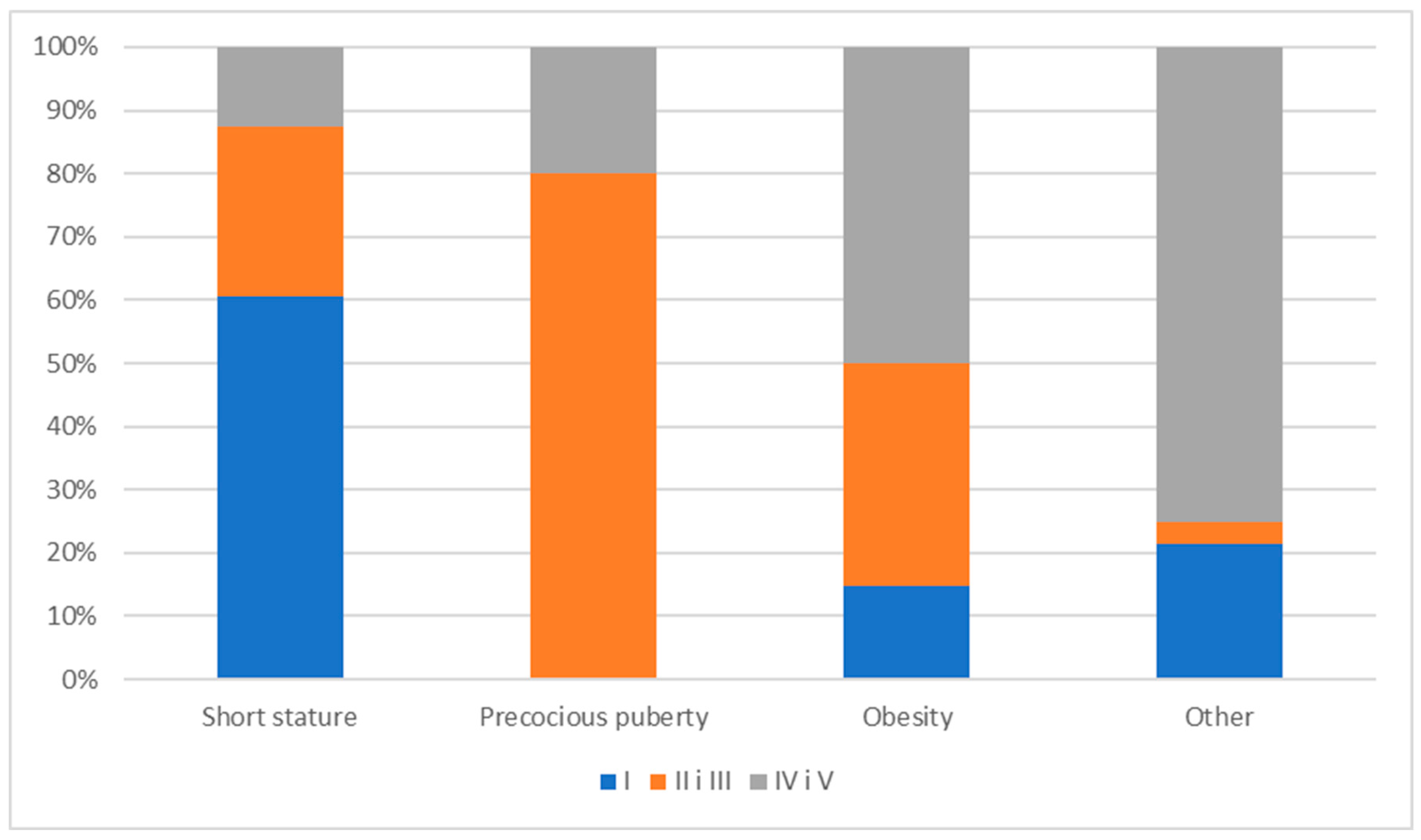
3.1. Prevalence of Bone Mineralization Disorders
3.2. The Impact of Pubertal Stage on Bone Mineral Density in the Study Population
3.3. A Comparison of Anthropometric Parameters, Laboratory Indicators of Calcium–Phosphate and Hormonal Metabolism, and DXA Parameters by Patient Diagnosis
3.4. An Analysis of Factors Influencing Bone Mineral Density and DXA Parameters in the Entire Study Group of Patients
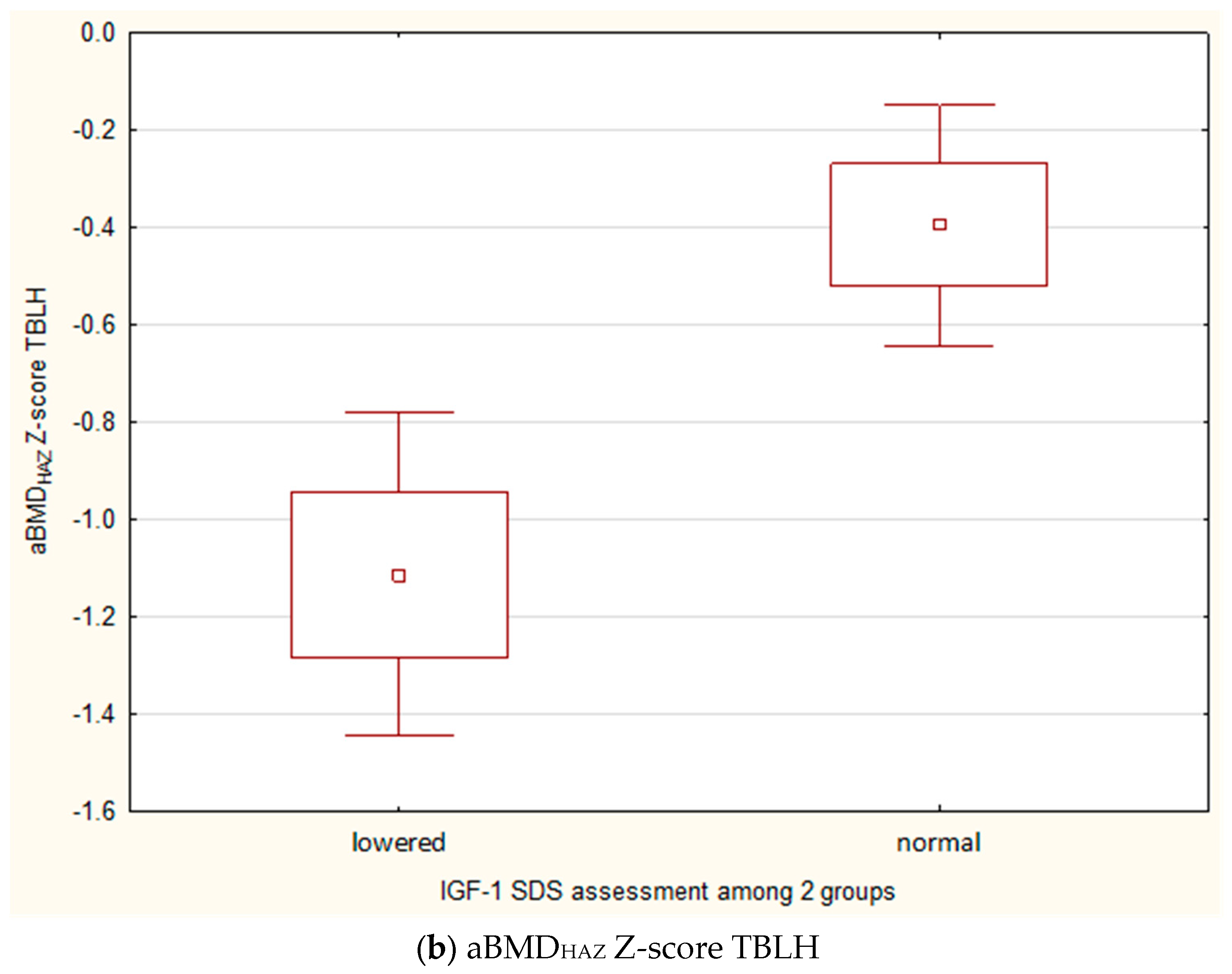
3.5. An Analysis of Factors Affecting Bone Mineral Density in Children with Growth Retardation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Formosa, M.M.; Christou, M.A.; Mäkitie, O. Bone Fragility and Osteoporosis in Children and Young Adults. J. Endocrinol. Investig. 2024, 47, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Di Iorgi, N.; Maruca, K.; Patti, G.; Mora, S. Update on Bone Density Measurements and Their Interpretation in Children and Adolescents. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 477–498. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.J.; Beaupré, G.S.; Carter, D.R. A Theoretical Analysis of the Relative Influences of Peak BMD, Age-Related Bone Loss and Menopause on the Development of Osteoporosis. Osteoporos. Int. 2003, 14, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, N.J.; Arabi, A.; Bachrach, L.K.; Fewtrell, M.; El-Hajj Fuleihan, G.; Kecskemethy, H.H.; Jaworski, M.; Gordon, C.M.; International Society for Clinical Densitometry. Dual-Energy X-Ray Absorptiometry Interpretation and Reporting in Children and Adolescents: The Revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 225–242. [Google Scholar] [CrossRef]
- Zemel, B.S.; Leonard, M.B.; Kelly, A.; Lappe, J.M.; Gilsanz, V.; Oberfield, S.; Mahboubi, S.; Shepherd, J.A.; Hangartner, T.N.; Frederick, M.M.; et al. Height Adjustment in Assessing Dual Energy X-Ray Absorptiometry Measurements of Bone Mass and Density in Children. J. Clin. Endocrinol. Metab. 2010, 95, 1265–1273. [Google Scholar] [CrossRef]
- Binkovitz, L.A.; Henwood, M.J. Pediatric DXA: Technique and Interpretation. Pediatr. Radiol. 2007, 37, 21–31. [Google Scholar] [CrossRef]
- Jang, M.J.; Shin, C.; Kim, S.; Lee, J.W.; Chung, N.-G.; Cho, B.; Jung, M.H.; Suh, B.-K.; Ahn, M.B. Factors Affecting Bone Mineral Density in Children and Adolescents with Secondary Osteoporosis. Ann. Pediatr. Endocrinol. Metab. 2023, 28, 34–41. [Google Scholar] [CrossRef]
- Eapen, E.; Grey, V.; Don-Wauchope, A.; Atkinson, S.A. Bone Health in Childhood: Usefulness of Biochemical Biomarkers. EJIFCC 2008, 19, 123–136. [Google Scholar]
- Levine, M.A. Assessing Bone Health in Children and Adolescents. Indian J. Endocrinol. Metab. 2012, 16, S205–S212. [Google Scholar] [CrossRef]
- Zemel, B.S.; Kalkwarf, H.J.; Gilsanz, V.; Lappe, J.M.; Oberfield, S.; Shepherd, J.A.; Frederick, M.M.; Huang, X.; Lu, M.; Mahboubi, S.; et al. Revised Reference Curves for Bone Mineral Content and Areal Bone Mineral Density According to Age and Sex for Black and Non-Black Children: Results of the Bone Mineral Density in Childhood Study. J. Clin. Endocrinol. Metab. 2011, 96, 3160–3169. [Google Scholar] [CrossRef]
- Carter, D.R.; Bouxsein, M.L.; Marcus, R. New Approaches for Interpreting Projected Bone Densitometry Data. J. Bone Miner. Res. 1992, 7, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.; Muñoz, M.; Shibli-Rahhal, A. Anorexia Nervosa and Osteoporosis. Calcif. Tissue Int. 2022, 110, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Abrams, S.A.; Committee on Nutrition; Daniels, S.R.; Abrams, S.A.; Corkins, M.R.; De Ferranti, S.D.; Golden, N.H.; Magge, S.N.; Schwarzenberg, S.J. Optimizing Bone Health in Children and Adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef]
- Soyka, L.A.; Fairfield, W.P.; Klibanski, A. Hormonal Determinants and Disorders of Peak Bone Mass in Children. J. Clin. Endocrinol. Metab. 2000, 85, 3951–3963. [Google Scholar] [CrossRef]
- Simm, P.J.; Biggin, A.; Zacharin, M.R.; Rodda, C.P.; Tham, E.; Siafarikas, A.; Jefferies, C.; Hofman, P.L.; Jensen, D.E.; Woodhead, H.; et al. Consensus Guidelines on the Use of Bisphosphonate Therapy in Children and Adolescents. J. Paediatr. Child Health 2018, 54, 223–233. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Growth and Physiological Development during Adolescence. Annu. Rev. Med. 1968, 19, 283–300. [Google Scholar] [CrossRef]
- Palczewska, I.; Niedzwiedzka, Z. Somatic Development Indices in Children and Youth of Warsaw. Med. Wieku Rozw. 2001, 5, 18–118. (In Polish) [Google Scholar]
- Katzman, D.K.; Bachrach, L.K.; Carter, D.R.; Marcus, R. Clinical and Anthropometric Correlates of Bone Mineral Acquisition in Healthy Adolescent Girls. J. Clin. Endocrinol. Metab. 1991, 73, 1332–1339. [Google Scholar] [CrossRef]
- Elmlinger, M.W.; Kühnel, W.; Weber, M.M.; Ranke, M.B. Reference Ranges for Two Automated Chemiluminescent Assays for Serum Insulin-like Growth Factor I (IGF-I) and IGF-Binding Protein 3 (IGFBP-3). Clin. Chem. Lab. Med. 2004, 42, 654–664. [Google Scholar] [CrossRef]
- van der Sluis, I.M.; Boot, A.M.; Hop, W.C.; De Rijke, Y.B.; Krenning, E.P.; de Muinck Keizer-Schrama, S.M.P.F. Long-Term Effects of Growth Hormone Therapy on Bone Mineral Density, Body Composition, and Serum Lipid Levels in Growth Hormone Deficient Children: A 6-Year Follow-up Study. Horm. Res. 2002, 58, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ravn, P.; Cizza, G.; Bjarnason, N.H.; Thompson, D.; Daley, M.; Wasnich, R.D.; McClung, M.; Hosking, D.; Yates, A.J.; Christiansen, C. Low Body Mass Index Is an Important Risk Factor for Low Bone Mass and Increased Bone Loss in Early Postmenopausal Women. Early Postmenopausal Intervention Cohort (EPIC) Study Group. J. Bone Miner. Res. 1999, 14, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Asomaning, K.; Bertone-Johnson, E.R.; Nasca, P.C.; Hooven, F.; Pekow, P.S. The Association between Body Mass Index and Osteoporosis in Patients Referred for a Bone Mineral Density Examination. J. Womens Health 2006, 15, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Högler, W.; Shaw, N. Childhood Growth Hormone Deficiency, Bone Density, Structures and Fractures: Scrutinizing the Evidence. Clin. Endocrinol. 2010, 72, 281–289. [Google Scholar] [CrossRef]
- Schweizer, R.; Martin, D.D.; Schwarze, C.P.; Binder, G.; Georgiadou, A.; Ihle, J.; Ranke, M.B. Cortical Bone Density Is Normal in Prepubertal Children with Growth Hormone (GH) Deficiency, but Initially Decreases During GH Replacement Due to Early Bone Remodeling. J. Clin. Endocrinol. Metab. 2003, 88, 5266–5272. [Google Scholar] [CrossRef][Green Version]
- DiGirolamo, D.J.; Mukherjee, A.; Fulzele, K.; Gan, Y.; Cao, X.; Frank, S.J.; Clemens, T.L. Mode of Growth Hormone Action in Osteoblasts. J. Biol. Chem. 2007, 282, 31666–31674. [Google Scholar] [CrossRef]
- Chen, J.-F.; Lin, P.-W.; Tsai, Y.-R.; Yang, Y.-C.; Kang, H.-Y. Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy. Cells 2019, 8, 1318. [Google Scholar] [CrossRef]
- Guntur, A.R.; Rosen, C.J. IGF-1 Regulation of Key Signaling Pathways in Bone. Bonekey Rep. 2013, 2, 437. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rosen, C.J.; Visser, M.; Hannan, M.T.; Harris, T.; Wilson, P.W.; Kiel, D.P. Association between Insulin-like Growth Factor I and Bone Mineral Density in Older Women and Men: The Framingham Heart Study. J. Clin. Endocrinol. Metab. 1998, 83, 4257–4262. [Google Scholar] [CrossRef]
- Rusińska, A.; Chlebna-Sokół, D. Insulin-like Growth Factor-I and Mineral Metabolism Markers in Children with Idiopathic Decrease in Bone Mass. Clin. Chim. Acta 2006, 366, 257–263. [Google Scholar] [CrossRef]
- Garnett, S.P.; Högler, W.; Blades, B.; Baur, L.A.; Peat, J.; Lee, J.; Cowell, C.T. Relation Between Hormones and Body Composition, Including Bone, in Prepubertal Children. Am. J. Clin. Nutr. 2004, 80, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Andrés, M.N.; Papapietro, K.; Cañizo, F.J.; Rejas, J.; Larrodera, L.; Hawkins, F.G. Correlations Between Bone Mineral Density, Insulin-like Growth Factor I and Auxological Variables. Eur. J. Endocrinol. 1995, 132, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yoo, J.W.; Lee, J.W.; Jung, M.H.; Cho, B.; Suh, B.-K.; Ahn, M.B.; Chung, N.-G. Association of Insulin-like Growth Factor-1 with Bone Mineral Density in Survivors of Childhood Acute Leukemia. Cancers 2024, 16, 1296. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.B.; Shults, J.; Wilson, B.A.; Tershakovec, A.M.; Zemel, B.S. Obesity during Childhood and Adolescence Augments Bone Mass and Bone Dimensions. Am. J. Clin. Nutr. 2004, 80, 514–523. [Google Scholar] [CrossRef]
- van Leeuwen, J.; Koes, B.W.; Paulis, W.D.; van Middelkoop, M. Differences in Bone Mineral Density Between Normal-Weight Children and Children with Overweight and Obesity: A Systematic Review and Meta-Analysis. Obes. Rev. 2017, 18, 526–546. [Google Scholar] [CrossRef]
- Fintini, D.; Cianfarani, S.; Cofini, M.; Andreoletti, A.; Ubertini, G.M.; Cappa, M.; Manco, M. The Bones of Children with Obesity. Front. Endocrinol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Fornari, E.D.; Suszter, M.; Roocroft, J.; Bastrom, T.; Edmonds, E.W.; Schlechter, J. Childhood Obesity as a Risk Factor for Lateral Condyle Fractures over Supracondylar Humerus Fractures. Clin. Orthop. Relat. Res. 2013, 471, 1193–1198. [Google Scholar] [CrossRef]
- Rinonapoli, G.; Pace, V.; Ruggiero, C.; Ceccarini, P.; Bisaccia, M.; Meccariello, L.; Caraffa, A. Obesity and Bone: A Complex Relationship. Int. J. Mol. Sci. 2021, 22, 13662. [Google Scholar] [CrossRef]
- Clarke, B.L.; Khosla, S. Female Reproductive System and Bone. Arch. Biochem. Biophys. 2010, 503, 118–128. [Google Scholar] [CrossRef]
- Saggese, G.; Bertelloni, S.; Baroncelli, G.I. Sex Steroids and the Acquisition of Bone Mass. Horm. Res. 1997, 48 (Suppl. S5), 65–71. [Google Scholar] [CrossRef]
- Grimston, S.K.; Morrison, K.; Harder, J.A.; Hanley, D.A. Bone Mineral Density during Puberty in Western Canadian Children. Bone Miner. 1992, 19, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, S.B.; Pereira, F.d.A.; Villela, R.A.; Antonini, S.R.R.; Elias, P.C.L.; Martinelli, C.E.; de Castro, M.; Moreira, A.C.; de Paula, F.J.A. Bone Mineral Density and Body Composition in Girls with Idiopathic Central Precocious Puberty before and after Treatment with a Gonadotropin-Releasing Hormone Agonist. Clinics 2012, 67, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L. The Mechanisms of Estrogen Regulation of Bone Resorption. J. Clin. Investig. 2000, 106, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and Bone Health in Men and Women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian Hormones and Obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Kanbur, N.O.; Derman, O.; Sen, T.A.; Kinik, E. Osteocalcin. A Biochemical Marker of Bone Turnover During Puberty. Int. J. Adolesc. Med. Health 2002, 14, 235–244. [Google Scholar] [CrossRef]
- El-Dorry, G.; Ashry, H.; Ibrahim, T.; Elias, T.; Alzaree, F. Bone Density, Osteocalcin and Deoxypyridinoline for Early Detection of Osteoporosis in Obese Children. Open Access Maced. J. Med. Sci. 2015, 3, 413–419. [Google Scholar] [CrossRef]
- Paldánius, P.M.; Ivaska, K.K.; Mäkitie, O.; Viljakainen, H. Serum and Urinary Osteocalcin in Healthy 7- to 19-Year-Old Finnish Children and Adolescents. Front. Pediatr. 2021, 9, 610227. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Huang, X.; Yu, X.; Li, Y.; Yu, F.; Zhou, W. Variation of Bone Turnover Markers in Childhood and Adolescence. Int. J. Clin. Pract. 2023, 2023, 5537182. [Google Scholar] [CrossRef]
- Korpysz, A.; Jaworski, M.; Skorupa, E.; Szalecki, M.; Walczak, M.; Petriczko, E. Bone Turnover Markers During Growth Hormone Therapy for Short Stature Children Born Small for Gestational Age. Biomedicines 2024, 12, 1919. [Google Scholar] [CrossRef]
- Furman, K.; Gut, P.; Sowińska, A.; Ruchała, M.; Fichna, M. Predictors of Bone Mineral Density in Patients Receiving Glucocorticoid Replacement for Addison’s Disease. Endocrine 2024, 84, 711–719. [Google Scholar] [CrossRef]
- Karimian, P.; Ebrahimi, H.K.; Jafarnejad, S.; Delavar, M.A. Effects of Vitamin D on Bone Density in Healthy Children: A Systematic Review. J. Fam. Med. Prim. Care 2022, 11, 870–878. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Dawson-Hughes, B. Positive Association between 25-Hydroxy Vitamin D Levels and Bone Mineral Density: A Population-Based Study of Younger and Older Adults. Am. J. Med. 2004, 116, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, N.; Pratelli, L.; Frigato, M.; Mulè, R.; Mascia, M.L.; Gnudi, S. The Relationship of Vitamin D Status to Bone Mineral Density in an Italian Population of Postmenopausal Women. Osteoporos. Int. 2005, 16, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Zhang, J.-R.; Mao, C.-J.; Li, K.; Wang, F.; Chen, J.; Liu, C.-F. Relationship Between 25-Hydroxyvitamin D, Bone Density, and Parkinson’s Disease Symptoms. Acta Neurol. Scand. 2019, 140, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; Toussaint, N.; de Regt, M.; Tieland, M.; van Loon, L.J.C.; de Groot, L.C.P.G.M. The Association Between 25-Hydroxyvitamin D Concentration, Physical Performance and Frailty Status in Older Adults. Eur. J. Nutr. 2019, 58, 1173–1181. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Rosen, C.J. Vitamin D: 100 Years of Discoveries, yet Controversy Continues. Lancet Diabetes Endocrinol. 2023, 11, 362–374. [Google Scholar] [CrossRef]

| Diagnosis | Short Stature Mean ± SD Median (Q1–Q3) | Precocious Puberty Mean ± SD Median (Q1–Q3) | Obesity Mean ± SD Median (Q1–Q3) | Other Mean ± SD Median (Q1–Q3) |
|---|---|---|---|---|
| Age [years] | 10.96 ± 3.07 * 11.16 (8.59–13.31) | 9.58 ± 1.68 # 9.50 (8.58–10.70) | 12.74 ± 3.12 # 13.34 (11.30–14.60) | 14.22 ± 3.35 *# 15.14 (12.76–16.15) |
| HSDS | −2.56 ± 1.16 *#^ −2.56 (−3.13–(−2.0)) | 0.14 ± 1.26 * 0.08 (−0.92–1.05) | 0.74 ± 1.47 # 0.94 (-0.29–1.67) | −0.45 ± 1.75 ^ −0.17 (−1.57–0.52) |
| BMI SDS | −0.42 ± 2.51 * −0.75 (−1.91–0.12) | 0.57 ± 2.26 # 0.16 (−1.24–0.68) | 6.22 ± 4.12 *#^ 6.37 (3.42–8.58) | −0.51 ± 1.56 ^ −0.73 (−1.85–0.68) |
| Serum calcium [mmol/L] | 2.41 ± 0.10 * 2.41 (2.34–2.47) | 2.42 ± 0.13 2.41 (2.34–2.50) | 2.40 ± 0.11 2.41 (2.31–2.47) | 2.33 ± 0.14 * 2.32 (2.23–2.42) |
| Serum phosphate [mmol/L] | 1.75 ± 0.18 1.74 (1.63–1.88) | 1.79 ± 0.16 1.81 (1.64–1.86) | 1.69 ± 0.27 1.72 (1.51–1.90) | 1.61 ± 0.23 1.57 (1.49–1.76) |
| Serum Magnesium [mmol/L] | 0.84 ± 0.06 0.84 (0.81–0.87) | 0.83 ± 0.05 0.83 (0.79–0.86) | 0.84 ± 0.07 0.84 (0.78–0.86) | 0.81 ± 0.07 0.80 (0.76–0.84) |
| ALP [U/L] | 153.33 ± 33.72 152.00 (119.00–179.00) | 205.17 ± 60.39 * 194.00 (163.00–270.00) | 205.71 ± 92.01 # 217.00 (98.00–302.00) | 88.60 ± 29.07 *# 78.00 (75.00–96.00) |
| Ca/Cr ratio | 0.11 ± 0.08 0.09 (0.03–0.16) | 0.12 ± 0.12 0.08 (0.03–0.15) | 0.08 ± 0.10 0.05 (0.03–0.10) | 0.10 ± 0.07 0.10 (0.05–0.15) |
| PTH [pg/mL] | 38.73 ± 104.28 25.15 (19.90–30.50) | 32.69 ± 10.33 32.30 (26.60–40.70) | 32.26 ± 8.95 32.00 (24.90–35.80) | 37.75 ± 30.35 30.25 (25.70–38.40) |
| 25OHD [ng/mL] | 28.43 ± 13.37 26.05 (20.40–32.60) | 21.08 ± 11.05 17.50 (11.50–29.70) | 21.45 ± 7.03 20.40 (17.25–25.97) | 25.98 ± 10.25 23.70 (19.60–28.10) |
| Osteocalcin [ng/mL] | 81.68 ± 27.98 75.10 (62.00–97.20) | 109.03 ± 32.38 97.10 (83.70–142.40) | 76.34 ± 35.10 79.55 (39.80–100.50) | 80.46 ± 59.16 64.90 (41.10–99.40) |
| Crosslaps [pg/mL] | 1889.69 ± 506.57 1939.00 (1571.00–2185.00) | 2176.67 ± 502.03 2136.00 (1951.00–2532.00) | 1679.57 ± 694.04 1580.50 (1027.0–2283.00) | 1719.15 ± 843.80 1654.00 (1044.0–2096.00) |
| Diagnosis | Short Stature Mean ± SD Median (Q1–Q3) | Precocious Puberty Mean ± SD Median (Q1–Q3) | Obesity Mean ± SD Median (Q1–Q3) | Other Mean ± SD Median (Q1–Q3) |
|---|---|---|---|---|
| TSH [uIU/mL] | 2.51 ± 1.02 2.45 (1.72–3.07) | 2.73 ± 1.35 2.55 (1.66–3.52) | 2.42 ± 1.26 2.06 (1.59–3.33) | 2.27 ± 1.66 1.94 (1.28–3.18) |
| fT4 [ng/dL] | 1.24 ± 0.17 1.22 (1.14–1.35) | 1.27 ± 0.20 1.30 (1.12–1.41) | 1.16±0.16 1.11 (1.04–1.26) | 1.27 ± 0.35 1.22 (1.11–1.32) |
| fT3 [pg/mL] | 3.85 ± 0.60 3.76 (3.44–4.11) | 4.11 ± 0.67 4.12 (3.63–4.22) | 3.82 ± 0.60 3.79 (3.42–4.10) | 3.57 ± 0.78 3.43 (3.07–4.03) |
| Estradiol | 18.88 ± 28.36 5.00 (5.00–20.40) | 18.27 ± 18.20 7.90 (5.00–32.80) | 29.37 ± 32.97 19.85 (5.00–42.40) | 39.88 ± 47.35 25.10 (7.10–42.90) |
| F: N = 55 | N = 17 | N = 10 | N = 17 | N = 11 |
| M: N = 31 | F: 25.16 ± 34.20 5.00 (5.00–43.30) | F: 19.18 ± 19.64 8.30 (5.00–38.10) | F: 38.68 ± 37.46 41.60 (14.40–44.20) | F: 55.05 ± 57.57 28.90 (6.10–94.20) |
| [pg/mL] | N = 11 M: 9.15 ± 11.3 5.00 (5.00–7.00) | N = 3 M: 15.23 ± 15.28 7.90 (5.00–32.80) | N = 9 M:11.79 ± 7.57 7.90 (5.00–18.40) | N = 8 M: 19.03 ± 13.03 15.25 (9.05–27.85) |
| Testosterone | ||||
| F: N = 46 | 0.84 ± 1.42 0.075 (0.025–1.35) | 0.85 ± 2.00 0.06 (0.03–0.14) | 0.97 ± 1.23 0.48 (1.20–1.23) | 1.93 ± 2.54 0.31 (0.12–4.23) |
| M: N = 51 | F: 0.17 ± 0.15 0.12 (0.04–0.26) | F: 0.053 ± 0.04 * 0.04 (0.03–0.07) | F: 0.04 ± 0.05 * 0.30 (0.11–0.63) | F: 0.22 ± 0.18 0.14 (0.10–0.27) |
| [ng/mL] | N = 10 | N = 10 | N = 16 | N = 10 |
| M: 1.11 ± 1.61 * 0.08 (0.03–1.74) N = 25 | M: 2.84 ± 3.15 2.25 (0.15–5.52) N = 4 | M: 1.72 ± 1.55 1.13 (0.45–3.06) N = 12 | M: 3.65 ± 2.65 * 4.23 (1.17–5.71) N = 10 | |
| IGF-1 SDS | −1.13 ± 1.05 * −0.95 (−1.66–(−0.46)) | 0.10 ± 1.02 * −0.08 (−0.63–0.54) | −0.52 ± 1.08 −0.41 (−1.49–0.24) | −0.90 ± 1.05 −0.71 (−1.40–(−0.31)) |
| IGF-1 [ng/mL] | 179.01 ± 88.30 *# 161.25 (110.90–221.40) | 313.00 ± 175.69 325.80 (168.40–418.60) | 285.44 ± 109.17 * 246.90 (190.40–380.60) | 276.56 ± 122.72 # 284.65 (173.40–362.60) |
| IGF-BP3 [ng/mL] | 3887.50 ± 924.49 3774.50 (3250.00–4429.00) | 4211.67 ± 719.06 4496.00 (3564.00–4749.00) | 4489.75 ± 1009.62 4693.50 (4178.0–4883.00) | 4156.21 ± 861.12 4139.00 (3089.0–4688.00) |
| Bone age [years] | 9.44 ± 3.48 10.00 (6.00–12.25) | 9.96 ± 2.15 10.00 (8.20–11.00) | 12.72 ± 2.88 13.00 (11.00–14.50) | 12.38 ± 4.35 14.00 (12.00–15.00) |
| Cortisol [ug/dL] | 13.81 ± 5.07 14.40 (10.70–17.10) | 13.72 ± 3.03 14.00 (12.80–15.50) | 13.53 ± 4.37 13.40 (10.80–16.70) | 11.62 ± 4.42 11.20 (7.50–14.70) |
| Diagnosis | Short Stature Mean ± SD Median (Q1–Q3) | Precocious Puberty Mean ± SD Median (Q1–Q3) | Obesity Mean ± SD Median (Q1–Q3) | Other Mean ± SD Median (Q1–Q3) |
|---|---|---|---|---|
| BMD Spine [g/cm2] | 0.58 ± 0.14 * 0.55 (0.49–0.64) | 0.63 ± 0.11 0.61 (0.54–0.71) | 0.85 ± 0.21 *# 0.86 (0.65–1.03) | 0.79 ± 0.21 # 0.81 (0.65–0.95) |
| aBMD for age Z-score Spine | −1.19 ± 1.19 *# −1.00 (−2.00–(−0.40)) | 0.28 ± 1.06 * 0.30 (−0.50–0.70) | 0.80 ± 1.16 # 0.95 (0.20–1.30) | −0.79 ± 1.66 −1.00 (−1.70–0.50) |
| aBMDHAZ Z-score Spine | −0.087 ± 1.13 0.10 (−0.93–0.54) | 0.08 ± 1.03 0.01 (−0.77–0.64) | 0.36 ± 1.04 * 0.30 (−0.27–0.94) | −0.77±1.46 * −0.94 (−1.38–0.39) |
| BMAD | 0.10 ± 0.02 *# 0.09 (0.08–0.11) | 0.10 ± 0.01 0.10 (0.08–0.11) | 0.12 ± 0.02 * 0.12 (0.10–0.14) | 0.11 ± 0.02 # 0.11 (0.09–0.13) |
| BMC Spine [g] | 21.99 ± 9.21 *# 20.42 (16.23–24.64) | 24.84 ± 6.52 22.78 (20.62–31.77) | 45.18 ± 17.94 * 44.97 (29.35–57.00) | 42.16 ± 17.85 # 43.13 (27.63–55.67) |
| BMD TBLH [g/cm2] | 0.63 ± 0.12 *# 0.62 (0.55–0.69) | 0.67 ± 0.09 0.65 (0.60–0.73) | 0.86 ± 0.14 * 0.86 (0.75–0.96) | 0.81 ± 0.15 # 0.87 (0.71–0.90) |
| aBMD for age Z-score TBLH | −2.19 ± 1.39 *#^ −2.20 (−2.90–(−1.20)) | −0.29 ± 1.11 * −0.40 (−0.90–0.20) | 0.41 ± 1.15 # 0.40 (−0.10–1.20) | −1.24 ± 1.39 ^ −1.10 (−2.25–(−0.45) |
| aBMDHAZ Z-score TBLH | −0.74 ± 1.19 −0.70 (−1.40–0.02) | −0.7 ± 0.78 * −0.64 (−1.18–(−0.26) | −0.17 ± 0.97 −0.02 (−0.49–0.43) | −1.16 ± 1.03 * −1.31 (−1.79–(−0.4) |
| BMC TBLH [g] | 691.99 ± 270.62 * 650.60 (514.78–784.82) | 773.23 ± 185.83 731.28 (634.68–910.76) | 1437.98 ± 516.97 1495.65 (969.82–1857.28) | 1776.44 ± 2949.76 * 1332.80 (874.82–1642.41) |
| BMC Spine | aBMD Spine | BMAD | BMD for Age Z-Score Spine | BMDHAZ Z-Score Spine | BMC TBLH | aBMD TBLH | BMD for Age Z-Score TBLH | BMDHAZ Z-Score TBLH | |
|---|---|---|---|---|---|---|---|---|---|
| Age [years] | 0.727 * | 0.684 * | 0.506 * | −0.066 | −0.169 * | 0.389 * | 0.796 * | −0.014 | −0.15 |
| HSDS | 0.519 * | 0.472 * | 0.334 * | 0.560 * | 0.007 | 0.199 * | 0.503 * | 0.719 * | 0.08 |
| BMI SDS | 0.431 * | 0.470 * | 0.450 * | 0.558 * | 0.348 * | 0.106 | 0.461 * | 0.638 * | 0.43 * |
| Serum calcium | −0.228 * | −0.256 * | −0.24 * | −0.028 | −0.013 | −0.195 * | −0.222 * | 0.001 | 0.07 |
| Serum phosphate | −0.298 * | −0.283 * | −0.223 * | 0.063 | 0.118 | −0.259 * | −0.277 * | −0.008 | 0.04 |
| Serum magnesium | −0.124 | −0.144 | −0.151 | −0.084 | −0.066 | −0.083 | −0.058 | 0.023 | 0.05 |
| ALP | −0.377 * | −0.320 | −0.242 | 0.137 | 0.091 | −0.198 * | −0.228 | 0.307 | 0.26 |
| Ca/Cr ratio | −0.196 * | −0.217 * | −0.199 * | −0.160 | −0.117 | −0.198 * | −0.238 * | −0.160 | −0.11 |
| PTH | −0.037 | 0.000 | 0.063 | 0.044 | 0.155 | −0.066 | −0.058 | −0.030 | 0.11 |
| 25OHD | −0.208 * | −0.245 * | −0.228 * | −0.332 * | −0.189 * | −0.212 * | −0.265 * | −0.426 * | −0.18 * |
| Osteocalcin | −0.279 * | −0.300 * | −0.304 * | −0.023 | −0.086 | −0.214 * | −0.238 * | −0.041 | −0.16 |
| Crosslaps | −0.287 * | −0.334 * | −0.358 * | −0.094 | −0.146 | −0.197 * | −0.252 * | −0.124 | −0.23 * |
| TSH | 0.005 | −0.032 | −0.038 | 0.095 | 0.119 | −0.063 | −0.108 | 0.003 | 0.04 |
| fT3 | −0.151 | −0.181 * | −0.194 * | 0.042 | −0.018 | −0.118 | −0.156 | 0.049 | −0.02 |
| fT4 | −0.012 | −0.031 | −0.040 | −0.080 | −0.072 | −0.058 | −0.060 | −0.069 | −0.06 |
| Estradiol (Total N = 86) | 0.418 * | 0.455 * | 0.436 * | 0.148 | 0.132 | 0.340 * | 0.399 * | 0.125 | 0.14 |
| Estradiol (F = 55) | 0.471 * | 0.470 * | 0.416 * | 0.154 | 0.137 | 0.420 * | 0.458 * | 0.132 | 0.120 |
| Estradiol (M = 31) | 0.615 * | 0.611 * | 0.522 * | 0.245 | 0.152 | 0.538 * | 0.576 * | 0.134 | 0.029 |
| Testosterone (Total N = 97) | 0.312 * | 0.219 * | 0.086 | 0.035 | −0.055 | 0.340 * | 0.351 * | 0.029 | −0.05 |
| Testosterone (F = 46) | 0.461 * | 0.459 * | 0.415 * | 0.257 | 0.245 | 0.443 * | 0.459 * | 0.233 | 0.281 |
| Testosterone (M = 51) | 0.550 * | 0.496 * | 0.368 * | 0.085 | −0.077 | 0.526 * | 0.585 * | 0.184 | 0.051 |
| IGF-1 | 0.577 * | 0.563 * | 0.439 * | 0.325 * | 0.088 | 0.608 * | 0.662 * | 0.423 * | 0.18 * |
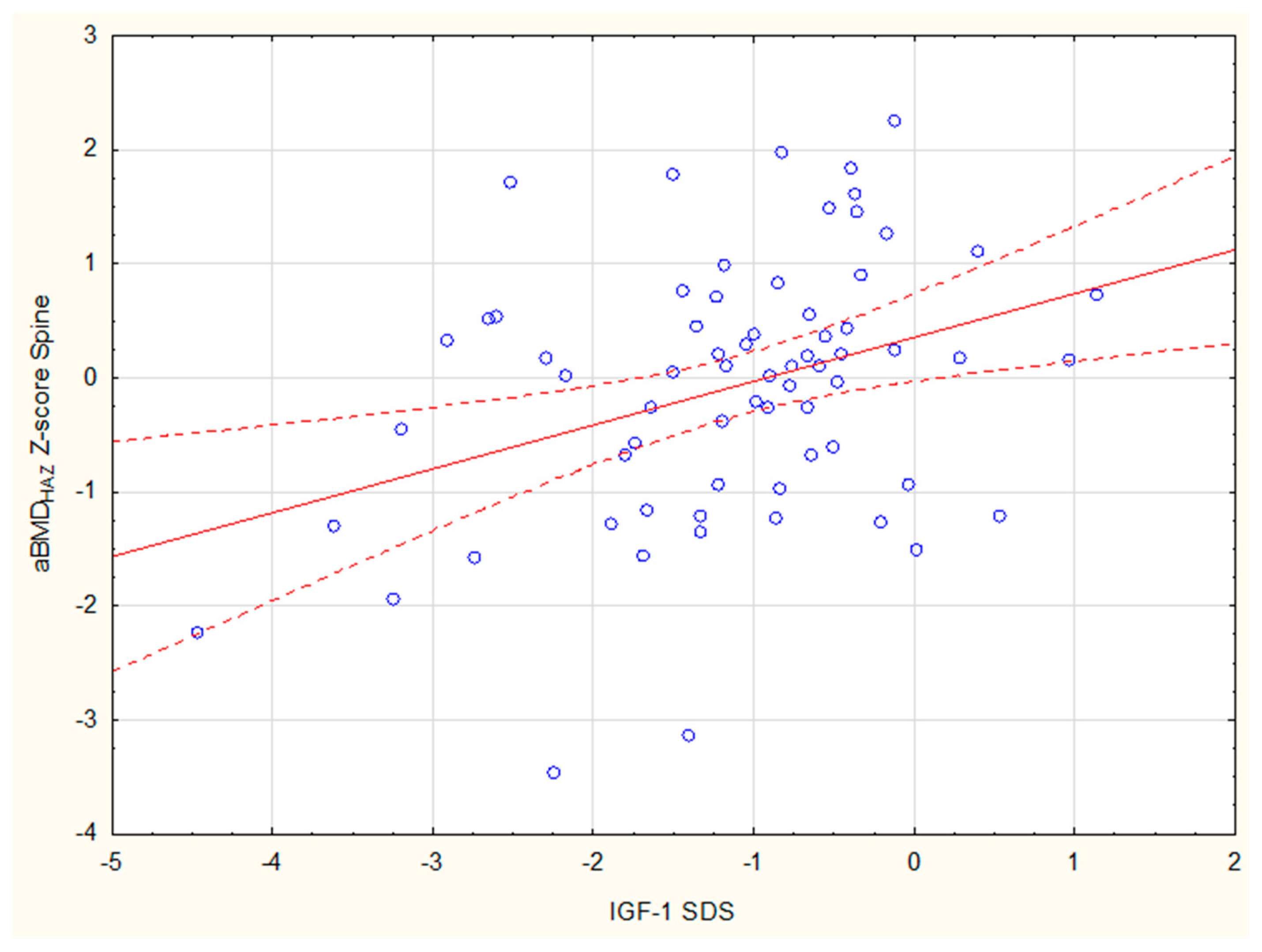
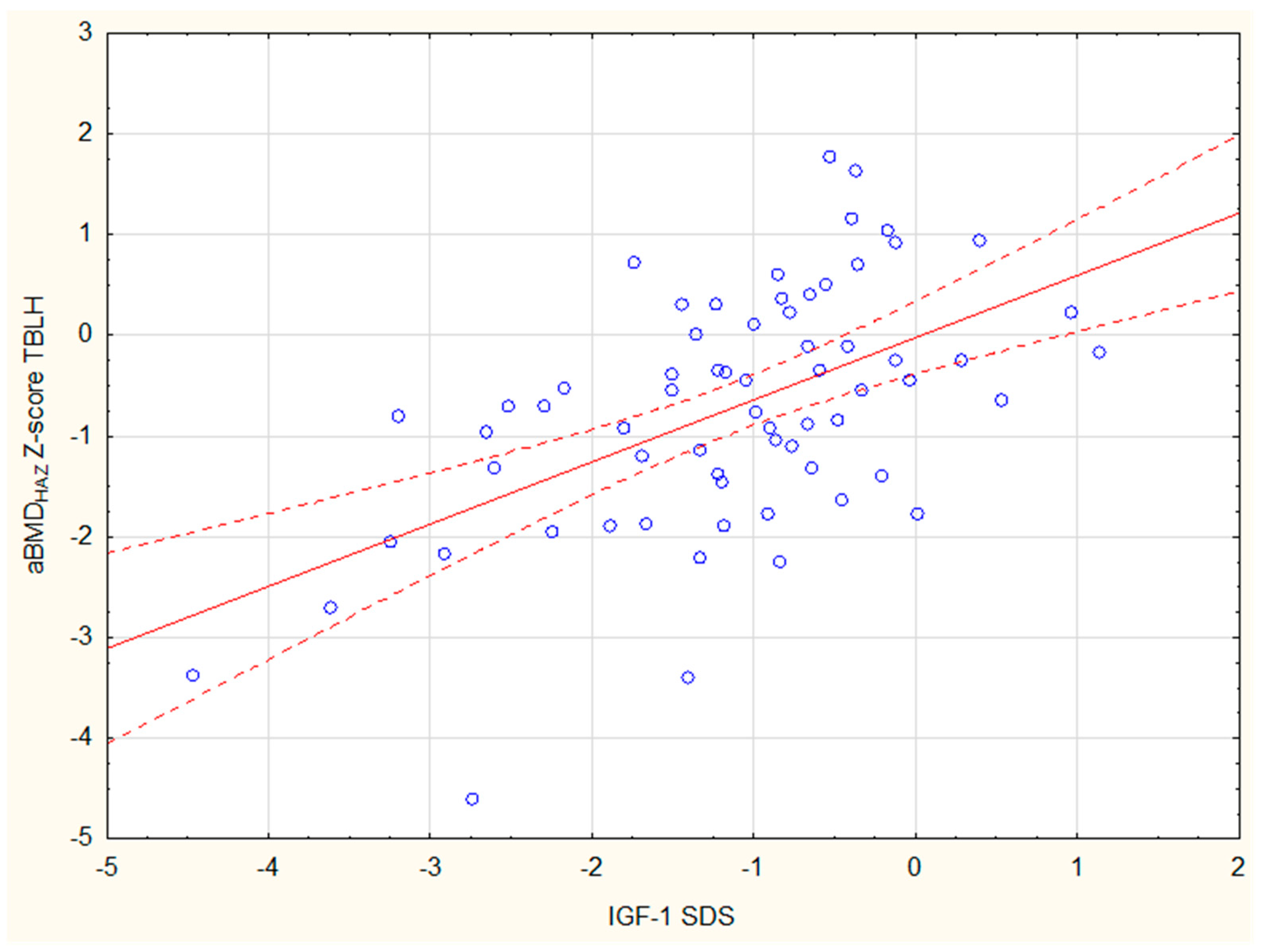
| IGF-1 SDS | 0.039 | 0.063 | 0.097 | 0.436 * | 0.288 * | 0.063 | 0.077 | 0.492 * | 0.43 * |
| IGF-BP3 | 0.474 * | 0.476 * | 0.397 * | 0.311 * | 0.211 * | 0.471 * | 0.525 * | 0.401 * | 0.30 * |
| Bone age | 0.767 * | 0.717 * | 0.495 * | 0.044 | −0.114 | −0.149 | 0.863 * | 0.166 | −0.05 |
| Cortisol | −0.125 | −0.083 | −0.016 | 0.037 | 0.067 | −0.149 | −0.120 | 0.066 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łupińska, A.; Aszkiełowicz, S.; Zygmunt, A.; Lewiński, A.; Stawerska, R. The Prevalence of Reduced Bone Mineral Density and the Impact of Specific Auxological Factors and Hormones on Bone Mass in Children with Endocrine Disorders. J. Clin. Med. 2025, 14, 2988. https://doi.org/10.3390/jcm14092988
Łupińska A, Aszkiełowicz S, Zygmunt A, Lewiński A, Stawerska R. The Prevalence of Reduced Bone Mineral Density and the Impact of Specific Auxological Factors and Hormones on Bone Mass in Children with Endocrine Disorders. Journal of Clinical Medicine. 2025; 14(9):2988. https://doi.org/10.3390/jcm14092988
Chicago/Turabian StyleŁupińska, Anna, Sara Aszkiełowicz, Arkadiusz Zygmunt, Andrzej Lewiński, and Renata Stawerska. 2025. "The Prevalence of Reduced Bone Mineral Density and the Impact of Specific Auxological Factors and Hormones on Bone Mass in Children with Endocrine Disorders" Journal of Clinical Medicine 14, no. 9: 2988. https://doi.org/10.3390/jcm14092988
APA StyleŁupińska, A., Aszkiełowicz, S., Zygmunt, A., Lewiński, A., & Stawerska, R. (2025). The Prevalence of Reduced Bone Mineral Density and the Impact of Specific Auxological Factors and Hormones on Bone Mass in Children with Endocrine Disorders. Journal of Clinical Medicine, 14(9), 2988. https://doi.org/10.3390/jcm14092988







