Salvage of the Mastectomy Pocket in Infected Implant-Based Breast Reconstruction Using Negative-Pressure Wound Therapy with Instillation and Dwell: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Extraction
2.3. Assessment of Risk of Bias and Quality of Evidence of the Included Studies
2.4. Statistical Analysis—Quantitative Synthesis
3. Results
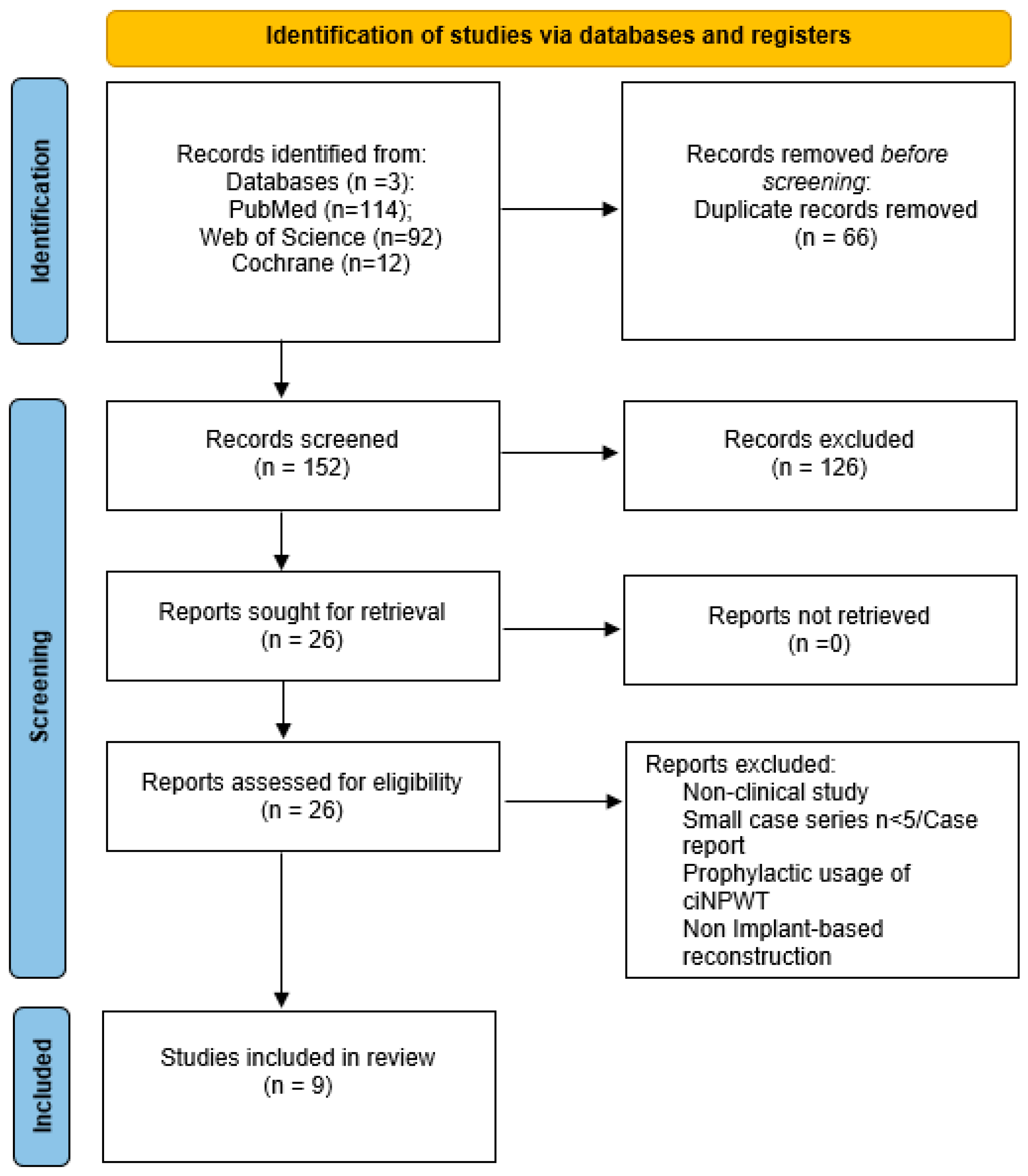
3.1. Literature Search Results
3.2. Details of the Included Studies
3.3. Therapeutic Settings
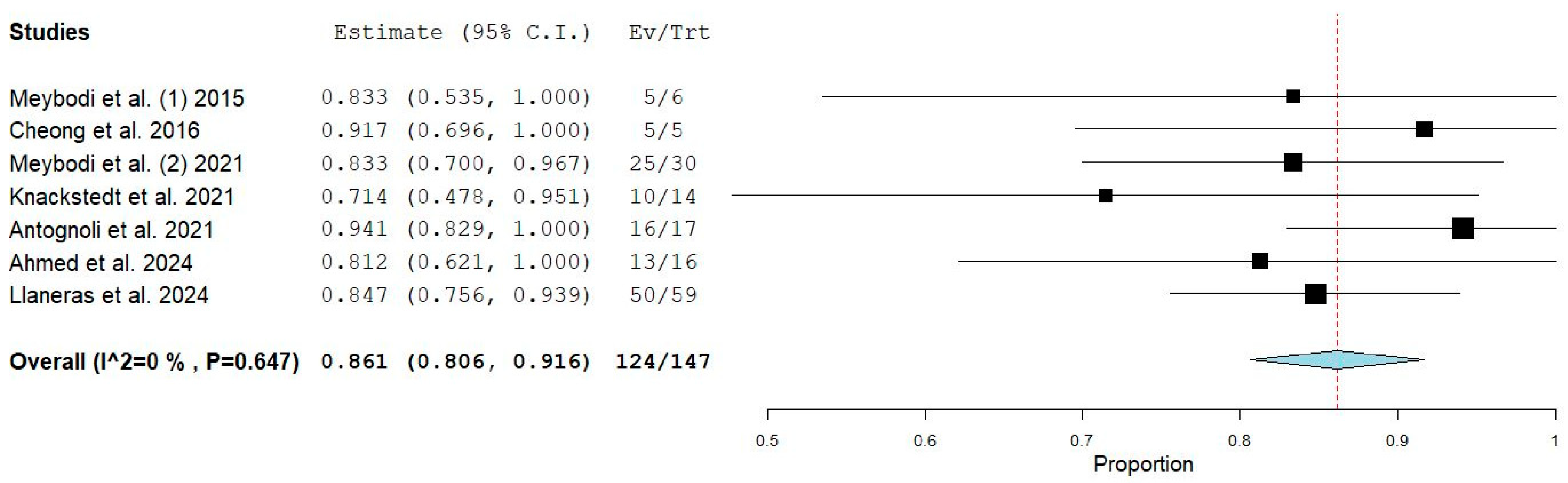
3.4. Quantitative Analysis
3.5. Mastectomy Pocket Volume Preservation and Surgical Technique
3.6. Change in Bioburden and Clinical Infection
3.7. Time Interval Between Implantation and Infection
3.8. Predictability of Mastectomy Pocket Salvage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPWTi-d | Negative pressure wound therapy with instillation and dwell |
| IBBR | Implant-based breast reconstruction |
| TE | Tissue Expander |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Hanlon, A.L.; Koshy, M.; Buras, R.; Chumsri, S.; Tkaczuk, K.H.; Cheston, S.B.; Regine, W.F.; Feigenberg, S.J. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann. Surg. Oncol. 2013, 20, 1436–1443. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Albornoz, C.R.; Bach, P.B.; Mehrara, B.J.; Disa, J.J.; Pusic, A.L.; McCarthy, C.M.; Cordeiro, P.G.; Matros, E. A paradigm shift in U.S. Breast reconstruction: Increasing implant rates. Plast. Reconstr. Surg. 2013, 131, 15–23. [Google Scholar] [CrossRef] [PubMed]
- van Verschuer, V.M.; Maijers, M.C.; van Deurzen, C.H.; Koppert, L.B. Koppert Oncological safety of prophylactic breast surgery: Skin-sparing and nipple-sparing versus total mastectomy. Gland Surg. 2015, 4, 467–475. [Google Scholar] [CrossRef]
- Paepke, S.; Schmid, R.; Fleckner, S.; Paepke, D.; Niemeyer, M.; Schmalfeldt, B.; Jacobs, V.R.; Kiechle, M. Subcutaneous mastectomy with conservation of the nipple-areola skin: Broadening the indications. Ann. Surg. 2009, 250, 288–292. [Google Scholar] [CrossRef] [PubMed]
- American Society of Plastic Surgeons. 2020 Plastic Surgery Statistics Report. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf (accessed on 3 February 2025).
- Gardani, M.; Bertozzi, N.; Grieco, M.P.; Pesce, M.; Simonacci, F.; Santi, P.; Raposio, E. Breast reconstruction with anatomical implants: A review of indications and techniques based on current literature. Ann. Med. Surg. 2017, 21, 96–104. [Google Scholar] [CrossRef]
- Spear, S.L.; Howard, M.A.; Boehmler, J.H.; Ducic, I.; Low, M.; Abbruzzesse, M.R. Abbruzzesse The infected or exposed breast implant: Management and treatment strategies. Plast. Reconstr. Surg. 2004, 113, 1634–1644. [Google Scholar] [CrossRef]
- Malekpour, M.; Malekpour, F.; Wang, H.T.-H. Breast reconstruction: Review of current autologous and implant-based techniques and long-term oncologic outcome. World J. Clin. Cases 2023, 11, 2201–2212. [Google Scholar] [CrossRef]
- Kanapathy, M.; Faderani, R.; Arumugam, V.; Haque, S.; Mosahebi, A. Management of periprosthetic breast infection: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2831–2845. [Google Scholar] [CrossRef]
- Xue, A.S.; Kania, K.E.; Brown, R.H.; Bullocks, J.M.; Hollier, L.; Izaddoost, S.A. Salvage of Infected Prosthetic Breast Reconstructions. Semin. Plast. Surg. 2016, 30, 55–59. [Google Scholar] [CrossRef]
- Bramhall, R.J.; Hernan, I.; Harris, P.A. A single-centre, retrospective proof-of-concept review of salvage of infected or exposed implant breast reconstructions with explantation and one-stage free flap replacement. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, B.; Walklet, E.; Bradley, E.; Thrush, S.; Skillman, J.; Whisker, L.; Barnes, N.; Holcombe, C.; Potter, S. Experiences of implant loss after immediate implant-based breast reconstruction: Qualitative study. BJS Open 2020, 4, 380–390. [Google Scholar] [CrossRef]
- Bennett, S.; Fitoussi, A.; Berry, M.; Couturaud, B.; Salmon, R. Management of exposed, infected implant-based breast reconstruction and strategies for salvage. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 1270–1277. [Google Scholar] [CrossRef]
- O’connor, M.J.B.; Huffman, K.N.B.; Ho, K.B.; Marzouk, S.M.; Fuentes, R.J.B.C.; Zhang, K.L.B.; Melnick, B.A.B.; Sparks, P.J.B.; Harris, R.B.; Bartler, A.V.; et al. Negative Pressure Wound Therapy with Instillation for Periprosthetic Infection after Breast Reconstruction: A Systematic Review. Plast. Reconstr. Surg.-Glob. Open 2024, 12, e6267. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, J.; Willy, C.; Fagerdahl, A.-M.; Fraccalvieri, M.; Malmsjö, M.; Piaggesi, A.; Probst, A.; Vowden, P. EWMA Document: Negative Pressure Wound Therapy. J. Wound Care 2017, 26 (Suppl. S3), S1–S154. [Google Scholar] [CrossRef]
- Gabriel, A.; Shores, J.; Heinrich, C.; Baqai, W.; Kalina, S.; Sogioka, N.; Gupta, S. Negative pressure wound therapy with instillation: A pilot study describing a new method for treating infected wounds. Int. Wound J. 2008, 5, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, F.; Sedaghat, N.; French, J.; Keighley, C.; Mitchell, D.; Elder, E. Implant salvage in breast reconstruction with severe peri-prosthetic infection. ANZ J. Surg. 2017, 87, E293–E299. [Google Scholar] [CrossRef]
- Available online: www.prisma-statement.org (accessed on 3 February 2025).
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Antognoli, L.E.; Singh, D.P.; Choudhry, S.; Turcotte, J.P.; Holton, L.H.I. Rinse But Don’t Repeat: Single Application V.A.C. VERAFLO Salvages Infected Breast Prostheses. Plast. Reconstr. Surg.-Glob. Open 2021, 9, e3896. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.Y.; Goltsman, D.; Warrier, S. A New Method of Salvaging Breast Reconstruction After Breast Implant Using Negative Pressure Wound Therapy and Instillation. Aesthetic Plast. Surg. 2016, 40, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Gruener, J.S.; Horch, R.E.; Geierlehner, A.; Mueller-Seubert, W.; Cai, A.; Arkudas, A.; Ludolph, I. Is Instillational Topical Negative Pressure Wound Therapy in Peri-Prosthetic Infections of the Breast Effective? A Pilot Study. J. Pers. Med. 2022, 12, 2054. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Kanapathy, M.; Bollen, E.; Mosahebi, A.; Younis, I. Patient-reported outcome and cost implication of acute salvage of infected implant-based breast reconstruction with negative pressure wound therapy with Instillation (NPWTi) compared to standard care. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 3300–3306. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.; Scomacao, I.; Djohan, R. Utilization of irrigating negative pressure wound therapy for breast implant salvage: Long-term results and success. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 407–447. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, F.M.; Sedaghat, N.B.; Elder, E.M.; French, J.M.; Adams, K.B.; Hsu, J.M.; Kanesalingam, K.M.; Brennan, M.F. Salvaging the Unsalvageable: Negative Pressure Wound Therapy for Severe Infection of Prosthetic Breast Reconstruction. Plast. Reconstr. Surg.-Glob. Open 2021, 9, e3456. [Google Scholar] [CrossRef]
- Ahmed, S.; Hulsman, L.B.; Imeokparia, F.; Ludwig, K.; Fisher, C.M.; Bamba, R.; Danforth, R.; VonDerHaar, R.J.; Lester, M.E.; Hassanein, A.H.M. Implant-based Breast Reconstruction Salvage with Negative Pressure Wound Therapy with Instillation: An Evaluation of Outcomes. Plast. Reconstr. Surg.-Glob. Open 2024, 12, e6116. [Google Scholar] [CrossRef]
- Llaneras, J.C.; Clark, R.C.; Antognoli, L.; Finkelstein, E.; Hulsman, L.B.; Holton, L.; Singh, D.; VonderHaar, R.J.; Djohan, R.; Hassanein, A.H.; et al. The Efficacy of Single-Application NPWTi-d for the Salvage of Infected Breast Prostheses: A Multi-Center Study. Plast. Reconstr. Surg.-Glob. Open 2025, 13, e6467. [Google Scholar] [CrossRef]
- Kim, P.J.; Attinger, C.E.; Constantine, T.; Crist, B.D.; Faust, E.; Hirche, C.R.; Lavery, L.A.; Messina, V.J.; Ohura, N.; Punch, L.J.; et al. Negative pressure wound therapy with instillation: International consensus guidelines update. Int. Wound J. 2020, 17, 174–186. [Google Scholar] [CrossRef]
- De Pellegrin, L.; Feltri, P.; Filardo, G.; Candrian, C.; Harder, Y.; Galetti, K.; De Monti, M. Effects of negative pressure wound therapy with instillation and dwell time (NPWTi-d) versus NPWT or standard of care in orthoplastic surgery: A systematic review and meta-analysis. Int. Wound J. 2023, 20, 2402–2413. [Google Scholar] [CrossRef]
- Accurso, A.; Rocco, N.; Accardo, G.; Reale, P.; Salerno, C.; Mattera, E.; D’andrea, F. Innovative Management of Implant Exposure in ADM/Implant-Based Breast Reconstruction with Negative Pressure Wound Therapy. Aesthetic Plast. Surg. 2017, 41, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Malahias, M.; Jordan, D.J.; Hughes, L.C.; Hindocha, S.; Juma, A. A literature review and summary of capsular contracture: An ongoing challenge to breast surgeons and their patients. Int. J. Surg. Open 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Headon, H.; Kasem, A.; Mokbel, K. Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch. Plast. Surg. 2015, 42, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Reish, R.G.; Damjanovic, B.; Austen, W.G.J.; Winograd, J.; Liao, E.C.M.; Cetrulo, C.L.; Balkin, D.M.; Colwell, A.S. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: Salvage rates and predictors of success. Plast. Reconstr. Surg. 2013, 131, 1223–1230. [Google Scholar] [CrossRef]
| Author | Publication Year | Country | Study Design | N° of Patients | N° of Breasts | Instillation Solution | Mastectomy Pocket Salvage Rate (n/Breasts) |
|---|---|---|---|---|---|---|---|
| Antognoli et al. [23] | 2021 | USA | Retrospective comparative | NPWTid (n = 16) vs. standard of care (n = 9) | NPWTid = 17 vs. non NPWTid = 9 | 0.1% polyhexanide + 0.1% betaine (Prontosan) | 16/17 |
| Cheong et al. [24] | 2016 | Australia | Case series | NPWTid n = 5 | 5 | Saline | 5/5 |
| Gruener et al. [25] | 2022 | Germany | Retrospective non-comparative | NPWTid n = 12 | 13 | Polyhexanid 0.4 mg/mL (Lavasept) | n.o. |
| Haque et al. [26] | 2021 | UK | Retrospective comparative | NPWTid (n = 20) vs. standard of care (n = 20) | NPWTid = 20 vs. non NPWTid = 20 | Saline | n.o. |
| Knackstedt et al. [27] | 2021 | USA | Case series | NPWTid n = 12 | 14 | n.r. | 10/14 |
| Meybodi et al. [28] | 2021 | Australia | Case series | NPWTid n = 28 | 30 | Saline, Acetic acid, 0.1% polyhexanide + 0.1% betaine (Prontosan) | 25/30 |
| Meybodi et al. [19] | 2015 | Australia | Case series | NPWTid n = 5 | 6 | Saline (changed accordingly to germs to Acetic acid or Prontosan) | 5/6 |
| Ahmed et al. [29] | 2024 | USA | Retrospective comparative | NPWTid (n = 13) vs. standard of care (n = 34) | NPWTid = 16 vs. non NPWTid = 60 | Oxychlorosene | 13/16 |
| Llaneras et al. [30] | 2024 | USA | Multicentric retrospective cohort | NPWTid n = 56 | 59 | Saline, Prontosan and oxychlorosene | 50/56 |
| IBBR Details | Presence of Allogenic Material for Implant Coverage | Previous Radiotherapy | Surgical Treatment in Addition to Implant Removal | Implant/Expander | Type of Surgery in Case of Failure | |
|---|---|---|---|---|---|---|
| Meybodi et al. [19] | Sub-pectoral placement (n = 6/6, 100%) | Biologic † (n = 1/6, 17%) Synthetic * (n = 2/6, 33%) None (n = 3/6, 50%) | n = 1/6 (17%) | Debridement | 3/6 (50%) infected tissue expanders → 3/6 expander insertion instead (1 failure) 3/6 (50%) infected implants → 3/6 expander insertion instead | Bilateral autologous breast reconstruction |
| Antognoli et al. [23] | Sub-pectoral placement (n = 13/16, 81%) Pre-pectoral placement (n = 3/16, 19%) | NR | n = 0/12 | Debridement | 9/16 (56%) infected tissue expanders → 3/9 implant insertion instead, 6/9 expander insertion instead 7/16(44%) infected implants → 3/7 expander insertion instead, 4/7 implant insertion instead (1 failure) | 1 flat chest for 5 months followed by delayed breast reconstruction with tissue expansion and subsequent exchange for implant |
| Haque et al. [26] | NR | NR | NR | Debridement | 20/20 infected implants → 20/20 new implant insertion instead | NR |
| Cheong et al. [24] | Sub-pectoral placement (n = 2/5 expanders, 40%) NR for implants | NR | n = 2/5 (40%) | NR | 3/5 (60%) infected implants, 2/5 (40%) infected tissue expanders → 5/5 implant insertion instead | NR |
| Gruener et al. [25] | NR | NR | n = 4/13 (31%) | Total capsulectomy | NR | NR |
| Meybodi et al. [28] | Sub-pectoral placement (n = 25/30, 83%) | Synthetic * (n = 17, 57%) Biologic † (n = 6, 20%) Autologous ‡ (n = 5, 17%) None (n = 2, 6%) | n = 2/30 (7%) | Debridement | 16/30 (53%) infected tissue expanders, 14/30 infected implants (47%) → 24/30 (80%) tissue expander, 5/30 (13%) implant and 1/30 (3%) expansion without replacement due to concerns about delay of AC instead | NR |
| Knackstedt et al. [27] | NR | NR | n = 2/12 (17%) | NR | 12/12 infected implants → 10/12 implant insertion instead | Autologous reconstruction in 12 cases, of which 1 chose for autologous reconstruction following second IBBR failure |
| Ahmed et al. [29] | NPWTid: Pre-pectoral n = 12 (92.3%), Subpectoral n = 1 (7.7%) Non NPWTid: Pre-pectoral n = 32 (94.1%), Subpectoral n = 2 (5.9%) | Acellular dermal matrix or mesh NPWTid n = 12 (92.3%), non NPWTid n = 34 (100%) | NPWTid n = 0 vs. non NPWTid n = 2 (5.9%) | Capsulectomy and complete removal of mesh if present | NPWTid: TE to TE 8/16 (50%), TE to implant 2/16 (12.5%), Implant to implant 6/16 (37.5%) | Autologous reconstruction or delayed implant reinsertion |
| Llaneras et al. [30] | Pre-pectoral n = 44 (78.6%) | Mesh/ADM n = 51 (91.1%) | n= 3 (5.4%) | Debridement | TE in 65% (n = 38); Implants 36% (n = 21) | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pellegrin, L.; Zucal, I.; Treglia, G.; Parodi, C.; Schweizer, R.; De Monti, M.; Harder, Y. Salvage of the Mastectomy Pocket in Infected Implant-Based Breast Reconstruction Using Negative-Pressure Wound Therapy with Instillation and Dwell: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2730. https://doi.org/10.3390/jcm14082730
De Pellegrin L, Zucal I, Treglia G, Parodi C, Schweizer R, De Monti M, Harder Y. Salvage of the Mastectomy Pocket in Infected Implant-Based Breast Reconstruction Using Negative-Pressure Wound Therapy with Instillation and Dwell: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(8):2730. https://doi.org/10.3390/jcm14082730
Chicago/Turabian StyleDe Pellegrin, Laura, Isabel Zucal, Giorgio Treglia, Corrado Parodi, Riccardo Schweizer, Marco De Monti, and Yves Harder. 2025. "Salvage of the Mastectomy Pocket in Infected Implant-Based Breast Reconstruction Using Negative-Pressure Wound Therapy with Instillation and Dwell: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 8: 2730. https://doi.org/10.3390/jcm14082730
APA StyleDe Pellegrin, L., Zucal, I., Treglia, G., Parodi, C., Schweizer, R., De Monti, M., & Harder, Y. (2025). Salvage of the Mastectomy Pocket in Infected Implant-Based Breast Reconstruction Using Negative-Pressure Wound Therapy with Instillation and Dwell: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(8), 2730. https://doi.org/10.3390/jcm14082730