Poroelastic Characterization of Human Vertebral Metastases to Inform a Transdisciplinary Assessment of Spinal Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Demographics
2.1.1. Demographic Data
2.1.2. Metastases Classification
2.1.3. Surgical Approaches
2.2. Sample Preparation
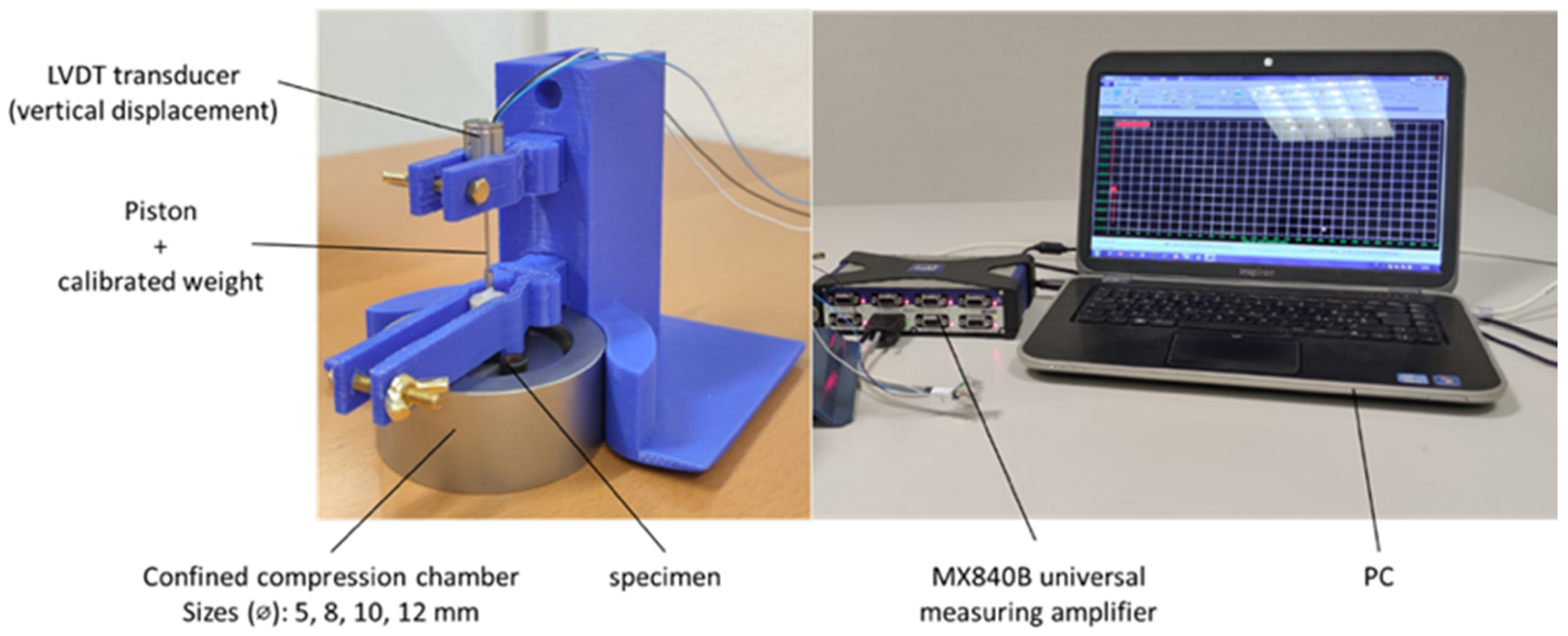
2.3. Creep Tests in Uniaxial Confined Compression
2.4. Histology
2.5. Data Analysis
- The biomechanical properties (namely aggregate modulus Ha and permeability ) and fracture (namely, fractured vs. unfractured vertebrae);
- Biomechanical properties and SINS (firmly unstable vs. potentially unstable);
- Percentage lesion’s volume and fracture;
- Percentage lesion’s volume and SINS elements.
3. Results
3.1. Patient Demographics
3.1.1. Demographic Data
3.1.2. Metastases Classification
3.1.3. Surgical Approaches
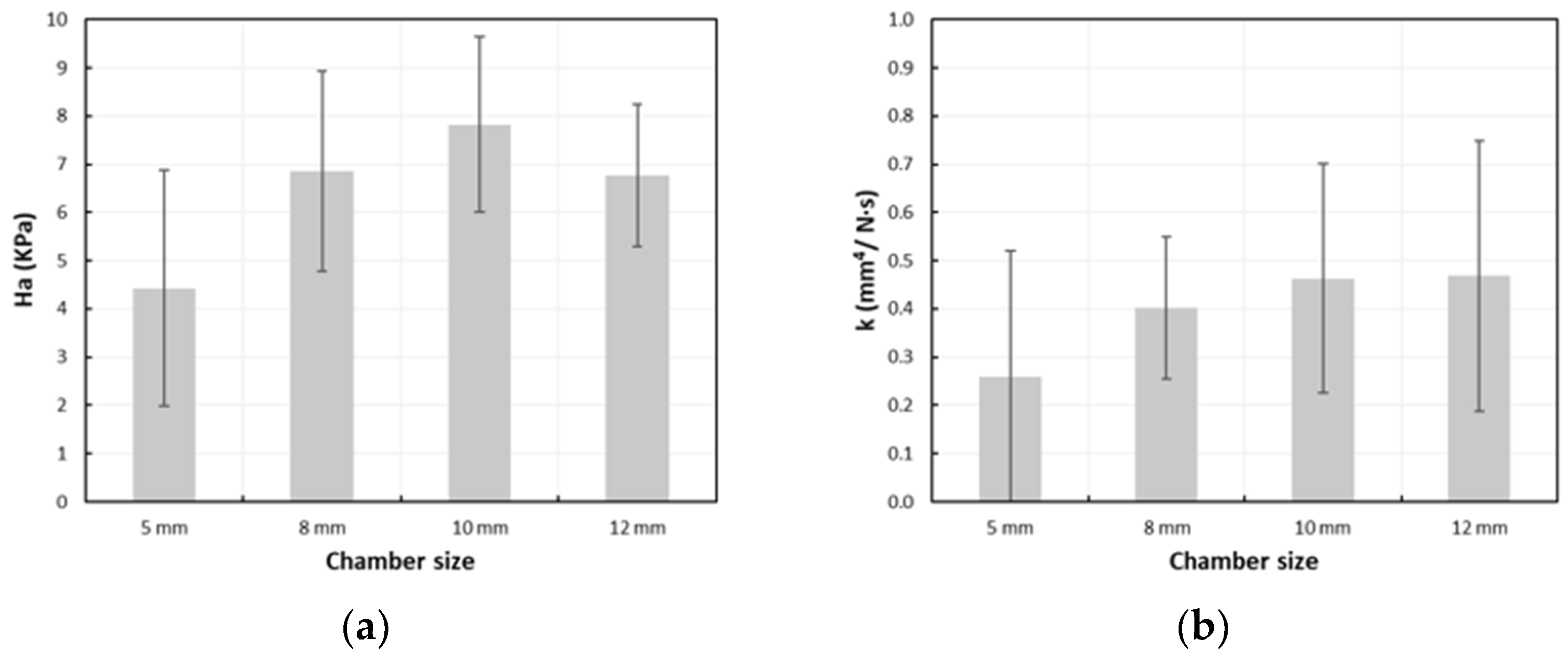
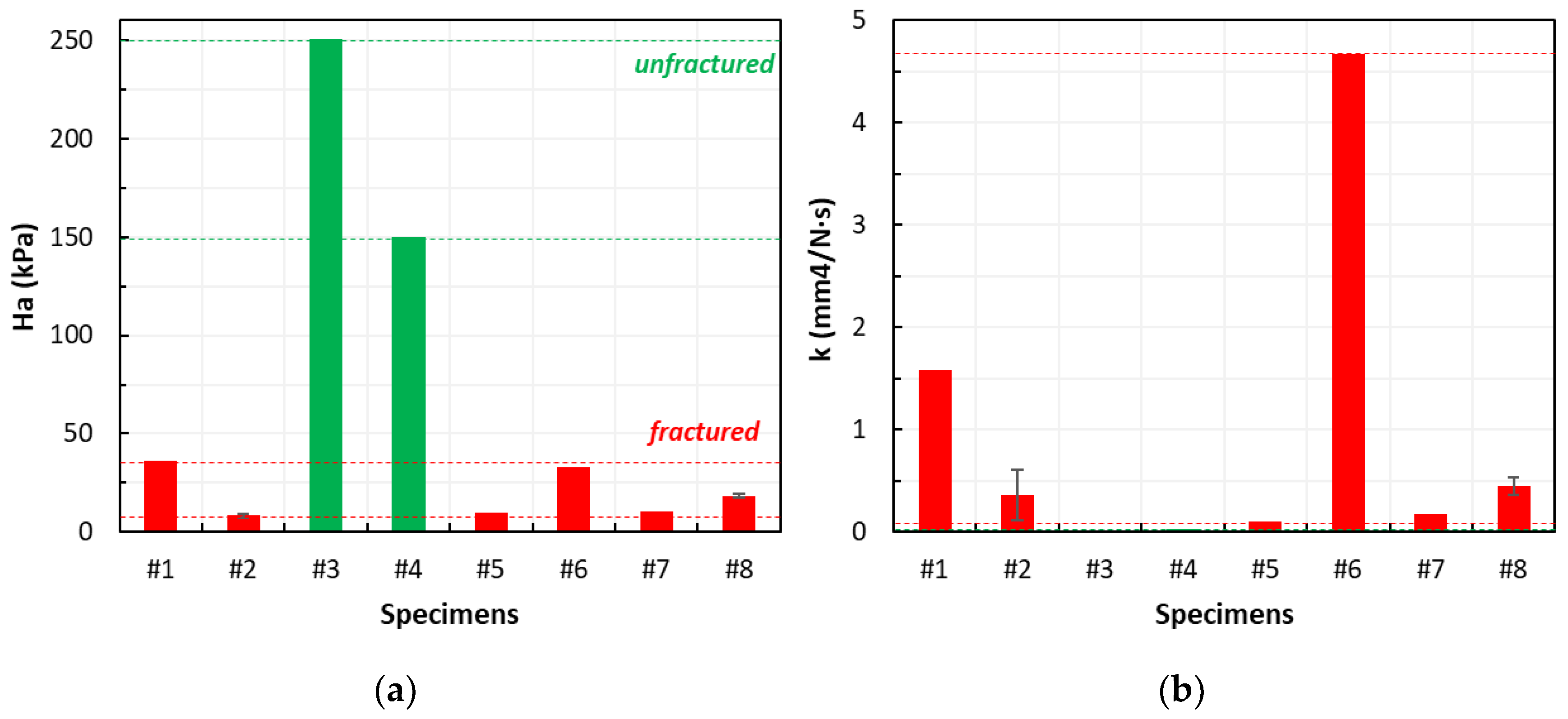
3.2. Creep Tests in Uniaxial Confined Compression
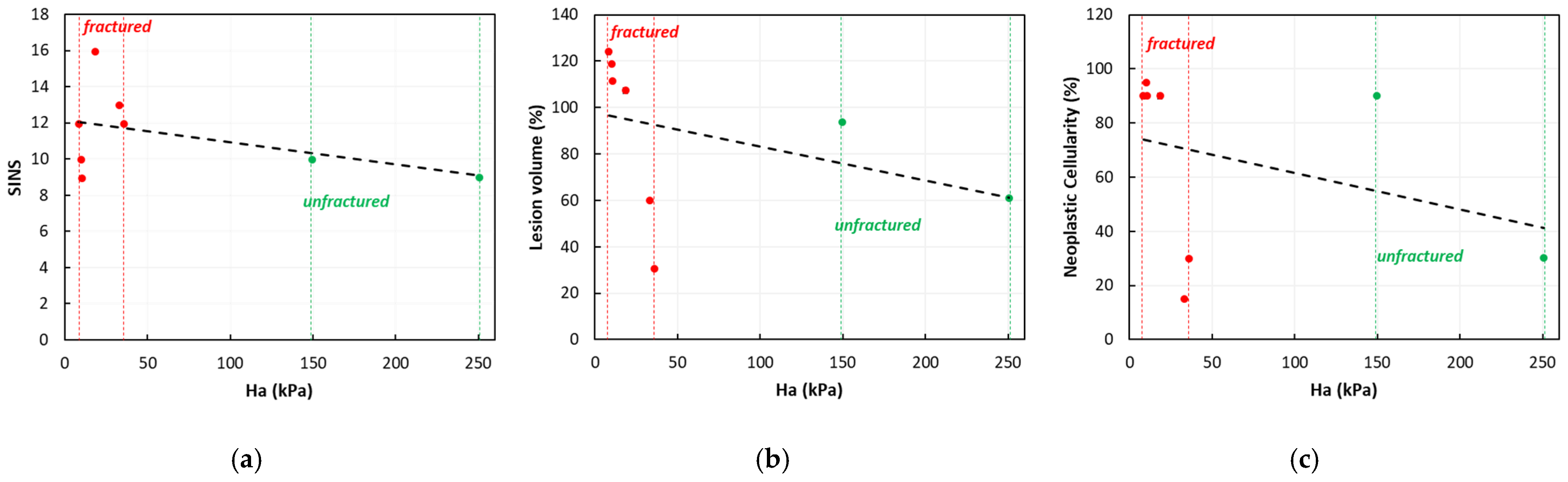
3.2.1. Correlation Between the Biomechanical Properties and Fracture
3.2.2. Correlation Between the Biomechanical Properties and SINS
3.2.3. Correlation Between the Percentage Lesion’s Volume and Fracture
3.2.4. Correlation Between the Percentage Lesion’s Volume and SINS Elements
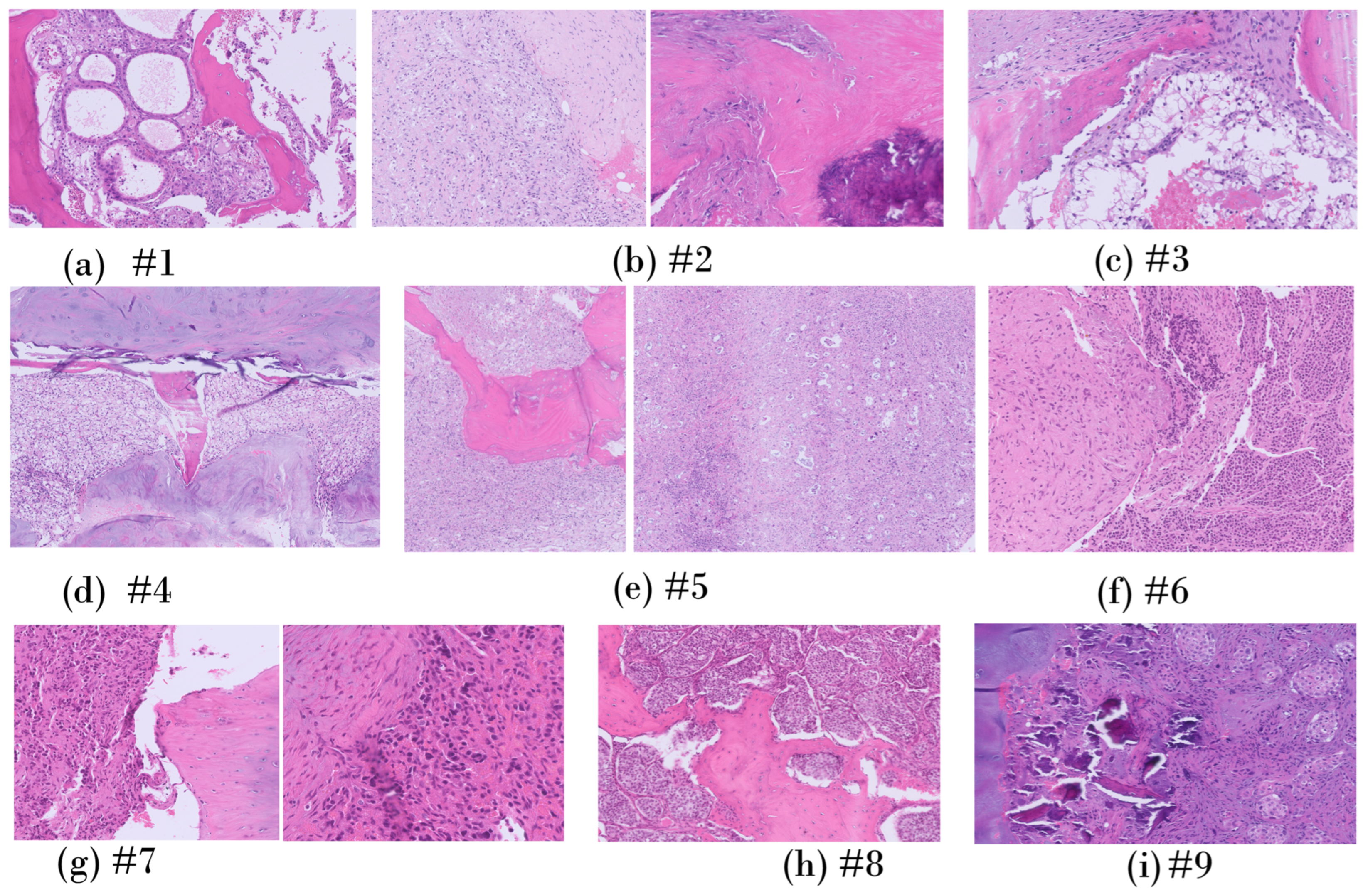
3.3. Histology
3.3.1. Patient-Specific Analysis
3.3.2. Cellularity
3.3.3. Correlation Between the Biomechanical Properties and Cellularity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Overview of Clinical Images for Each Patient
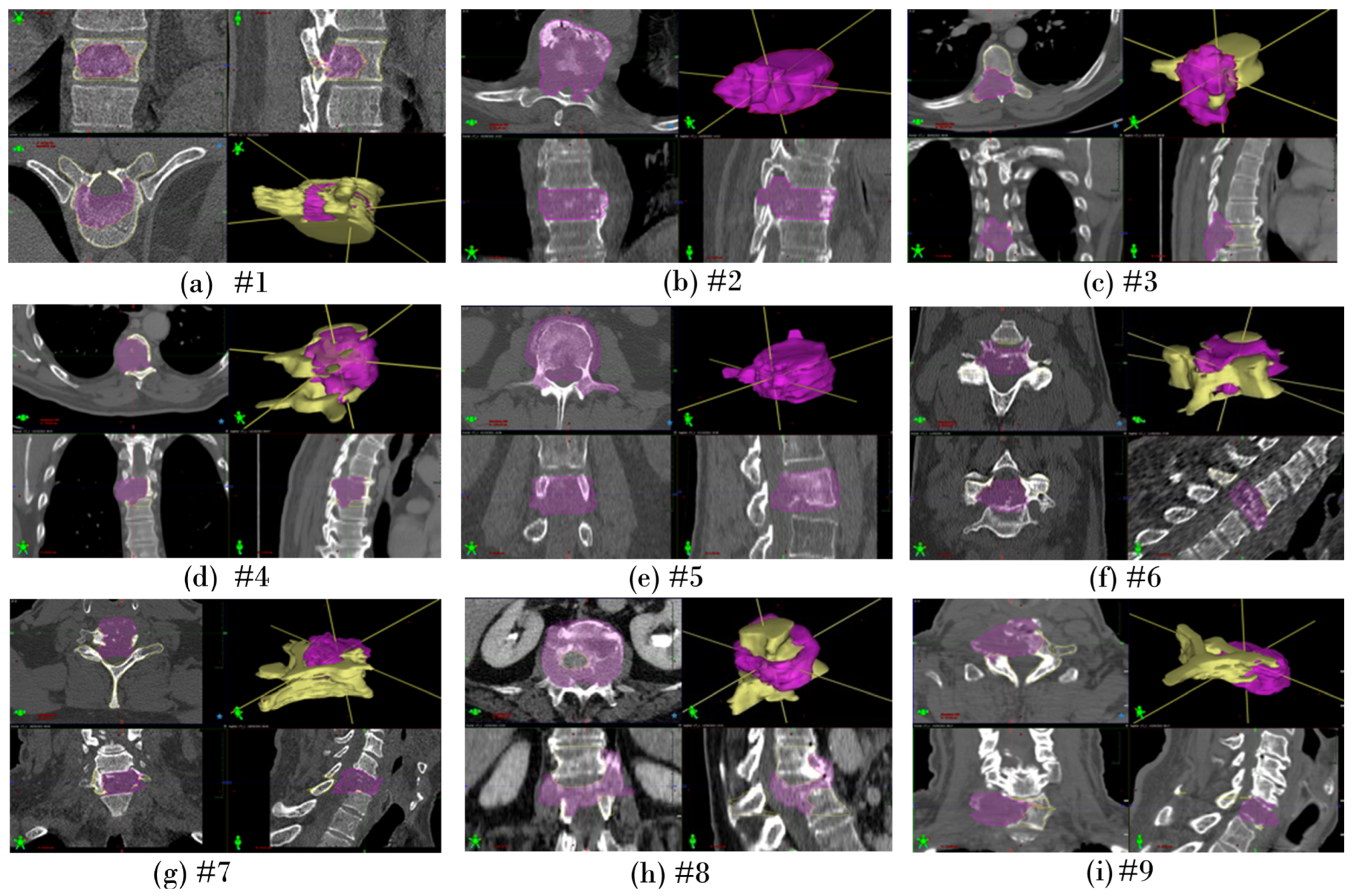
Appendix A.2. Segmentation of Lesion Volume on Clinical Images
| Patient ID | Upper Vertebra | Metastatic Vertebra | |||
|---|---|---|---|---|---|
| Level | Volume (cc) | Level | Volume (cc) | Volume (% vs. Upper Vertebra) | |
| #1 | T10 | 36.2 | T11 | 11.1 | 30.7% |
| #2 | T9 | 26.6 | T8 | 33.0 | 124.1% * |
| #3 | T9 | 37.6 | T8 | 22.8 | 60.6% |
| #4 | T6 | 25.7 | T7 | 24.0 | 93.4% |
| #5 | L2 | 45.9 | L3 | 54.5 | 118.7% * |
| #6 | C6 | 14.7 | C5 | 8.8 | 59.9% |
| #7 | C5 | 11.3 | C6 | 12.6 | 111.5% * |
| #8 | L3 | 42.3 | L1 | 45.4 | 107.3% * |
| #9 | C6 | 14.7 | C7 | 12.6 | 85.7% |
Appendix B
Preliminary Creep Tests in Uniaxial Confined Compression on Agarose Gel Samples
References
- Newman, W.C.; Patel, A.; Goldberg, J.L.; Bilsky, M.H. The importance of multidisciplinary care for spine metastases: Initial tumor management. Neuro-Oncol. Pract. 2020, 7 (Suppl. S1), i25–i32. [Google Scholar] [CrossRef]
- Hernandez, R.K.; Adhia, A.; Wade, S.W.; O’Connor, E.; Arellano, J.; Francis, K.; Alvrtsyan, H.; Million, R.P.; Liede, A. Prevalence of bone metastases and bone-targeting agent use among solid tumor patients in the United States. Clin. Epidemiol. 2015, 7, 335–345. [Google Scholar] [CrossRef]
- North, R.B.; LaRocca, V.R.; Schwartz, J.; North, C.A.; Zahurak, M.; Davis, R.F.; McAfee, P.C. Surgical management of spinal metastases: Analysis of prognostic factors during a 10-year experience. J. Neurosurg. Spine 2005, 2, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Primers 2020, 6, 83. [Google Scholar] [CrossRef]
- Burke, M.; Atkins, A.; Kiss, A.; Akens, M.; Yee, A.; Whyne, C. The impact of metastasis on the mineral phase of vertebral bone tissue. J. Mech. Behav. Biomed. Mater. 2017, 69, 75–84. [Google Scholar] [CrossRef]
- Confavreux, C.B.; Follet, H.; Mitton, D.; Pialat, J.B.; Clézardin, P. Fracture Risk Evaluation of Bone Metastases: A Burning Issue. Cancers 2021, 13, 5711. [Google Scholar] [CrossRef]
- Esperança-Martins, M.; Roque, D.; Barroso, T.; Abrunhosa-Branquinho, A.; Belo, D.; Simas, N.; Costa, L. Multidisciplinary Approach to Spinal Metastases and Metastatic Spinal Cord Compression—A New Integrative Flowchart for Patient Management. Cancers 2023, 15, 1796. [Google Scholar] [CrossRef]
- Di Perna, G.; Cofano, F.; Mantovani, C.; Badellino, S.A.; Marengo, N.; Ajello, M.; Comite, L.M.; Palmieri, G.; Tartara, F.; Zenga, F.; et al. Separation surgery for metastatic epidural spinal cord compression: A qualitative review. J. Bone Oncol. 2020, 25, 100320. [Google Scholar] [CrossRef]
- Tancioni, F.; Navarria, P.; Pessina, F.; Attuati, L.; Mancosu, P.; Alloisio, M.; Scorsetti, M.; Santoro, A.; Rodriguez, R.; Baena, Y. Assessment of prognostic factors in patients with metastatic epidural spinal cord compression (MESCC) from solid tumor after surgery plus radiotherapy: A single institution experience. Eur. Spine J. 2012, 21 (Suppl. S1), S146–S148. [Google Scholar] [CrossRef]
- Palanca, M.; Cavazzoni, G.; Dall’Ara, E. The role of bone metastases on the mechanical competence of human vertebrae. Bone 2023, 173, 116814. [Google Scholar] [CrossRef]
- Palanca, M.; Barbanti-Bròdano, G.; Marras, D.; Marciante, M.; Serra, M.; Gasbarrini, A.; Dall’Ara, E.; Cristofolini, L. Type, size, and position of metastatic lesions explain the deformation of the vertebrae under complex loading conditions. Bone 2021, 151, 116028. [Google Scholar] [CrossRef]
- Stadelmann, M.; Schenk, D.; Maquer, G.; Lenherr, C. Conventional finite element models estimate the strength of metastatic human vertebrae despite alterations of the bone’s tissue and structure. Bone 2020, 141, 115598. [Google Scholar] [CrossRef]
- Costa, M.C.; Eltes, P.; Lazary, A.; Varga, P.P.; Viceconti, M.; Dall’Ara, E. Biomechanical assessment of vertebrae with lytic metastases with subject-specific finite element models. J. Mech. Behav. Biomed. Mater. 2019, 98, 268–290. [Google Scholar] [CrossRef]
- Galbusera, F.; Qian, Z.; Casaroli, G.; Bassani, T.; Costa, F.; Schlager, B.; Wilke, H.J. The Role of the Size and Location of the Tumors and of the Vertebral Anatomy in Determining the Structural Stability of the Metastatically Involved Spine: A Finite Element Study. Transl. Oncol. 2018, 11, 639–646. [Google Scholar] [CrossRef]
- Groenen, K.H.J.; Bitter, T.; van Veluwen, T.C.G.; van der Linden, Y.M.; Verdonschot, N.; Tanck, E.; Janssen, D. Case-specific non-linear finite element models to predict failure behavior in two functional spinal units. J. Orthop. Res. 2018, 36, 3208–3218. [Google Scholar] [CrossRef]
- Tschirhart, C.E.; Finkelstein, J.A.; Whyne, C.M. Biomechanics of vertebral level, geometry, and transcortical tumors in the metastatic spine. J. Biomech. 2007, 40, 46–54. [Google Scholar] [CrossRef]
- Tschirhart, C.E.; Nagpurkar, A.; Whyne, C.M. Effects of tumor location, shape and surface serration on burst fracture risk in the metastatic spine. J. Biomech. 2004, 37, 653–660. [Google Scholar] [CrossRef]
- Whyne, C.M.; Hu, S.S.; Lotz, J.C. Parametric finite element analysis of vertebral bodies affected by tumors. J. Biomech. 2001, 34, 1317–1324. [Google Scholar] [CrossRef]
- Nazarian, A.; von Stechow, D.; Zurakowski, D.; Müller, R.; Snyder, B.D. Bone volume fraction explains the variation in strength and stiffness of cancellous bone affected by metastatic cancer and osteoporosis. Calcif. Tissue Int. 2008, 83, 368–379. [Google Scholar] [CrossRef]
- Whyne, C.; Hu, S.; Workman, K.; Lotz, J. Biphasic material properties of lytic bone metastases. Ann. Biomed. Eng. 2020, 28, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; Di Paola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S. A Novel Classification System for Spinal Instability in Neoplastic Disease An Evidence-Based Approach and Expert Consensus From the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef]
- Stefan, U.; Michael, B.; Werner, S. Effects of three different preservation methods on the mechanical properties of human and bovine cortical bone. Bone 2010, 47, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.Y.; Yao, H.; Huang, C.Y.; Cheung, H.S. New insight into deformation-dependent hydraulic permeability of gels and cartilage, and dynamic behavior of agarose gels in confined compression. J. Biomech. 2003, 36, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Swabb, E.A.; Wei, J.; Gullino, P.M. Diffusion and convection in normal and neoplastic tissues. Cancer Res. 1974, 34, 2814–2821. [Google Scholar]
- Boucher, Y.; Netti, P.A.; Baxter, L.T.; Jain, R.K. Intratumoral infusion of fluid: Estimation of hydraulic conductivity and implications for the delivery of therapeutic agents. Br. J. Cancer 1998, 78, 1442–1448. [Google Scholar] [CrossRef]
- Di Resta, G.R.; Lee, J.; Larson, S.M.; Arbit, E. Characterization of neuroblastoma xenograft in rat flank. I. Growth, interstitial fluid pressure, and interstitial fluid velocity distribution profiles. Microvasc. Res. 1993, 46, 158–177. [Google Scholar] [CrossRef]
- Serratrice, N.; Faddoul, J.; Tarabay, B.; Attieh, C.; Chalah, M.A.; Ayache, S.S.; Abi Lahoud, G.N. Ten Years After SINS: Role of Surgery and Radiotherapy in the Management of Patients With Vertebral Metastases. Front. Oncol. 2022, 12, 802595. [Google Scholar] [CrossRef]
- Amelot, A.; Terrier, L.M.; Cristini, J.; LeNail, L.R.; Buffenoir, K.; Pascal-Moussellard, H.; Bonaccorsi, R.; Mathon, B. Approaching spinal metastases spread profile. Surg. Oncol. 2019, 31, 61–66. [Google Scholar] [CrossRef]
- Boehling, N.S.; Grosshans, D.R.; Allen, P.K.; McAleer, M.F.; Burton, A.W.; Azeem, S.; Rhines, L.D.; Chang, E.L. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J. Neurosurg. Spine 2012, 16, 379–386. [Google Scholar] [CrossRef]
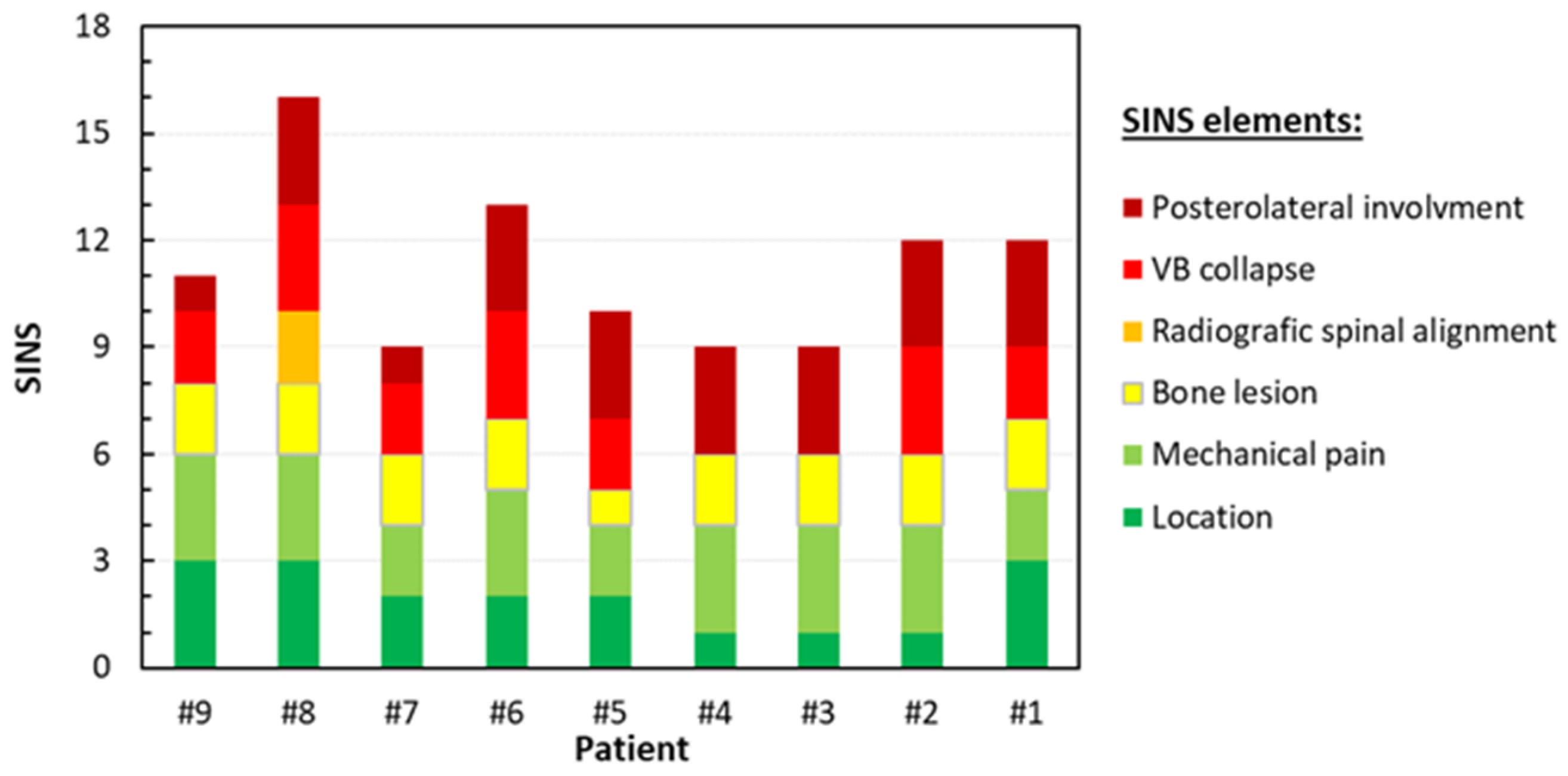
| Patient ID | Sex | Age (years) | BMI | Smoking Status | Steroids | Chemotherapy | Primary Tumor | Spinal Level | Lesion Type | Volume (% vs. Upper Vertebra) | SINS | Fracture | Surgical Approach |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | M | 42 | 26.54 | Past | Yes | No | Kidney | T11 | Lytic | 30.7% | 12 | Yes | C |
| #2 | F | 77 | 29.30 | Never | Yes | No | Kidney | T8 | Lytic | 124.1% * | 12 | Yes | C |
| #3 | M | 48 | 25.35 | Past | Yes | No | Kidney | T8 | Lytic | 60.6% | 9 | No | P |
| #4 | M | 61 | 25.47 | Smoker | Yes | No | Kidney | T7 | Lytic | 93.4% | 10 | No | P |
| #5 | M | 51 | 26.37 | Never | Yes | Yes | Lung | L3 | Mixed | 118.7% * | 10 | Yes | P |
| #6 | M | 74 | 25.25 | Smoker | No | No | Plasmo- cyithome | C5 | Lytic | 59.9% | 13 | Yes | A |
| #7 | M | 56 | 27.76 | Past | No | No | NET | C6 | Lytic | 111.5% * | 9 | Yes | A |
| #8 | F | 74 | 29.33 | Past | No | Yes | Breast | L1 | Lytic | 107.3% * | 16 | Yes | C |
| #9 | M | 72 | 22.53 | Smoker | No | No | Lung | C7 | Lytic | 85.7% | 11 | Yes | A |
| Patient ID | Cellularity (%) | Histological Diagnosis |
|---|---|---|
| #1 | 30% | Renal |
| #2 | 90% | Renal |
| #3 | 30% | Renal |
| #4 | 90% | Renal |
| #5 | 95% (30% tumor necrosis) | Pulmonary |
| #6 | 15% | Plasmacytoma |
| #7 | 90% | NET |
| #8 | 90% | Breast |
| #9 | 100% (extensive desmoplasia) | Pulmonary |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Barbera, L.; Villa, T.; Costa, F.; Boschetti, F.; De Robertis, M.; Anselmi, L.; Capo, G.; Pancetti, S.; Fornari, M. Poroelastic Characterization of Human Vertebral Metastases to Inform a Transdisciplinary Assessment of Spinal Tumors. J. Clin. Med. 2025, 14, 2913. https://doi.org/10.3390/jcm14092913
La Barbera L, Villa T, Costa F, Boschetti F, De Robertis M, Anselmi L, Capo G, Pancetti S, Fornari M. Poroelastic Characterization of Human Vertebral Metastases to Inform a Transdisciplinary Assessment of Spinal Tumors. Journal of Clinical Medicine. 2025; 14(9):2913. https://doi.org/10.3390/jcm14092913
Chicago/Turabian StyleLa Barbera, Luigi, Tomaso Villa, Francesco Costa, Federica Boschetti, Mario De Robertis, Leonardo Anselmi, Gabriele Capo, Saverio Pancetti, and Maurizio Fornari. 2025. "Poroelastic Characterization of Human Vertebral Metastases to Inform a Transdisciplinary Assessment of Spinal Tumors" Journal of Clinical Medicine 14, no. 9: 2913. https://doi.org/10.3390/jcm14092913
APA StyleLa Barbera, L., Villa, T., Costa, F., Boschetti, F., De Robertis, M., Anselmi, L., Capo, G., Pancetti, S., & Fornari, M. (2025). Poroelastic Characterization of Human Vertebral Metastases to Inform a Transdisciplinary Assessment of Spinal Tumors. Journal of Clinical Medicine, 14(9), 2913. https://doi.org/10.3390/jcm14092913





