Non-Melanoma Skin Cancer: Dermatoscopic Diagnostic Clues in Mexican Individuals Based on Fitzpatrick Skin Phototypes
Abstract
1. Introduction
2. Methodology
2.1. Selection of Patients
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sol, S.; Boncimino, F.; Todorova, K.; Waszyn, S.E.; Mandinova, A. Therapeutic Approaches for Non-Melanoma Skin Cancer: Standard of Care and Emerging Modalities. Int. J. Mol. Sci. 2024, 25, 7056. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Fang, L.; Ni, R.; Zhang, H.; Pan, G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer 2022, 22, 836. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef] [PubMed]
- Roky, A.H.; Islam, M.M.; Ahasan, A.M.F.; Mostaq, S.; Mahmud, Z.; Amin, M.N.; Mahmud, A. Overview of skin cancer types and prevalence rates across continents. Cancer Pathog. Ther. 2024, 3, 89–100. [Google Scholar] [CrossRef]
- Zambrano-Román, M.; Padilla-Gutiérrez, J.R.; Valle, Y.; Muñoz-Valle, J.F.; Valdés-Alvarado, E. Non-Melanoma Skin Cancer: A Genetic Update and Future Perspectives. Cancers 2022, 14, 2371. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, M.; Guida, S.; Ferretta, A.; Pellacani, G.; Porcelli, L.; Azzariti, A.; Guida, G. Behind the Scene: Exploiting MC1R in Skin Cancer Risk and Prevention. Genes 2021, 12, 1093. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Chauhan, D.; Singh, A.K.; Chatterjee, M. Melanin Based Classification of Skin Types and Their Susceptibility to UV-Induced Cancer. In Skin Cancer: Pathogenesis and Diagnosis; Springer: Singapore, 2021; pp. 41–67. [Google Scholar]
- Bolick, N.L.; Huang, L.; Trepanowski, N.; Hartman, R.I. Sun protective behaviors in sun-sensitive individuals: A cross-sectional study examining for ethnic and racial differences. Arch. Dermatol. Res. 2022, 315, 1023–1027. [Google Scholar] [CrossRef]
- Rangel-Villalobos, H.; Muñoz-Valle, J.F.; González-Martín, A.; Gorostiza, A.; Magaña, M.T.; Páez-Riberos, L.A. Genetic admixture, relatedness, and structure patterns among Mexican populations revealed by the Y-chromosome. Am. J. Phys. Anthropol. 2008, 135, 448–461. [Google Scholar] [CrossRef]
- Sławińska, M.; Żółkiewicz, J.; Behera, B.; Ding, D.D.; Lallas, A.; Chauhan, P.; Khare, S.; Enechukwu, N.A.; Akay, B.N.; Ankad, B.S.; et al. Dermoscopy of Inflammatory Dermatoses (Inflammoscopy) in Skin of Color—A Systematic Review by the International Dermoscopy Society “Imaging in Skin of Color” Task Force. Dermatol. Pract. Concept. 2023, 13, e2023297S. [Google Scholar] [CrossRef]
- Behera, B.; Kumari, R.; Thappa, D.M.; Gochhait, D.; Srinivas, B.H.; Ayyanar, P. Dermoscopic features of basal cell carcinoma in skin of color: A retrospective cross-sectional study from Puducherry, South India. Indian J. Dermatol. Venereol. Leprol. 2021, 89, 254–260. [Google Scholar] [CrossRef]
- Wojtowicz, I.; Żychowska, M. Application of Ultraviolet-Enhanced Fluorescence Dermoscopy in Basal Cell Carcinoma. Cancers 2024, 16, 2685. [Google Scholar] [CrossRef]
- Martín, J.M.; Bella-Navarro, R.; Jordá, E. Vascular Patterns in Dermoscopy. Actas Dermo-Sifiliogr. (Engl. Ed.) 2012, 103, 357–375. [Google Scholar] [CrossRef]
- Karampinis, E.; Georgopoulou, K.-E.; Kampra, E.; Zafiriou, E.; Lallas, A.; Lazaridou, E.; Apalla, Z.; Behera, B.; Errichetti, E. Clinical and Dermoscopic Patterns of Basal Cell Carcinoma and Its Mimickers in Skin of Color: A Practical Summary. Medicina 2024, 60, 1386. [Google Scholar] [CrossRef]
- Scope, A.; Bevenuto-Andrade, C.; Agero, A.L.C.; Marghoob, A.A. Nonmelanocytic Lesions Defying the Two-Step Dermoscopy Algorithm. Dermatol. Surg. 2006, 32, 1398–1406. [Google Scholar] [PubMed]
- Farré, X.; Blay, N.; Cortés, B.; Carreras, A.; Iraola-Guzmán, S.; de Cid, R. Skin Phototype and Disease: A Comprehensive Genetic Approach to Pigmentary Traits Pleiotropy Using PRS in the GCAT Cohort. Genes 2023, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Cabaço, L.C.; Tomás, A.; Pojo, M.; Barral, D.C. The Dark Side of Melanin Secretion in Cutaneous Melanoma Aggressiveness. Front. Oncol. 2022, 12, 887366. [Google Scholar] [CrossRef] [PubMed]
- Savoye, I.; Olsen, C.M.; Whiteman, D.C.; Bijon, A.; Wald, L.; Dartois, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Kvaskoff, M. Patterns of Ultraviolet Radiation Exposure and Skin Cancer Risk: The E3N-SunExp Study. J. Epidemiol. 2018, 28, 27–33. [Google Scholar] [CrossRef]
- Paver, E.C.; Ahmed, T.; Burke, H.; Saw, R.P.M.; Stretch, J.R.; Spillane, A.J.; Shannon, K.F.; Vergara, I.A.; Elder, D.E.; Lo, S.N.; et al. Prognostic Significance of Incipient Ulceration in Primary Cutaneous Melanoma. JAMA Dermatol. 2023, 159, 1359–1367. [Google Scholar] [CrossRef]
- Davies, J.; Muralidhar, S.; Randerson-Moor, J.; Harland, M.; O’shea, S.; Diaz, J.; Walker, C.; Nsengimana, J.; Laye, J.; Mell, T.; et al. Ulcerated melanoma: Systems biology evidence of inflammatory imbalance towards pro-tumourigenicity. Pigment Cell Melanoma Res. 2022, 35, 252–267. [Google Scholar] [CrossRef]
- Menzies, S.W.; Westerhoff, K.; Rabinovitz, H.; Kopf, A.W.; McCarthy, W.H.; Katz, B. Surface Microscopy of Pigmented Basal Cell Carcinoma. Arch. Dermatol. 2000, 136, 1012–1016. [Google Scholar] [CrossRef]
- Suppa, M.; Micantonio, T.; Di Stefani, A.; Soyer, H.; Chimenti, S.; Fargnoli, M.; Peris, K. Dermoscopic variability of basal cell carcinoma according to clinical type and anatomic location. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Horimoto, K.; Sato, S.; Minowa, T.; Uhara, H. Dermoscopy of Melanoma and Non-melanoma Skin Cancers. Front. Med. 2019, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Benati, E.; Persechino, F.; Piana, S.; Argenziano, G.; Lallas, A.; Moscarella, E.; Castagnetti, F.; Longo, C. Dermoscopic features of squamous cell carcinoma on the lips. Br. J. Dermatol. 2017, 177, e41–e43. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Filoni, A.; Bonamonte, D.; Giudice, G.; Fanelli, M.; Vestita, M. Vascular Patterns in Cutaneous Ulcerated Basal Cell Carcinoma: A Retrospective Blinded Study Including Dermoscopy. Acta Derm.-Venereol. 2017, 97, 612–616. [Google Scholar] [CrossRef]

| Total % (n = 53) | |
|---|---|
| Patient age (years) | 69.37 ± 14.18 |
| Sex | |
| Female | 45.3 (24) |
| Male | 54.7 (29) |
| Skin phototype | |
| II | 15.1 (8) |
| III | 60.4 (32) |
| IV | 24.5 (13) |
| Sun exposure history | 100 (53) |
| Use of sun protection | 0 |
| Duration of lesion (years) | 2.39 ± 1.57 |
| Symptoms | |
| Asymptomatic | 52.8 (28) |
| Pruritus | 30.2 (16) |
| Pain | 17.0 (9) |
| Morphology type | |
| Nodular | 26.6 (14) |
| Ulcerated nodular | 5.7 (3) |
| Nodular with telangiectasias | 1.9 (1) |
| Papule | 5.7 (3) |
| Pigmented papule | 1.9 (1) |
| Patch shape | 11.3 (6) |
| Warty appearance | 1.9 (1) |
| Exulceration | 41.5 (22) |
| Neoformation | 1.9 (1) |
| Exophytic tumor mass | 1.9 (1) |
| Ulcerated lesions | 32.1 (17) |
| Non-ulcerated lesions | 56.6 (30) |
| Partially ulcerated lesions | 11.3 (6) |
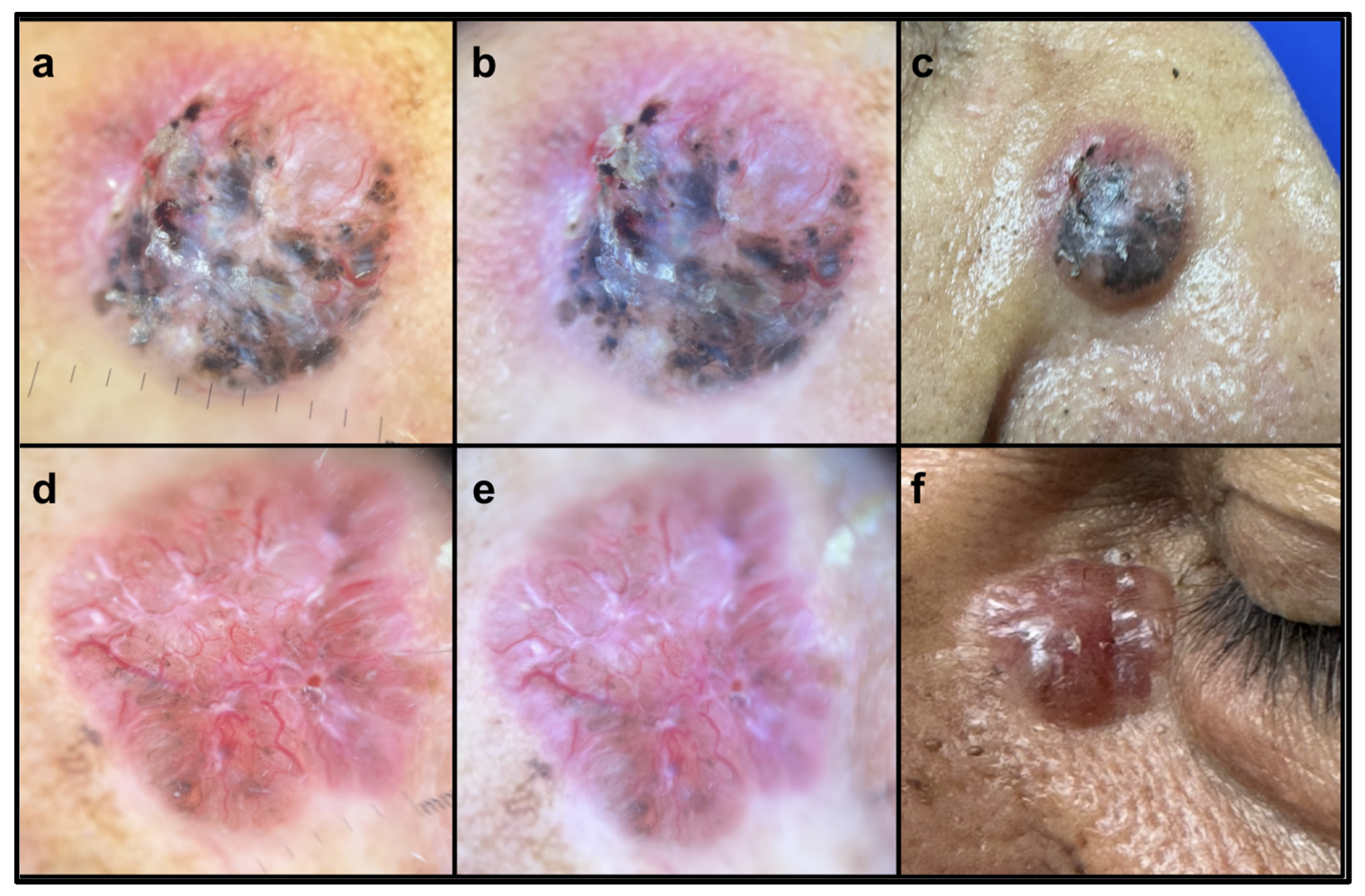
| Pattern Analysis of Lesions Pigmented by Melanin | BCC % (n = 43) | SCC % (n = 9) | Ulcerate % (n = 28) | Non-Ulcerate % (n = 25) | Nodular % (n = 32) | Non-Nodular % (n = 21) |
|---|---|---|---|---|---|---|
| Pigmented lesion | 60.5 (26) * | 0 | 50.0 (14) | 48.0 (12) | 65.6 (21) ** | 23.8 (5) |
| Monomorphic | 30.2 (13) | 55.6 (5) | 28.6 (8) | 40.0 (10) | 31.3 (10) | 38.1 (8) |
| Polymorphic | 67.4 (29) | 44.4 (4) | 71.4 (20) | 56.0 (14) | 65.6 (21) | 61.9 (13) |
| Pigmented areas | 32.6 (14) | 33.3 (3) | 32.1 (9) | 32.0 (8) | 46.9 (15) | 28.6 (6) |
| Clods (includes dots) | 7.0 (3) | 0 | 3.6 (1) | 8.0 (2) | 9.4 (3) | 0 |
| Depigmented areas | 14.0 (6) | 0 | 10.7 (3) | 12.0 (3) | 6.3 (2) | 19.0 (4) |
| White structures | 65.1 (28) | 66.7 (6) | 57.1 (16) | 72.0 (18) | 59.4 (19) | 71.4 (15) |
| Ulcerated Tumors % (n = 28) | Non-Ulcerated Tumors % (n = 25) | pa | pa′ | pb | pb′ | pc | pc′ | pd | pd′ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | BCC % (n = 24) | SCC % (n = 3) | BCC % (n = 19) | SCC % (n = 6) | ||||||||
| Symptoms Associated | ||||||||||||
| Asymptomatic | 58.3 (14) | 33.3 (1) | 57.9 (11) | 33.3 (2) | ||||||||
| Pruritus | 25.0 (6) | 33.3 (1) | 36.8 (7) | 16.7 (1) | ||||||||
| Pain | 16.7 (4) | 33.3 (1) | 5.3 (1) | 50.0 (3) | 0.009 | 0.03 | ||||||
| Edges of the lesion | ||||||||||||
| Well defined | 12.5 (3) | 0 | 52.6 (10) | 16.7 (1) | 0.004 | 0.007 | ||||||
| Irregular | 37.5 (9) | 0 | 15.8 (3) | 16.7 (1) | 0.051 | |||||||
| Poorly defined | 37.5 (9) | 100 (3) | 26.3 (5) | 66.7 (4) | 0.073 | |||||||
| Melanin pigment | ||||||||||||
| Monomorphous | 25.0 (6) | 66.7 (2) | 36.8 (7) | 50.0 (3) | ||||||||
| Polymorphous | 75.0 (18) | 33.3 (1) | 57.9 (11) | 50.0 (3) | ||||||||
| Pigmented areas | 37.5 (9) | 66.7 (2) | 42.1 (8) | 33.3 (2) | ||||||||
| Gray or blue color | 8.3 (2) | 0 | 10.5 (2) | 0 | ||||||||
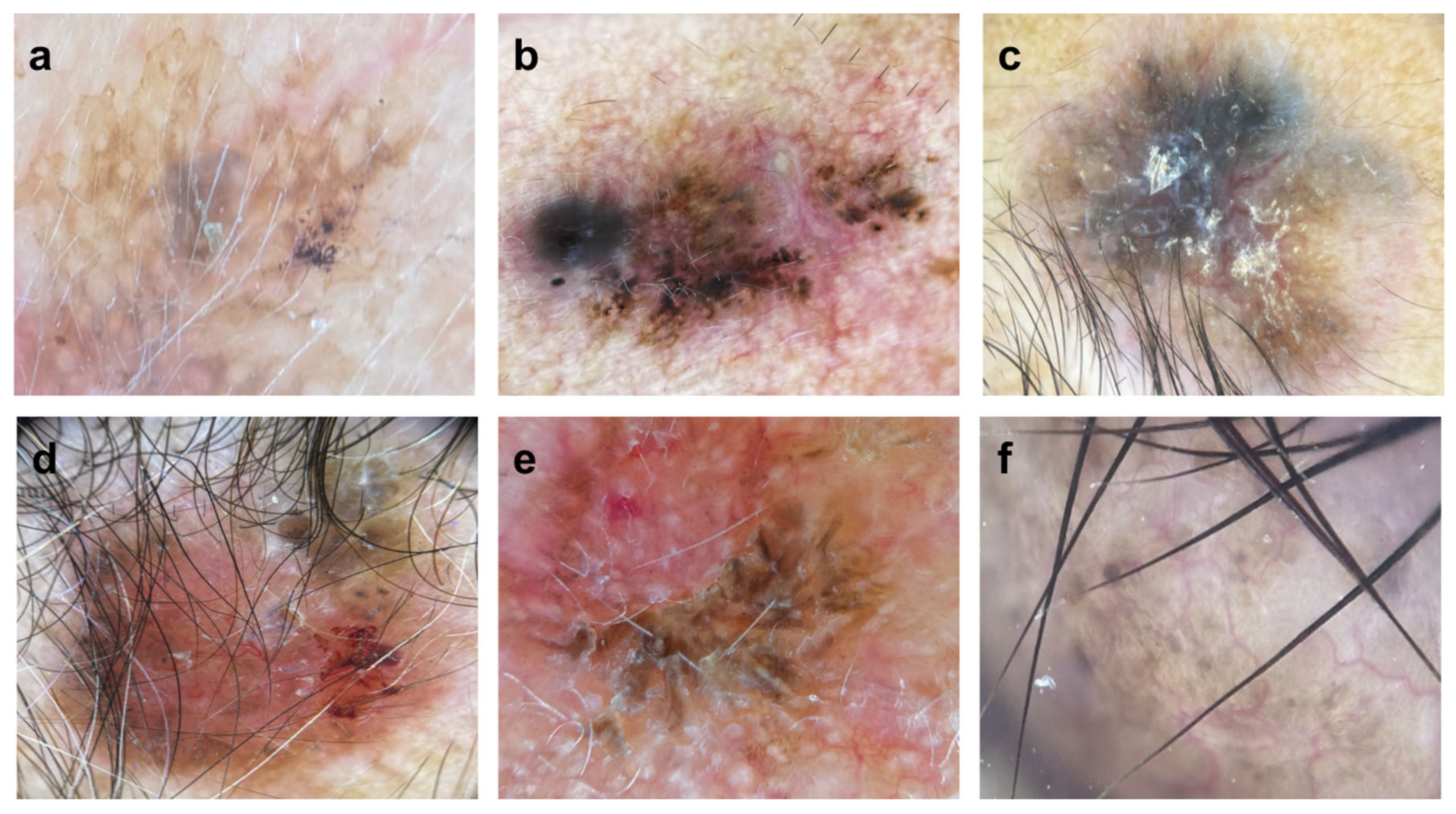
| Dotted pigment | 79.2 (19) | 0 | 42.1 (8) | 83.3 (5) | 0.013 | 0.025 | 0.017 | 0.048 | 0.004 | 0.019 | ||
| Peripheral black lumps | 8.3 (2) | 0 | 26.3 (5) | 16.7 (1) | ||||||||
| Clods (includes dots) | 4.2 (1) | 0 | 10.5 (2) | 0 | ||||||||
| Depigmented areas | 12.5 (3) | 0 | 15.8 (3) | 0 | ||||||||
| Types of lines | ||||||||||||
| Thick reticular lines | 83.3 (20) | 66.7 (2) | 36.8 (7) | 83.3 (5) | 0.002 | 0.004 | 0.047 | 0.07 | ||||
| Segmental radial lines | 25.0 (6) | 33.3 (1) | 15.8 (3) | 16.7 (1) | ||||||||
| white lines | 58.3 (14) | 66.7 (2) | 66.7 (13) | 66.7 (4) | ||||||||
| Angulated lines | 0 | 0 | 0 | 0 | ||||||||
| Lines parallel | 0 | 0 | 0 | 0 | ||||||||
| Melanin pigment distribution | ||||||||||||
| Central | 16.7 (4) | 0 | 21.1 (4) | 0 | ||||||||
| Peripheral | 12.5 (3) | 66.7 (2) | 21.1 (4) | 33.3 (2) | ||||||||
| Peripheral and central | 8.3 (2) | 0 | 10.5 (2) | 0 | ||||||||
| Not applicable | 62.5 (15) | 33.3 (1) | 47.4 (9) | 66.7 (4) | ||||||||
| White structures presence | 54.2 (13) | 100 (3) | 78.9 (15) | 50.0 (3) | 0.09 | |||||||
| Pattern of white structures | ||||||||||||
| Amorphous | 25.0 (6) | 0 | 26.3 (5) | 33.3 (2) | ||||||||
| Irregular | 25.0 (5) | 66.7 (2) | 26.3 (5) | 16.7 (1) | ||||||||
| Dotted | 4.2 (1) | 0 | 5.3 (1) | 0 | ||||||||
| Reticulated | 0 | 0 | 10.5 (2) | 0 | ||||||||
| Linear | 0 | 33.3 (1) | 0 | 0 | ||||||||
| Homogeneous | 0 | 0 | 5.3 (1) | 0 | ||||||||
| Radial | 0 | 0 | 5.3 (1) | 0 | ||||||||
| Disrupted | 8.3 (2) | 0 | 0 | 0 | ||||||||
| Ulcerated Tumors % (n = 28) | Non-Ulcerated Tumors % (n = 25) | pa | pa′ | pb | pb′ | pc | pc′ | pd | pd′ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular Variables | BCC % (n = 24) | SCC % (n = 3) | BCC % (n = 19) | SCC % (n = 6) | ||||||||
| Vessel Structures | ||||||||||||
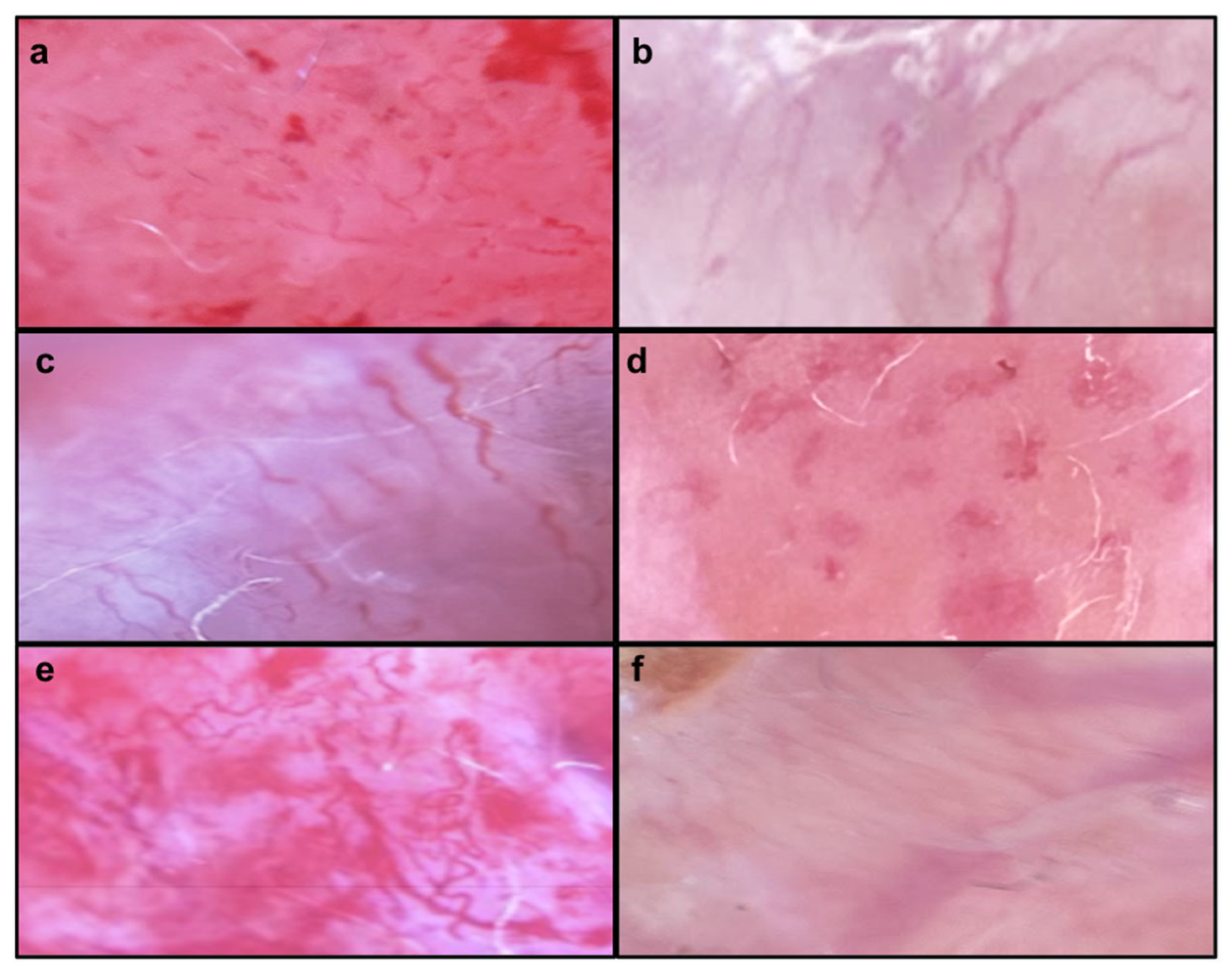
| No structure | 25.0 (6) | 66.7 (2) | 47.4 (9) | 50.0 (3) | ||||||||
| Dott | 66.7 (16) | 0 | 47.4 (9) | 83.3 (5) | 0.018 | 0.048 | 0.027 | 0.057 | ||||
| Clod | 4.2 (1) | 0 | 5.3 (1) | 0 | ||||||||
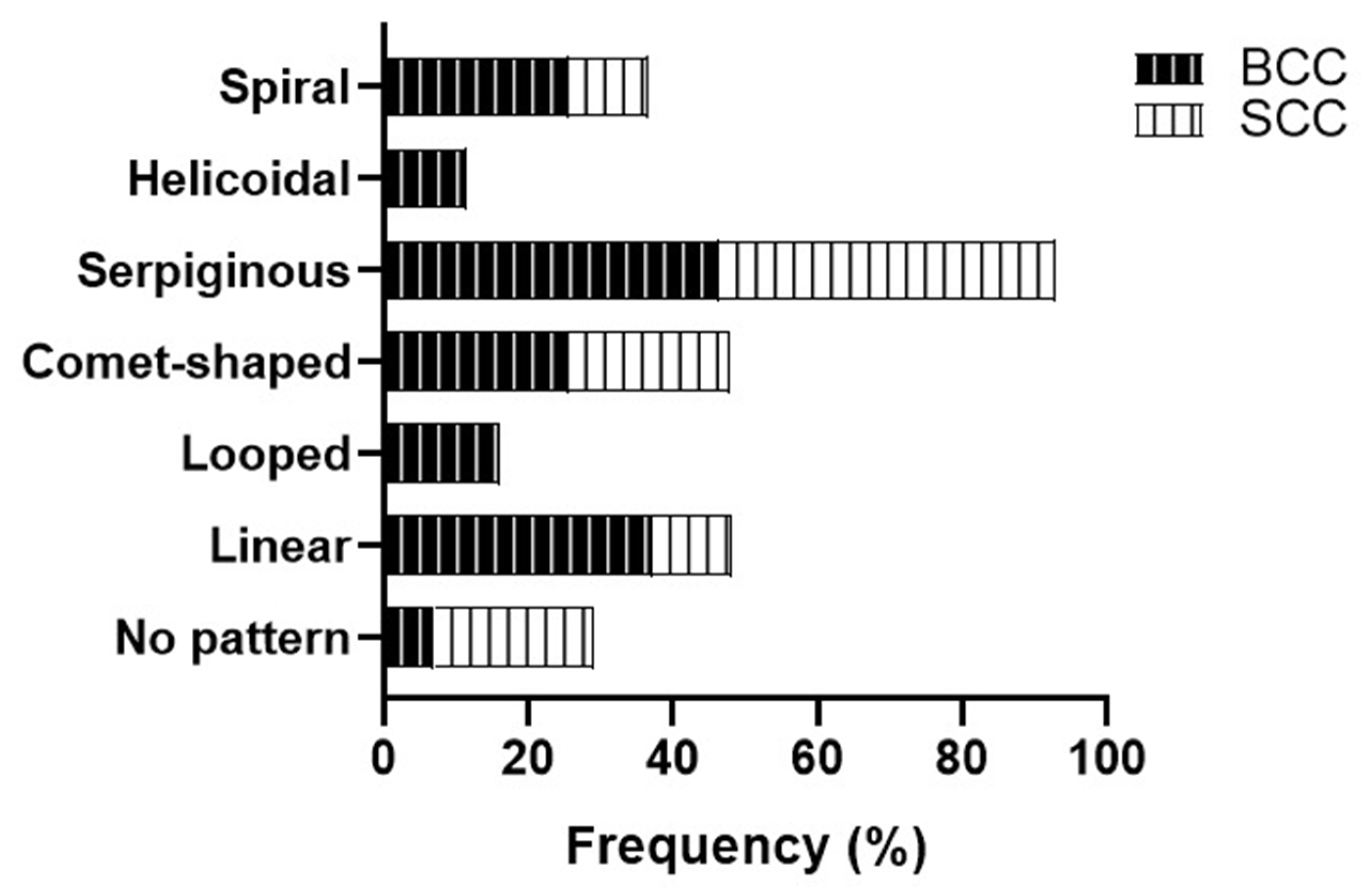
| Linear | 25.0 (6) | 66.7 (2) | 42.1 (8) | 50.0 (3) | ||||||||
| Linear vessel subtypes | ||||||||||||
| No pattern | 8.3 (2) | 0 | 10.5 (2) | 50.0 (3) | ||||||||
| Linear straight | 33.3 (8) | 0 | 42.1 (8) | 16.7 (1) | ||||||||
| Linear looped | 12.5 (3) | 0 | 21.1 (4) | 0 | ||||||||
| Linear curved | 29.2 (7) | 33.3 (1) | 21.1 (4) | 16.7 (1) | ||||||||
| Linear serpentine | 54.2 (13) | 66.7 (2) | 31.6 (6) | 33.3 (2) | ||||||||
| Linear helical | 12.5 (3) | 0 | 15.8 (3) | 0 | ||||||||
| Linear coiled | 25.0 (6) | 33.3 (1) | 26.3 (5) | 0 | ||||||||
| Arrangement of vessels and specific patterns | ||||||||||||
| Random | 8.3 (2) | 33.3 (1) | 21.1 (4) | 0 | ||||||||
| Clustered | 0 | 0 | 0 | 0 | ||||||||
| Serpinginous | 0 | 0 | 0 | 0 | ||||||||
| Radial | 70.8 (17) | 33.3 (1) | 57.9 (11) | 50.0 (3) | ||||||||
| Reticular | 0 | 0 | 0 | 0 | ||||||||
| Branched | 0 | 0 | 5.3 (1) | 0 | ||||||||
| Other variables | ||||||||||||
| Maple Leaf Structure | 0 | 0 | 0 | 0 | ||||||||
| Radiated Areas | 37.5 (9) | 66.7 (2) | 21.1 (4) | 16.7 (1) | ||||||||
| Blue-Gray Ovoid Nests | 33.3 (8) | 33.3 (1) | 42.1 (8) | 50.0 (3) | ||||||||
| Unstructured eccentric area | 0 | 33.3 (1) | 5.3 (1) | 0 | ||||||||
| Millium pseudocysts and follicular pseudoapertures | 8.3 (2) | 0 | 10.5 (2) | 0 | ||||||||
| Pigmented Reticulum, Aggregations of Black Dots and Whitish Veil | 12.5 (3) | 66.7 (2) | 26.3 (5) | 0 | 0.023 | 0.083 | 0.023 | 0.079 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Moreno, E.C.; Machado Sulbaran, A.C.; Leal, L.R.; Ortiz García, Y.M.; Olivas Román, L.R.; Riera Leal, A. Non-Melanoma Skin Cancer: Dermatoscopic Diagnostic Clues in Mexican Individuals Based on Fitzpatrick Skin Phototypes. J. Clin. Med. 2025, 14, 2966. https://doi.org/10.3390/jcm14092966
Sánchez Moreno EC, Machado Sulbaran AC, Leal LR, Ortiz García YM, Olivas Román LR, Riera Leal A. Non-Melanoma Skin Cancer: Dermatoscopic Diagnostic Clues in Mexican Individuals Based on Fitzpatrick Skin Phototypes. Journal of Clinical Medicine. 2025; 14(9):2966. https://doi.org/10.3390/jcm14092966
Chicago/Turabian StyleSánchez Moreno, Esli Camila, Andrea Carolina Machado Sulbaran, Lizbeth Riera Leal, Yveth Marlene Ortiz García, Luis Roberto Olivas Román, and Annie Riera Leal. 2025. "Non-Melanoma Skin Cancer: Dermatoscopic Diagnostic Clues in Mexican Individuals Based on Fitzpatrick Skin Phototypes" Journal of Clinical Medicine 14, no. 9: 2966. https://doi.org/10.3390/jcm14092966
APA StyleSánchez Moreno, E. C., Machado Sulbaran, A. C., Leal, L. R., Ortiz García, Y. M., Olivas Román, L. R., & Riera Leal, A. (2025). Non-Melanoma Skin Cancer: Dermatoscopic Diagnostic Clues in Mexican Individuals Based on Fitzpatrick Skin Phototypes. Journal of Clinical Medicine, 14(9), 2966. https://doi.org/10.3390/jcm14092966









