Immunomodulation in Pediatric Sepsis: A Narrative Review
Abstract
1. Background
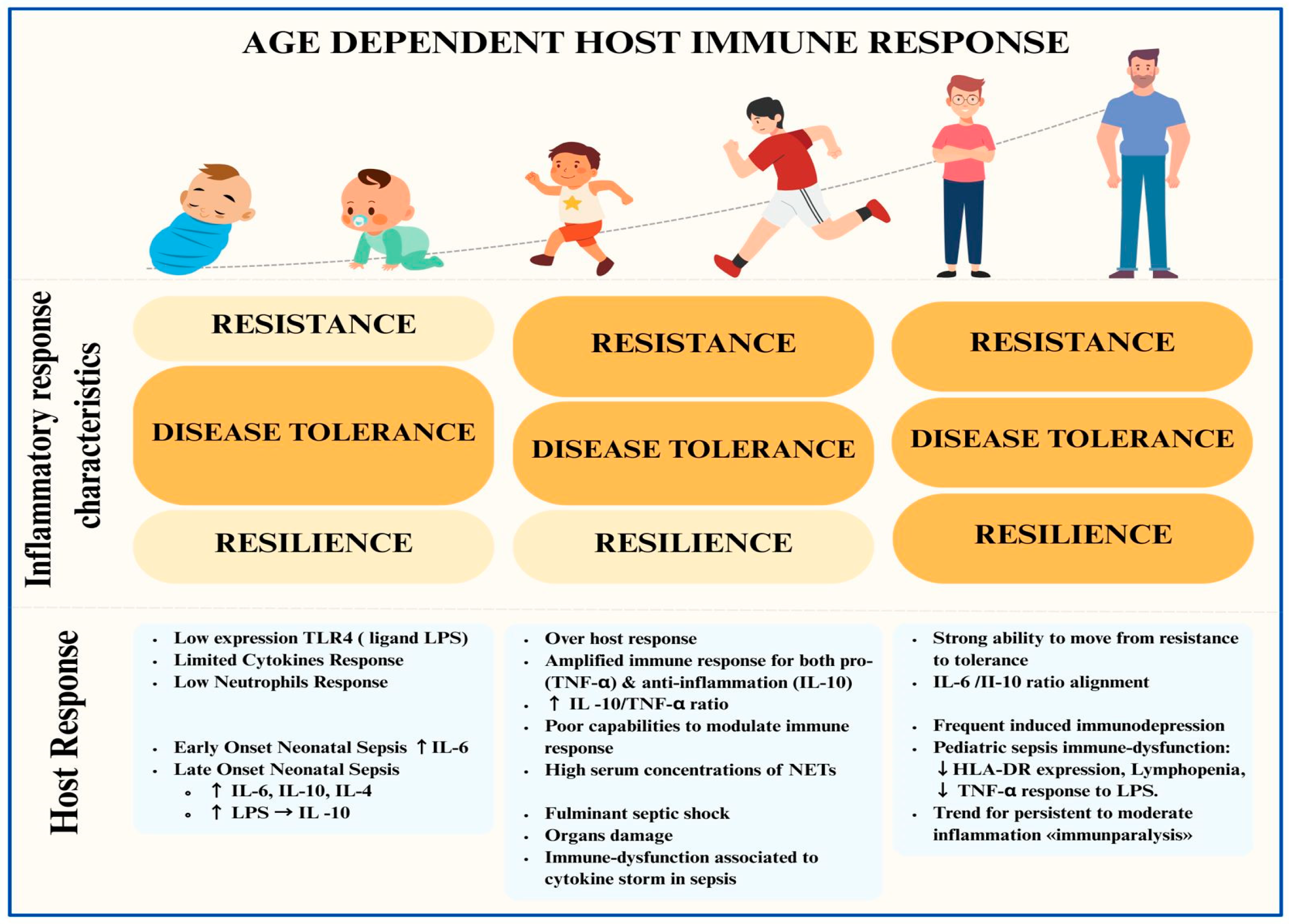
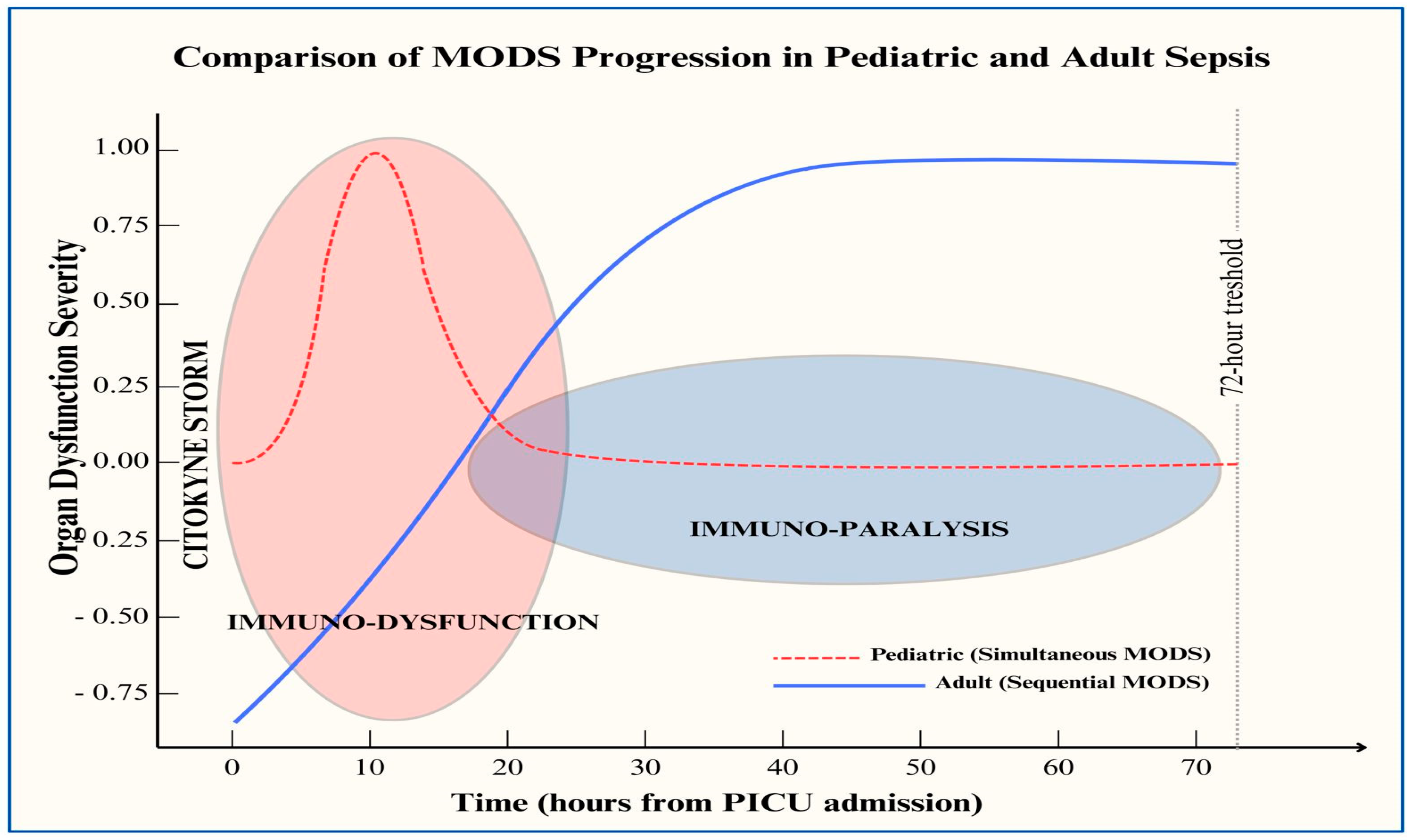
2. Host Response in Children with Sepsis and Differences from Adult Populations
3. Management of Pediatric Septic Shock: Exploring New Perspectives Beyond Standard Care
- Cytokine modulation: Techniques such as extracorporeal blood purification therapies or the administration of immunoglobulins aim to control the overwhelming cytokine response.
- Targeted immunomodulation: Selective drugs such as monoclonal antibodies block key mediators of septic shock.
- Immune stimulation: Strategies to counteract immune paralysis through immunostimulatory therapies.
4. Immunomodulation in Pediatric Septic Shock: Current Evidence
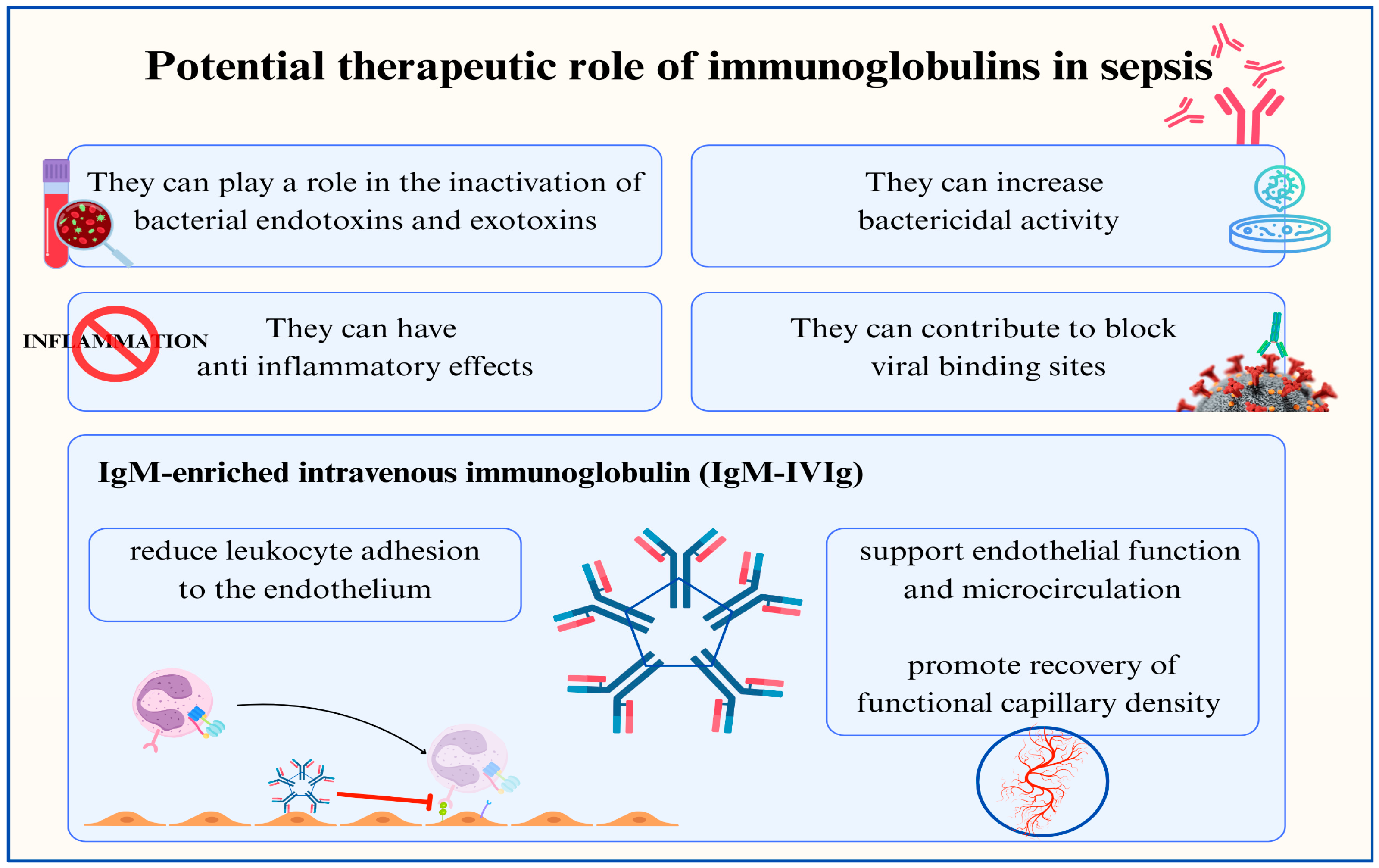
4.1. Immunoglobulins
4.2. Immunoglobulins and Toxic Shock Syndrome
4.3. Corticosteroids
4.4. Monoclonal Antibodies
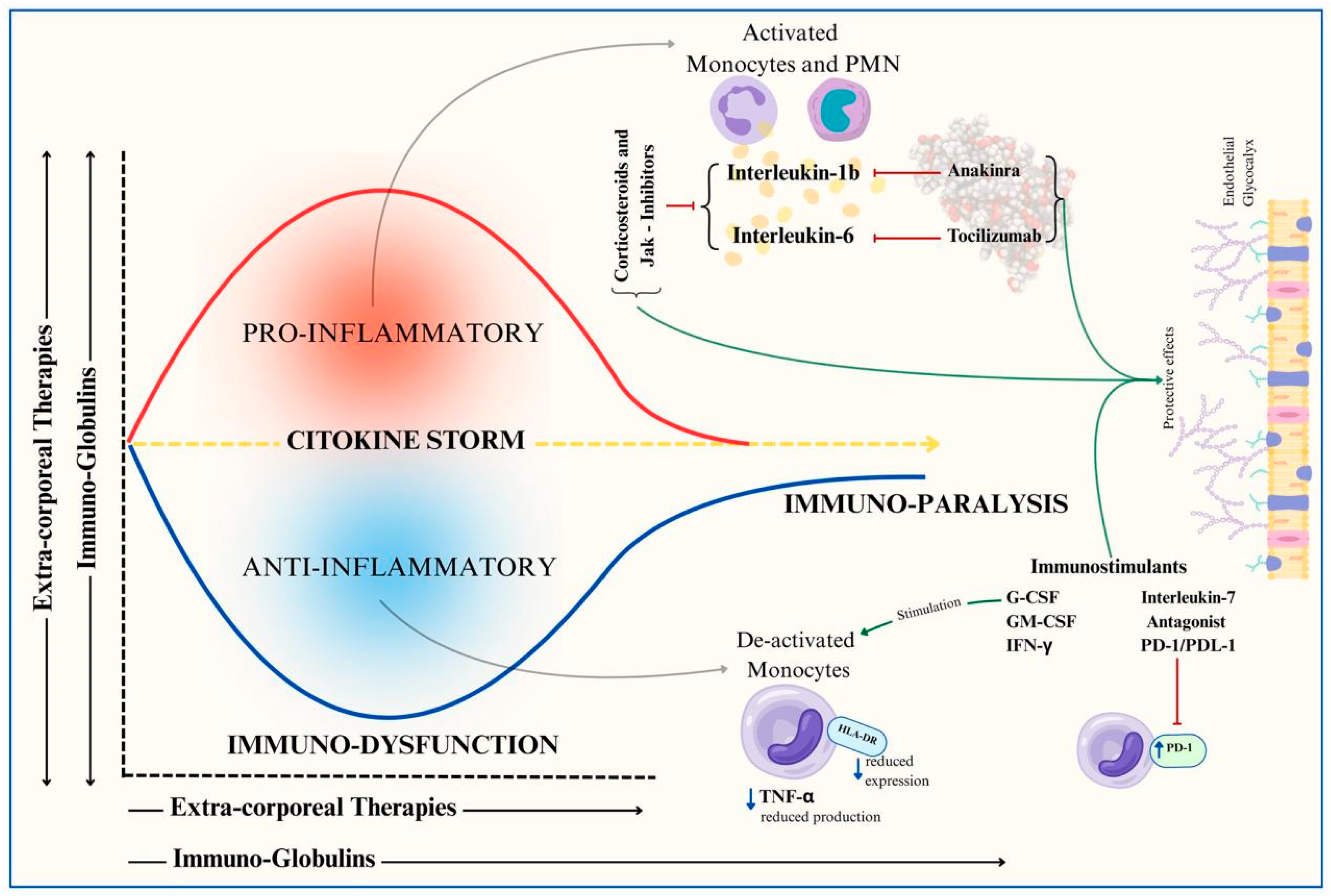
4.5. Immunostimulation
4.6. Extracorporeal Blood Purification Techniques in Pediatric Septic Shock
5. Knowledge Gaps and Research Opportunities in Pediatric Sepsis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| CI | Confidence Interval |
| CRRT | Continuous Renal Replacement Therapy |
| DIC | Disseminated Intravascular Coagulation |
| ELBW | Extremely Low Birth Weight |
| EOS | Early-Onset Sepsis |
| GCR | Glucocorticoid Receptor |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GLUT-1 | Glucose Transporter 1 |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| GPX4 | Glutathione Peroxidase 4 |
| HBD | Hepatobiliary Disfunction |
| HF | Hemofiltration |
| HLA | Human Leukocyte Antigen |
| HLH | Hemophagocytic Lymphohistiocytosis |
| HR | Hazard Ratio |
| HVHF | High-Volume Hemofiltration |
| ICU | Intensive Care Unit |
| IFN | Interferon |
| IL | Interleukin |
| IQR | Interquartile Range |
| IVIG | Intravenous Immunoglobulins |
| JAK | Janus Kinase |
| kDa | Kilodalton |
| LOS | Late-Onset Sepsis, Length of Stay |
| LPS | Lipopolysaccharide |
| MAS | Macrophage Activation Syndrome |
| MODS | Multiple-Organ Dysfunction Syndrome |
| MV | Mechanical Ventilation |
| NETs | Neuthrophil Extracellular Traps |
| NO | Nitric Oxide |
| OR | Odds Ratio |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death Ligand 1 |
| PE | Plasma Separation Techniques |
| PEI | Polyethyleneimine |
| PICU | Pediatric Intensive Care Unit |
| PMX | Polymyxin |
| PRISM | Pediatric Risk of Mortality |
| RCT | Randomized Controlled Trial |
| rhIL-1ra | Recombinant Human IL-1 Receptor Antagonists |
| RR | Relative Risk |
| RRTs | Renal Replacement Therapies |
| ST | Surface Treatment |
| TAMOF | Thrombocytopenia-Associated Multiple-Organ Failure |
| Th | T Helper |
| TLR | Toll-Like Receptor |
| TNF | Tumor Necrosis Factor |
| TPE | Plasma Exchange |
| TSS | Toxic Shock Syndrome |
References
- Cavaillon, J.M.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L. Improved survival in critically ill patients: Are large RCTs more useful than personalized medicine? No. Intensive Care. Med. 2016, 42, 1778–1780. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; van der Poll, T.; Marshall, J.C. The End of “One Size Fits All” Sepsis Therapies: Toward an Individualized Approach. Biomedicines. 2022, 10, 2260. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Watson, R.S.; Sorce, L.R.; Argent, A.C.; Menon, K.; Hall, M.W.; Akech, S.; Albers, D.J.; Alpern, E.R.; Balamuth, F.; et al. Society of Critical Care Medicine Pediatric Sepsis Definition Task Force. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA. 2024, 331, 665–674. [Google Scholar] [CrossRef]
- Sanchez-Pinto, L.N.; Bennett, T.D.; DeWitt, P.E.; Russell, S.; Rebull, M.N.; Martin, B.; Akech, S.; Albers, D.J.; Alpern, E.R.; Balamuth, F.; et al. Development and Validation of the Phoenix Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024, 331, 675–686. [Google Scholar] [CrossRef]
- Jabornisky, R.; Kuppermann, N.; González-Dambrauskas, S. Transitioning from SIRS to Phoenix with the updated pediatric sepsis criteria—the difficult task to simplyfing the complex. JAMA 2024, 331, 650–651. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Calandra, T.; Soares, M.P.; Bauer, M.; Wiersinga, W.J.; Prescott, H.C.; Knight, J.C.; Baillie, K.J.; Bos, L.D.J.; Derde, L.P.G.; et al. Reframing sepsis immunobiology for translation: Towards informative subtyping and targeted immunomodulatory therapies. Lancet Respir. Med. 2024, 12, 323–336. [Google Scholar] [CrossRef]
- Greenfield, K.G.; Badovinac, V.P.; Griffith, T.S.; Knoop, K.A. Sepsis, Cytokine Storms, and Immunopathology: The Divide between Neonates and Adults. Immunohorizons 2021, 5, 512–522. [Google Scholar] [CrossRef]
- Zhang, X.; Zhivaki, D.; Lo-Man, R. Unique aspects of the perinatal immune system. Nat. Rev. Immunol. 2017, 17, 495–507. [Google Scholar] [CrossRef]
- Wynn, J.L.; Guthrie, S.O.; Wong, H.R.; Lahni, P.; Ungaro, R.; Lopez, M.C.; Baker, H.V.; Moldawer, L.L. Postnatal Age Is a Critical Determinant of the Neonatal Host Response to Sepsis. Mol. Med. 2015, 21, 496–504. [Google Scholar] [CrossRef]
- Khaertynov, K.S.; Boichuk, S.V.; Khaiboullina, S.F.; Anokhin, V.A.; Andreeva, A.A.; Lombardi, V.C.; Satrutdinov, M.A.; Agafonova, E.A.; Rizvanov, A.A. Comparative Assessment of Cytokine Pattern in Early and Late Onset of Neonatal Sepsis. J. Immunol. Res. 2017, 2017, 8601063. [Google Scholar] [CrossRef]
- Carter, M.J.; Carrol, E.D.; Ranjit, S.; Mozun, R.; Kissoon, N.; Watson, R.S.; Schlapbach, L.J. Susceptibility to childhood sepsis, contemporary management, and future directions. Lancet Child Adolesc. Health 2024, 8, 682–694. [Google Scholar] [CrossRef]
- Mandel, J.; Casari, M.; Stepanyan, M.; Martyanov, A.; Deppermann, C. Beyond homeostasis: Platelet innate immune interactions and thrombo-inflammation. Int. J. Mol. Sci. 2022, 23, 3868. [Google Scholar] [CrossRef]
- Meier, A.; Sakoulas, G.; Nizet, V.; Ulloa, E.R. Neutrophil extracellular traps: An emerging therapeutic target to improve infectious disease outcomes. J. Infect. Dis. 2024, 230, 514–521. [Google Scholar] [CrossRef]
- Colon, D.F.; Wanderley, C.W.; Franchin, M.; Silva, C.M.; Hiroki, C.H.; Castanheira, F.V.S.; Donate, P.B.; Lopes, A.H.; Volpon, L.C.; Kavaguti, S.K.; et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care 2019, 23, 113. [Google Scholar] [CrossRef]
- Mithal, L.B.; Arshad, M.; Swigart, L.R.; Khanolkar, A.; Ahmed, A.; Coates, B.M. Mechanisms and Modulation of Sepsis-Induced Immune Dysfunction in Children. Pediatr. Res. 2022, 91, 447–453. [Google Scholar] [CrossRef]
- Barsness, K.A.; Bensard, D.D.; Partrick, D.A.; Calkins, C.M.; Hendrickson, R.J.; Banerjee, A.; McIntyre, R.C., Jr. IL-1beta induces an exaggerated pro- and anti-inflammatory response in peritoneal macrophages of children compared with adults. Pediatr. Surg. Int. 2004, 20, 238–242. [Google Scholar] [CrossRef]
- Barsness, K.A.; Bensard, D.D.; Partrick, D.A.; Calkins, C.M.; Hendrickson, R.J.; McIntyre, R.C., Jr. Endotoxin Induces an Exaggerated Interleukin-10 Response in Peritoneal Macrophages of Children Compared with Adults. J. Pediatr. Surg. 2004, 39, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Muszynski, J.A.; Nofziger, R. Early immune function and duration of organ dysfunction in critically ill children with sepsis. Am. J. Respir. Crit. Care Med. 2018, 198, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Remy, S.; Koley-Descamps, K. Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: A pilot study. Ann. Intensive Care 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.; Ray, S.; Wilson, C.; Remy, S.; Benissa, M.R.; Jansen, N.J.G.; Javouhey, E.; Peters, M.J.; Kneyber, M.; De Luca, D.; et al. A European Society of Paediatric and Neonatal Intensive Care Definition. Intensive Care Med. 2016, 42, 1948–1957. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; MacLaren, G.; Festa, M.; Alexander, J.; Erickson, S.; Beca, J.; Slater, A.; Schibler, A.; Pilcher, D.; Millar, J.; et al. Prediction of Pediatric Sepsis Mortality Within 1 Hour of Intensive Care Admission. Intensive Care Med. 2017, 43, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Balamuth, F.; Hensley, J.; Fitzgerald, J.C.; Bush, J.; Nadkarni, V.M.; Thomas, N.J.; Hall, M.; Muszynski, J. The Epidemiology of Hospital Death Following Pediatric Severe Sepsis: When, Why, and How Children with Sepsis Die. Pediatr. Crit. Care Med. 2017, 18, 823–830. [Google Scholar] [CrossRef]
- Cvetkovic, M.; Lutman, D.; Ramnarayan, P.; Pathan, N.; Inwald, D.P.; Peters, M.J. Timing of death in children referred for intensive care with severe sepsis: Implications for interventional studies. Pediatr. Crit. Care Med. 2015, 16, 410–417. [Google Scholar] [CrossRef]
- Hanna, W.; Wong, H.R. Pediatric sepsis: Challenges and adjunctive therapies. Crit. Care Clin. 2013, 29, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Lukaszewicz, A.C.; Payen, D.; Hotchkiss, R.; Monneret, G. Monitoring the immune response in sepsis: A rational approach to administration of immunoadjuvant therapies. Curr. Opin. Immunol. 2013, 25, 477–483. [Google Scholar] [CrossRef]
- Puchwein-Schwepcke, A.; Genzel-Boroviczeny, O.; Nussbaum, C. The endothelial glycocalyx: Physiology and pathology in neonates, infants and children. Front. Cell Dev. Biol. 2021, 9, 733557. [Google Scholar] [CrossRef]
- Fernandez-Sarmiento, J.; Hernandez-Sarmiento, R.; Salazar, M.P.; Barrera, S.; Castilla, V.; Duque, C. The association between hypoalbuminemia and microcirculation, endothelium, and glycocalyx disorders in children with sepsis. Microcirculation 2023, 30, e12829. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, C.; Wang, Y.; Liu, T.; Yu, Z.; Wang, K.; Chen, W.; Zhou, Y.; Lu, G. Sulodexide improves vascular permeability via glycocalyx remodeling in endothelial cells during sepsis. Front. Immunol. 2023, 14, 1172892. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Willmann, K.; Moita, L.F. Physiologic disruption and metabolic reporgramming in infection and sepsis. Cell Metab. 2024, 36, 927–946. [Google Scholar] [CrossRef] [PubMed]
- Burgunder, L.; Heyrend, C.; Olson, J.; Stidham, C.; Lane, R.D.; Workman, J.K.; Larsen, G.Y. Medication and fluid management of pediatric sepsis and septic shock. Paediatr. Drugs 2022, 24, 193–205. [Google Scholar] [CrossRef]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020, 46 (Suppl. S1), 10–67. [Google Scholar] [CrossRef]
- Ohlsson, A.; Lacy, J.B. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst. Rev. 2020, 1, CD001239. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Dang, H.; Liu, C.; Huo, J.; Fu, Y.Q. Effect of intravenous immunoglobulin on the outcome of children with septic shock in a PICU: A retrospective cohort study. Eur. J. Pediatr. 2023, 182, 5315–5323. [Google Scholar] [CrossRef]
- Berlot, G.; Vassallo, M.C.; Busetto, N.; Nieto Yabar, M.; Istrati, T.; Baronio, S.; Quarantotto, G.; Bixio, M.; Barbati, G.; Dattola, R.; et al. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann. Intensive Care 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Cavazzuti, I.; Serafini, G.; Busani, S.; Rinaldi, L.; Biagioni, E.; Buoncristiano, M.; Girardis, M. Early therapy with IgM-enriched polyclonal immunoglobulin in patients with septic shock. Intensive Care Med. 2014, 40, 1888–1896. [Google Scholar] [CrossRef]
- Kakoullis, L.; Pantzaris, N.-D.; Platanaki, C.; Lagadinou, M.; Papachristodoulou, E.; Velissaris, D. The use of IgM-enriched immunoglobulin in adult patients with sepsis. J. Crit. Care 2018, 47, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wei, X.; Lv, H.; Li, Y.; Li, P.; Chen, Z.; Liu, G. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: A meta-analysis with trial sequential analysis. Ann. Intensive Care 2019, 9, 27. [Google Scholar] [CrossRef]
- Pan, B.; Sun, P.; Pei, R.; Lin, F.; Cao, H. Efficacy of IVIG therapy for patients with sepsis: A systematic review and meta-analysis. J. Transl. Med 2023, 21, 765. [Google Scholar] [CrossRef] [PubMed]
- El-Nawawy, A.; El-Kinany, H.; Hamdy El-Sayed, M.; Boshra, N. Intravenous polyclonal immunoglobulin administration to sepsis syndrome patients: A prospective study in a pediatric intensive care unit. J. Trop. Pediatr. 2005, 51, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Kilic, O.; Bozan, G.; Kiral, E.; Iseri Nepesov, M.; Dinleyici, E.C. Clinical, laboratory features and prognosis of children receiving IgM-enriched immunoglobulin (3 days vs. 5 days) as adjuvant treatment for serious infectious disease in pediatric intensive care unit: A retrospective single-center experience (PIGMENT study). Hum. Vaccines Immunother. 2020, 16, 1997–2002. [Google Scholar]
- Atchade, E.; De Tymowski, C.; Grall, N.; Tanaka, S.; Montravers, P. Toxic Shock Syndrome: A Literature Review. Antibiotics 2024, 13, 96. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Steer, A.C.; Smeesters, P.R.; Curtis, N. Toxic shock syndrome—the seven Rs of management and treatment. J. Infect. 2017, 74 (Suppl. S1), S147–S152. [Google Scholar] [CrossRef]
- Parks, T.; Wilson, C.; Curtis, N.; Norrby-Teglund, A.; Sriskandan, S. Polyspecific Intravenous Immunoglobulin in Clindamycin-treated Patients with Streptococcal Toxic Shock Syndrome: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2018, 67, 1434–1436. [Google Scholar] [CrossRef]
- Adalat, S.; Dawson, T.; Hackett, S.J.; Clark, J.E. Toxic shock syndrome surveillance in UK children. Arch. Dis. Child. 2014, 99, 1078–1082. [Google Scholar] [CrossRef]
- Chen, K.Y.H.; Cheung, M.; Burgner, D.P.; Curtis, N. Toxic shock syndrome in Australian children. Arch. Dis. Child. 2016, 101, 736–740. [Google Scholar] [CrossRef]
- Shah, S.S.; Hall, M.; Srivastava, R.; Subramony, A.; Levin, J.E. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin. Infect. Dis. 2009, 49, 1369–1376. [Google Scholar] [CrossRef]
- Annane, D.; Sébille, V. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002, 288, 862–871, Erratum in JAMA 2008, 300, 1652. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Valoor, H.T.; Singhi, S. Low-dose hydrocortisone in pediatric septic shock: An exploratory study in a third world setting. Pediatr. Crit. Care Med. 2009, 10, 121–125. [Google Scholar] [CrossRef] [PubMed]
- El-Nawawy, A.; Khater, D. Evaluation of early corticosteroid therapy in management of pediatric septic shock in pediatric intensive care patients: A randomized clinical study. Pediatr. Infect Dis. J. 2017, 36, 155–159. [Google Scholar] [CrossRef]
- Menon, K.; McNally, D.; O’Hearn, K.; Acharya, A.; Wong, H.R.; Lawson, M.; Ramsay, T.; McIntyre, L.; Gilfoyle, E.; Tucci, M.; et al. A randomized controlled trial of corticosteroids in pediatric septic shock: A pilot feasibility study. Pediatr. Crit. Care Med. 2017, 18, 505–512. [Google Scholar]
- Alkhalaf, H.A.; Alhamied, N.A. The Association of Corticosteroid Therapy With Mortality and Length of Stay Among Children With Septic Shock: A Retrospective Cohort Study. Cureus 2023, 15, e33267. [Google Scholar] [CrossRef] [PubMed]
- Alder, M.N.; Opoka, A.M.; Wong, H.R. The glucocorticoid receptor and cortisol levels in pediatric septic shock. Crit. Care 2018, 22, 244. [Google Scholar] [CrossRef]
- Wong, H.R.; Cvijanovich, N.Z.; Anas, N.; Allen, G.L.; Thomas, N.J.; Bigham, M.T.; Weiss, S.L.; Fitzgerald, J.; Checchia, P.A.; Meyer, K.; et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am. J. Respir. Crit. Care Med. 2015, 191, 309–315. [Google Scholar] [PubMed]
- Wong, H.R.; Cvijanovich, N.Z.; Anas, N.; Allen, G.L.; Thomas, N.J.; Bigham, M.T.; Weiss, S.L.; Fitzgerald, J.C.; Checchia, P.A.; Meyer, K.; et al. Endotype transitions during the acute phase of pediatric septic shock reflect changing risk and treatment response. Crit. Care Med. 2018, 46, e242–e249. [Google Scholar]
- Fisher, C.J.; Dhainaut, J.F.A. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 1994, 271, 1836–1843. [Google Scholar] [CrossRef]
- Shakoory, B.; Carcillo, J.A. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar] [CrossRef]
- Opal, S.M.; Fisher, C.J., Jr. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 1997, 25, 1115–1124. [Google Scholar] [CrossRef]
- Manchikalapati, R.; Schening, J. Clinical utility of interleukin-1 inhibitors in pediatric sepsis. Shock. 2024, 61, 340–345. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Kruse, K.; Kovey, K. Therapeutic role of anakinra, an interleukin-1 recptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr. Crit. Care Med. 2014, 15, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Greenberg, J.; Basu, S. Outcomes analysis of children diagnosed with hemophagocytic lymphohistiocytosis in the PICU. Pediatr. Crit. Care Med. 2019, 20, e185–e190. [Google Scholar] [CrossRef]
- Eloseily, E.M.; Weiser, P.; Crayne, C.B. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020, 72, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, J.E.; Wilson, S.; Qureshi, A.; Blanco, E.; Mitchell, A.; Segal, S.; Kelly, D.; Weitz, J.; O’Shea, D.; Bailey, K.; et al. Continuous intravenous anakinra for treating severe secondary haemophagocytic lymphohistiocytosis/macrophage activation syndrome in critically ill children. Pediatr. Blood Cancer 2021, 68, e29102. [Google Scholar] [CrossRef] [PubMed]
- Kotsaki, A.; Pickkers, P.; Bauer, M.; Calandra, T.; Wiersing, W.J.; Meylan, S.; Bloos, F.; van der Poll, T.; Slim, M.A.; van Mpurik, N.; et al. ImmunoSep (Personalised Immunotherapy in Sepsis) international double-blind, double-dummy, placebo-controlled randomised clinical trial: Study protocol. BMJ Open 2022, 12, e067251. [Google Scholar] [CrossRef]
- Mark, H. Targeted Reversal of Inflammation in Pediatric Sepsis-Induced MPDS (TRIPS). NCT05267821. Available online: https://clinicaltrials.gov/study/NCT05267821 (accessed on 13 April 2025).
- Giamarellos-Bourboulis, E.J. Adjunctive treatment in COVID-19 and sepsis—What did we learn? Med. Klin. Intensivmed. Notfallmed. 2023, 118 (Suppl. S2), 80–85. [Google Scholar] [CrossRef]
- Ghosn, L.; Assi, R.; Evrenoglou, T. Interleukin-6 blocking agents for treating COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2023, 6, CD013881. [Google Scholar]
- Wang, B.; Wang, Q. Tocilizumab, an IL6-receptor antibody, proved effective as adjuvant therapy for cytokine storm induced by severe infection in patients with hematologic malignancy. Ann. Hematol. 2023, 102, 961–966. [Google Scholar] [CrossRef]
- Tomulic Brusich, K.; Juricic, K.; Bobinac, M.; Milosevic, M.; Protic, A.; Boban, A. Administration of tocilizumab in septic patients with pancytopenia and hyper-inflammatory syndrome. Ann. Hematol. 2023, 102, 2633–2634. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Sun, S. Efficacy and safety of baricitinib in patients with severe COVID-19: A systematic review and meta-analysis. Medicine 2023, 102, e36313. [Google Scholar] [CrossRef]
- Venet, F.; Rimmelé, T.; Monneret, G. Management of Sepsis-Induced Immunosuppression. Crit. Care Clin. 2018, 34, 97–106. [Google Scholar] [CrossRef]
- Hall, M.W.; Carcillo, J.A.; Cornell, T. Pediatric organ dysfunction information update mandte (PODIUM) collaborative. Pediatrics 2022, 149, S91–S98. [Google Scholar] [CrossRef] [PubMed]
- Bo, L. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: A meta-analysis. Crit. Care 2011, 15, 1–12. [Google Scholar] [CrossRef]
- Hall, M.W.; Greathouse, K.C. Immunoparalysis in Pediatric Critical Care. Pediatr. Clin. N. Am. 2017, 64, 1089–1102. [Google Scholar] [CrossRef]
- Lee, A.O.C.J.; Chua, A.H.Y. Immunomodulator use in paediatric severe sepsis and septic shock. Ann. Acad. Med. Singap. 2021, 50, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Drossou-Agakidou, V.; Kanakoudi-Tsakalidou, F. In vivo effect of rhGM-CSF And rhG-CSF on monocyte HLA-DR expression of septic neonates. Cytokine 2002, 18, 260–265. [Google Scholar] [CrossRef]
- Bilgin, K.; Yaramis, A.; Haspolat, K.; Tas, A.; Gunbey, S.; Derman, O. A randomized trial of granulocyte-macrophage colony-stimulating factor in neonates with sepsis and neutropenia. Pediatrics 2001, 107, 37–41. [Google Scholar]
- Fang, H.; Jiang, W. Balancing Innate Immunity and Inflammatory State via Modulation of Neutrophil Function: A Novel Strategy to Fight Sepsis. J. Immunol. Res. 2015, 2015, 187048. [Google Scholar] [CrossRef]
- Carr, R.; Modi, N. Dorè CJ. G-CFS and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst. Rev. 2003, 2003, CD003066. [Google Scholar]
- Venet, F.; Demaret, J. IL-7 Restores T Lymphocyte Immunometabolic Failure in Septic Shock Patients through mTOR Activation. J. Immunol. 2017, 199, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Francois, B.; Jeannet, R. Interleukin-7 restores lymphocytes in septic shock: The IRIS-7 randomized clinical trial. JCI Insight 2018, 3, e98960. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.K.; Bohannon, J.K. A promising approach to reverse sepsis-induced immunosuppression. Pharmacol. Res. 2016, 111, 688–702. [Google Scholar] [CrossRef]
- Döcke, W.D.; Randow, F. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat. Med 1997, 3, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Kox, M. Reversal of immunoparalysis in humans in vivo: A double-blind, placebo-controlled, randomized pilot study. Am. J. Respir. Crit. Care Med. 2012, 186, 838–845. [Google Scholar] [CrossRef]
- Payen, D.; Faivre, V. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. BMC Infect Dis. 2019, 19, 931. [Google Scholar] [CrossRef]
- Tissières, P.; Ochoda, A. Innate immune deficiency of extremely premature neonates can be reversed by interferon-γ. PLoS ONE 2012, 7, e32863. [Google Scholar] [CrossRef]
- Nakamori, Y.; Park, E.J. Immune Deregulation in Sepsis and Septic Shock: Reversing Immune Paralysis by Targeting PD-1/PD-L1 Pathway. Front. Immunol 2021, 11, 624279. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Colston, E. Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559). Crit. Care Med. 2019, 47, 632–642. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Colston, E. Immune checkpoint inhibition in sepsis: A Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med. 2019, 45, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Svabek, C. Targeting the programmed cell death 1: Programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit. Care 2014, 18, 1–15. [Google Scholar] [CrossRef]
- Qu, G.; Liu, H. GPX4 is a key ferroptosis biomarker and correlated with immune cell populations and immune checkpoints in childhood sepsis. Sci. Rep. 2023, 13, 11358. [Google Scholar] [CrossRef]
- Ronco, C.; Tetta, C. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: The peak concentration hypothesis. Artif. Organs 2003, 27, 792–801. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R. Acute renal failure and multiple organ dysfunction in the ICU: From renal replacement therapy (RRT) to multiple organ support therapy (MOST). Int. J. Artif. Organs 2002, 25, 733–747. [Google Scholar] [CrossRef]
- Ricci, Z.; Romagnoli, S. From Continuous Renal Replacement Therapies to Multiple Organ Support Therapy. Contrib. Nephrol. 2018, 194, 155–169. [Google Scholar] [PubMed]
- Ranieri, V.M.; Brodie, D. Extracorporeal Organ Support: From Technological Tool to Clinical Strategy Supporting Severe Organ Failure. JAMA 2017, 318, 1105–1106. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; Ricci, Z. Extracorporeal organ support (ECOS) in critical illness and acute kidney injury: From native to artificial organ crosstalk. Intensive Care Med. 2018, 44, 1447–1459. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Colardyn, F.A. Cytokine removal during continuous hemofiltration in septic patients. J. Am. Soc. Nephrol. 1999, 10, 846–853. [Google Scholar] [CrossRef]
- Morin, L.; Charbel, R. Blood Purification with oXiris© in Critically Ill Children with Vasoplegic Shock. Blood Purif. 2023, 52, 541–548. [Google Scholar] [CrossRef]
- Shimiz, T.; Miyake, T. History and current status of polymyxin B-immobilized fiber column for treatment of severe sepsis and septic shock. Ann. Gastroenterol. Surg. 2017, 1, 105–113. [Google Scholar] [CrossRef]
- Cruz, D.N.; Antonelli, M. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 2009, 301, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Payen, D.M.; Guilhot, J. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized control trial. Intensive Care Med. 2015, 41, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Coudroy, R.; Payen, D.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Collange, O.; Dewitte, A.; Martin-Lefevre, L.; Jabaundon, M.; et al. Modulation by Polymyxin-B Hemoperfusion of Inflammatory Response Related to Severe Peritonitis. Shock 2017, 47, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Payen, D.; Dupuis, C.; Deckert, V.; Pais de Barros, J.P.; Rerole, A.L.; Lukaszewicz, A.C.; Coudroy, R.; Robert, R.; Lagros, L.; for the ABDOMINIX group. Endotoxin mass concentration in plasma is associated with mortality in a multicentric cohort of peritonitis-induced shock. Front. Med. 2021, 8, 7494405. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Bagshaw, S.M. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients with Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA 2018, 320, 1455–1463. [Google Scholar] [CrossRef]
- Antonelli, M.; Cutuli, S.L. Polymixin B hemoperfusion in septic shock: Just look at the evidence! Intensive Care Med. 2015, 41, 1731–1732. [Google Scholar] [CrossRef]
- Klein, D.J.; Foster, D. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: A post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018, 44, 2205–2212. [Google Scholar] [CrossRef]
- Nishizaki, N.; Nagagawa, M. Effect of PMX-DHP for sepsis due to ESBL-producing E.coli in an extremely birth-weight infant. Pediatr. Int. 2016, 58, 411–414. [Google Scholar] [CrossRef]
- Tokumasu, H.; Watabe, S. Effect of hemodiafilatration therapy in a low birthweight infants with congenital sepsis. Pediatr. Int. 2016, 58, 237–240. [Google Scholar] [CrossRef]
- Morishita, J.; Kita, Y. Successful treatment of sepsis with polymiyxin b-immobilized fiber hemoperfusion in a child after living donor liver transplantation. Dig. Dis. Sci. 2005, 50, 757. [Google Scholar] [CrossRef] [PubMed]
- Nanishi, E.; Hirata, Y. Polymyxin B immobilized column-direct hemoperfusion for adolescent toxic shock syndrome. Pediatr. Int. 2016, 58, 1051–1086. [Google Scholar] [CrossRef] [PubMed]
- Yaroustovsky, M.; Abramyan, M. Selective polymyxin hemoperfusion in complex therapy of sepsis in children after cardiac surgery. Blood Purif. 2021, 50, 222–229. [Google Scholar] [CrossRef]
- Saetang, P.; Samransamruajkit, R. Polymyxin B hemoperfusion in pediatric septic shock: Single-center observational case series. Pediatr. Crit. Care Med. 2022, 23, e386–e391. [Google Scholar] [CrossRef]
- Ward, D.M. Conventional apheresis therapies: A review. J. Clin. Apher. 2011, 26, 230–238. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Han, Y.Y. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit. Care Med. 2008, 36, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.C.; Kiss, J.E. The role of plasmapheresis in critical illness. Crit. Care Clin. 2012, 28, 453–468. [Google Scholar] [CrossRef]
- Lee, O.P.E.; Kanesan, N.; Leow, E.H.; Sultana, R.; Chor, Y.K.; Gan, C.S.; Lee, J.H. Survival Benefits of Therapeutic Plasma Exchange in Severe Sepsis and Septic Shock: A Systematic Review and Meta-analysis. J. Intensive Care Med. 2023, 38, 598–611. [Google Scholar] [CrossRef]
- Taniguchi, T. Cytokine Adsorbing Columns. Contrib. Nephrol. 2010, 166, 134–141. [Google Scholar]
- Ankawi, G.; Bagshaw, S.M. Hemoadsorption: Consensus report of the 30th Acute Disease Quality Initiative workgroup. Nephrol. Dial Transpl. 2024, 39, 1945–1964. [Google Scholar]
- Poli, E.C.; Rimmele, T. Hemoadsorption with CytoSorb((R)). Intensive Care Med. 2019, 45, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Guzzo, I. Impact of CytoSorb and CKRT on hemodynamics in pediatric patients with septic shock: The PedCyto study. Front. Pediatr. 2023, 11, 1259384. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Cecchetti, C. Potential correlation between hemodynamic improvement and an immune-modulation effect in pediatric patients with septic shock: An insight from the PedCyto study. Crit. Care 2024, 28, 25. [Google Scholar] [CrossRef] [PubMed]
- Siripanadorn, T.; Samransamruajkit, R. The Role of Blood Purification by HA330 as Adjunctive Treatment in Children with Septic Shock. Blood Purif. 2023, 52, 549–555. [Google Scholar] [CrossRef]
- Sazonov, V.; Abylkassov, R. Case series: Efficacy and safety of he- moadsorption with HA-330 adsorber in septic pediatric patients with cancer. Front. Pediatr. 2021, 9, 672260. [Google Scholar] [CrossRef]
- Duchini, P.P.; Bottari, G. Hemadsorption in Critically Ill Children. Contrib. Nephrol. 2023, 200, 242–251. [Google Scholar] [CrossRef]
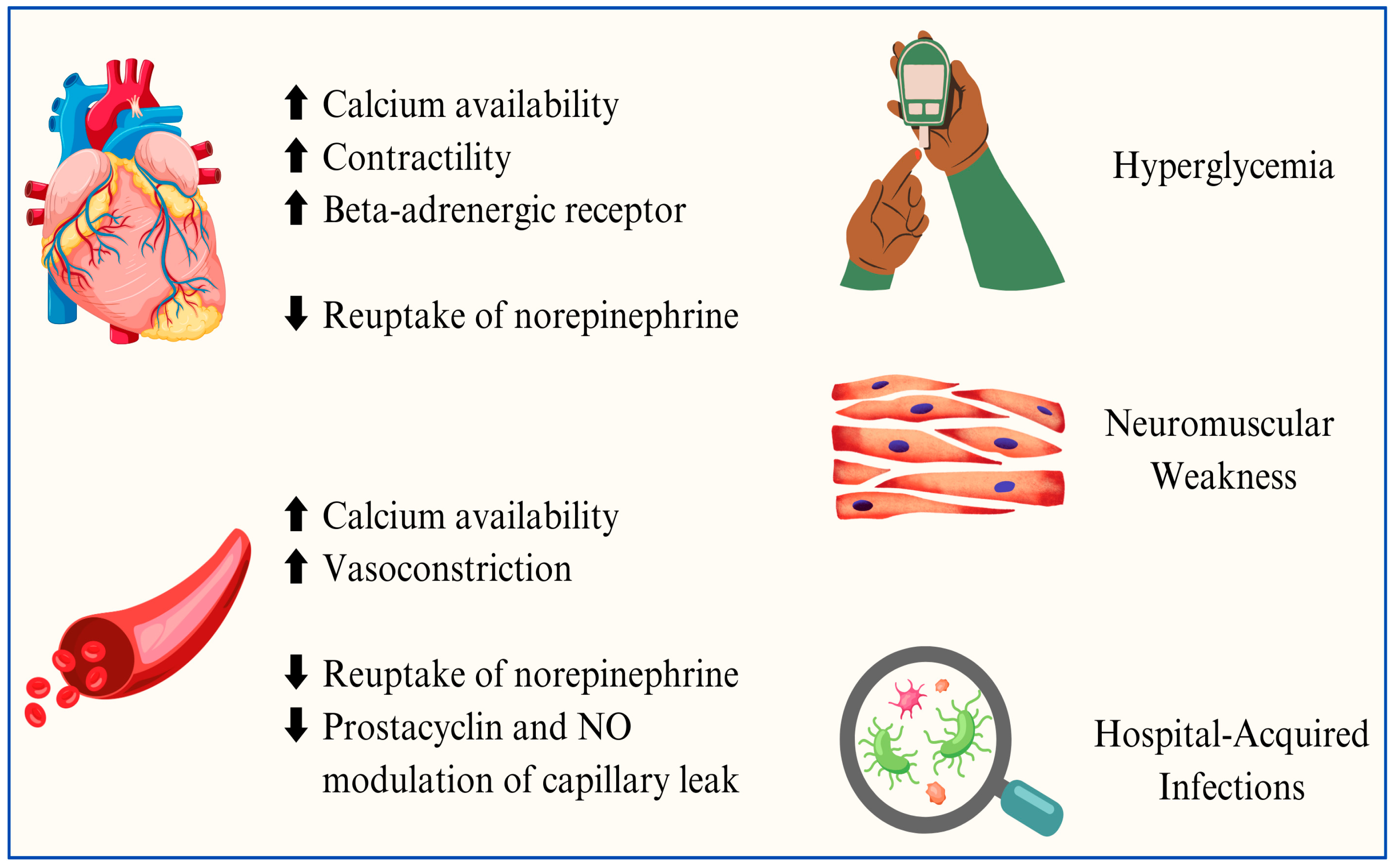
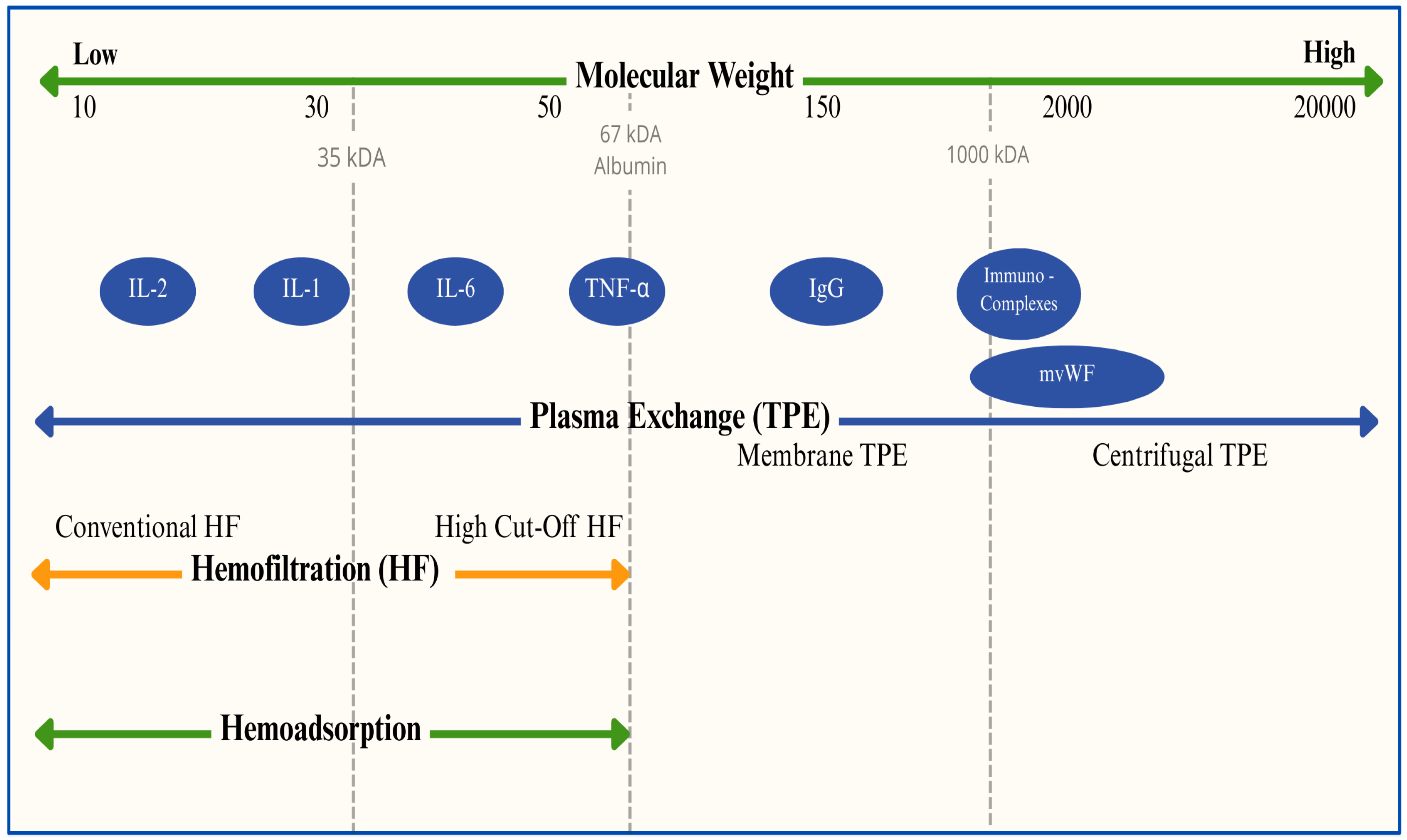
| IVIG | ||||||
|---|---|---|---|---|---|---|
| Reference | No. of Patients | Study Design | Study Period | Age | Outcomes | Mortality |
| Huang et al. (2023) [36] | 304 | Retrospective cohort study | 1 January 2017–31 December 2021 | 7–144 months | Primary: in-hospital mortality Secondary: PICU duration of stay, length of hospital stay, requirement for MV and CRRT | No-IVIG group: 112 (52%); IVIG group: 38 (43%) |
| IgM-enriched IVIG | ||||||
| Pan et al. (2023) [41] | 6276 | Systematic review and meta-analysis | Studies published up to 31 January 2023 | Neonates and adults | Primary: mortality at end of follow-up period Secondary: length of hospital stay | Inconclusive regarding effect of IVIG in reducing mortality among neonates (RR: 0.93; 95% CI 0.81–1.05); IgM-rich IVIG showed a positive effect in the treatment of neonatal sepsis (RR 0.45; 95% CI: 0.25–0.80) |
| El-Nawaway et al. (2005) [42] | 100 | Prospective study | 2022 | 1–24 months | To study differences between control group (standard treatment) and case group receiving polyclonal IVIG in addition | Controls had a smaller percentage of mortality at 14 (28%) vs. the control group at 28 (56%) |
| Abdullayev et al., PIGMENT study (2002) [43] | 254 | Retrospective study | January 2010–December 2017 | 1 month–18 years old | To evaluate clinical features and prognoses of children receiving IgM-enriched IVIG | Mortality rate was 28.7%; in particular, it was 40.3% (#42) for the 3-day treatment group and 20.6% (#31) for the 5-day treatment group (OR: 0.51; 95% CI 0.34–0.75) |
| Corticosteroids | ||||||
|---|---|---|---|---|---|---|
| Reference | No. of Patients | Study Design | Study Period | Age | Mortality N (%) | Dose and Type of Corticosteroids |
| Valoor et al. (2009) [52] | 38 | Open-label randomized pilot study | Subjects were enrolled within 30 min of the time that fluid refractory shock was diagnosed, and the time for shock reversal was calculated. | 2 months–12 years | Control group: 7 (37%). Placebo group: 6 (32%). | Control group: intravenous hydrocortisone 5 mg/kg/day in four divided doses, followed by half the dose for a total duration of 7 days |
| El-Nawawy et al. (2017) [53] | 96 | Prospective interventional randomized clinical trial | 30 day follow-up | 1 month–4 years | Group C: deceased (30-day mortality) 14 (43.75%). Group D: deceased (30-day mortality) 20 (55.55%) | Group C: intravenous hydrocortisone 50 mg/m2/24 h with continuous infusion for 5 days from admission and weaning of the drug over 5 days Group D: corticosteroids in the third stage of therapy |
| Kusum Menon et al. (2017) [54] | 101 | Randomized, double-blind, placebo-controlled, multicentric trial | Screening period: July 2014–March 2016. The total number of recruitment months was 90 across all study sites, with the site-specific recruitment period ranging from 2 to 20 months. | Children from newborn to 17 years old inclusive | Placebo group: 3 (6%). Control group: 1 (2%). p = 0.61. | Control group: an initial intravenous bolus of 2 mg/kg hydrocortisone, followed by 1 mg/kg of hydrocortisone every 6 h until the patient met stability criteria for at least 12 h. Hydrocortisone dosing was then reduced to 1 mg/kg every 8 h until all vasoactive infusions had been discontinued for at least 12 h for a maximum of 7 days. |
| Alkhalaf H.A. et al. (2023) [55] | 182 | Retrospective cohort study | Study period: January 2016–December 2021 | <14 years old | After adjusting for baseline characteristics, severity scores, and medical intervention, no statistical association was found between corticosteroid use and mortality (HR: 2.61; 95% CI 0.66–10.28). | Steroid regimen not specified |
| Alder et al. (2018) [56] | 164 | Prospective cohort study | 28 days follow-up | <18 years old | Mortality, n (%): SIRS: 2 (12); sepsis: 0 (0); septic shock: 6 (8) | No steroid administration |
| Wong H.R. et al. (2015) [57] | Study subjects (n = 168) Separate cohort (n = 132) | Development and validation study, prospective cohort study (for the validation and outcome analysis phase) | 28 days follow-up | 0.2–7.3 years old | Derivation Cohort: Subclass A: 12 (21); Subclass B: 11 (10). Test Cohort: Subclass A: 11 (17); Subclass B: 4 (5). Adjunctive corticosteroids increased risk of mortality in subclass A (OR = 4.1; p = 0.011), but not in subclass B. | Steroid regimen not specified |
| Wong H.R. et al. (2018) [58] | 375 | Observational cohort study | 28 days follow-up | ≤10 years | 28-day mortality, n (%): Endotype AA: 12 (16); Endotype AB: 10 (18); Endotype BB: 8 (5); Endotype BA: 1 (1). | Steroid regimen not specified |
| rhIL-1ra | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | No. of Patients | Study Design | Study Period | Age | Clinical Presentation | Outcomes | Mortality | Further Results |
| Rajasekaran et al. (2014) [63] | 8 | Retrospective case series | 1 January 2011–31 July 2012 | 8–21 years old | Patients with secondary HLH admitted to PICU | To study the role of anakinra in reducing systemic inflammation | 1 (12.5%) | 5 (62.5%) needed MV; 5 (62.5%) required vasoactive therapy; 1 (12.5%) needed RRT |
| Gregory et al. (2019) [64] | 33 | Retrospective electronic medical record review | 2007–2017 | 27–186 months | Patients with both familial and secondary HLH | To study both in-hospital mortality and 1-year mortality | 7 in-hospital deaths (21%); 1-year mortality was 27%. | 48% received anakinra (42% of survivors and 71% of non-survivors) |
| Eloseily et al. (2020) [65] | 44 | Retrospective review | January 2008–December 2016 | 1–19 years old | Children with secondary HLH | To analyze the role of anakinra in the treatment of secondary HLH | 12 (27%) | Early anakinra administration (<5 days of hospitalization) was associated with a reduction in mortality (p = 0.046). |
| Charlesworth et al. (2021) [66] | 3 | Case series | / | 9, 11, and 17 years old | Severe secondary HLH/MAS | To report 3 cases of critically ill children who received IV anakinra | 0 | The study underlines the safety and efficacy of anakinra in patients with infection. |
| G-CSF and GM-CSF | ||||||
|---|---|---|---|---|---|---|
| Reference | No. of Patients | Study Design | Study Period | Age | Outcomes | Mortality/Results |
| Lee et al. (2021) [78] | 109 | Retrospective review | 1 January 2010–31 October 2017 | Children | PICU mortality, 28-day ventilator-free days (VFD), and intensive care unit-free days (IFD) | PICU mortality was not different between the 2 groups (20/54 [37.0%] vs. 11/55 [20.0%], p = 0.058) |
| Bilgin et al. (2001) [80] | 60 | RCT | January 1994–March 1995 | Neonates | Assessing whether rhGM-CSF could reverse neutropenia and other hematologic parameters in septic neonates and improve neonatal survival, compared to conventional therapy in a control group | All neonates tolerated GM-CSF. Neutrophil numbers increased on day 7 after GM-CSF, compared with the conventionally treated group (8088 ± 2822/mm3 vs. 2757 ± 823/mm3) (p < 0.01). The mean platelet count was significantly higher on day 14 in the GM-CSF-group (266,867 ± 55,102/mm3 vs. 229,200 ± 52,317/mm3) (p < 0.01). Other hematologic parameters were similar between groups on day 28. Twenty-seven neonates in the rh-GMCSF group and 21 in the control group survived. The mortality rate in the rhGM-CSF group (10%) was significantly lower than in the conventionally treated group (30%) (p < 0.05). |
| Drossou-Agakidou et al. (2002) [79] | 60 | RCT | Follow-up during the study | Neonates | Assessing the increase in HLA-DR on monocytes after GM-CSF and G-CSF in septic neonates | On day 0, the HLA-DR expression of the septic neonates was significantly lower than the healthy control values (p < 0.0001, for both parameters). On follow-up (days 1, 3, and 5), a significant increase in HLA-DR expression was observed in all groups of septic neonates. |
| IFN-γ | ||||||
| Payen et al. (2019) [85] | 18 adults, 2 children | Multicenter case series | Three cohorts, collected in different periods | Both adults and children | The following were considered: monocyte expression of HLA-DR, lymphocyte immune-phenotyping, IL-6 and IL-10 plasma levels, bacterial cultures, disease severity, and mortality. | In 15 out of 18 patients, IFN-γ determined an increase in HLA-DR expression from 2666 [IQ 1547; 4991] to 12,451 [IQ 4166; 19,707], while the absolute number of lymphocyte subpopulations was not affected. Plasma levels of IL-6 (from 464 [201–770] to 108 [89–140] ng/mL (p = 0.04)) and IL-10 (from 29 [12–59] to 9 [1–15] pg/mL) decreased significantly. Three patients who received IFN-γ died. The other patients had clinical improvements (bacterial cultures became negative). The 2 pediatric cases improved rapidly, but 1 died due to hemorrhagic complications. |
| Tissières et al. (2012) [86] | 70 neonates, 20 adults | Longitudinal study | Follow-up during the study | Both adults and neonates | Demonstrating that innate immune function is impaired in premature infants (particularly in ELBW). Assessing whether innate immune deficiency in extremely premature infants can be reversed by treatment with IFN-γ. | A 12 h course of ex vivo treatment of whole blood with IFN-γ restored the LPS responsiveness of circulating leukocytes in premature infants to levels measured in control adults (11.2 ± 4.5 ng/mL IL-6 in conditioned supernatants from IFN-γ-treated neonate leukocytes stimulated with LPS vs. 16.7 ± 2.8 in untreated leukocytes from healthy adults stimulated with LPS). In contrast, IL-10 cytokine level was decreased. |
| GPX-4 | ||||||
| Qu et al. (2023) [91] | 283 (from four different datasets) | RCT | Follow-up during the study | Children | Assessing new biomarkers (involved in ferroptosis) in pediatric sepsis | GPX4 was markedly downregulated in sepsis in the training set relative to the control group (p < 0.05). The area under the curve (AUC) of the ROC of GPX4 in diagnosing sepsis was 0.64, with sensitivity and specificity of 0.79 and 0.5, respectively. |
| Subgroup | Biomarkers/Endpoints | Potential Interventions | Knowledge Gaps |
|---|---|---|---|
| Corticosteroids | Glucocorticoid receptor (GCR) | Corticosteroid therapy guided by endotype (A vs. B) | How to identify, at the bedside, children with sepsis who are likely to benefit from corticosteroid treatment, according to Wong’s endotype classification. |
| Sepsis with sHLH traits | Ferritin-soluble urokinase plasminogen receptor (suPAR) | Recombinant human IL-1 receptor antagonist (anakinra) | Role of anakinra in pediatric patients with sepsis and secondary hemophagocytic lymphohistiocytosis (sHLH) traits. |
| Pediatric septic shock | Organ dysfunction scores, mortality | IgM-enriched immunoglobulins | Can IgM-enriched immunoglobulins improve outcomes compared to standard IVIG in pediatric septic shock? |
| Sepsis with immune dysfunction | HLA-DR expression, leukocyte count | Immunostimulatory therapies (e.g., GM-CSF, IL-7), guided by PODIUM immune criteria | How to optimize immunostimulatory therapy in immune-dysregulated pediatric sepsis according to PODIUM-defined criteria. |
| Refractory septic shock | Organ dysfunction score, morbidity | Extracorporeal blood purification techniques | Can early extracorporeal purification reduce mortality, morbidity, and the need for ECMO in children with refractory septic shock? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottari, G.; Taccone, F.S.; Corrias, A.; Irrera, M.; Currao, P.; Salvagno, M.; Cecchetti, C.; Payen, D. Immunomodulation in Pediatric Sepsis: A Narrative Review. J. Clin. Med. 2025, 14, 2983. https://doi.org/10.3390/jcm14092983
Bottari G, Taccone FS, Corrias A, Irrera M, Currao P, Salvagno M, Cecchetti C, Payen D. Immunomodulation in Pediatric Sepsis: A Narrative Review. Journal of Clinical Medicine. 2025; 14(9):2983. https://doi.org/10.3390/jcm14092983
Chicago/Turabian StyleBottari, Gabriella, Fabio Silvio Taccone, Angelica Corrias, Mariangela Irrera, Paolo Currao, Michele Salvagno, Corrado Cecchetti, and Didier Payen. 2025. "Immunomodulation in Pediatric Sepsis: A Narrative Review" Journal of Clinical Medicine 14, no. 9: 2983. https://doi.org/10.3390/jcm14092983
APA StyleBottari, G., Taccone, F. S., Corrias, A., Irrera, M., Currao, P., Salvagno, M., Cecchetti, C., & Payen, D. (2025). Immunomodulation in Pediatric Sepsis: A Narrative Review. Journal of Clinical Medicine, 14(9), 2983. https://doi.org/10.3390/jcm14092983








