The Power of Heuristics in Predicting Fracture Nonunion
Abstract
1. Introduction
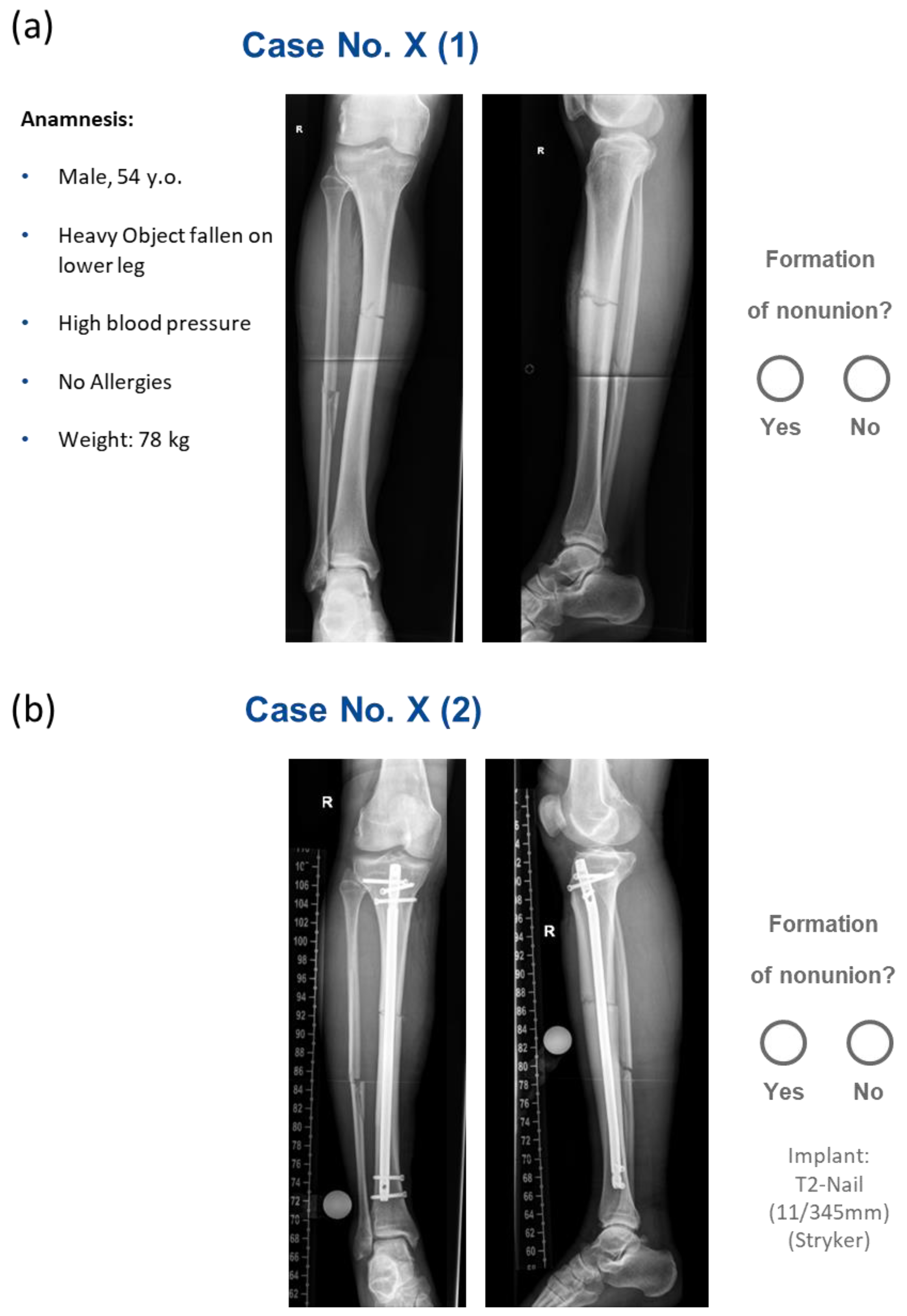
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Rater Characteristics
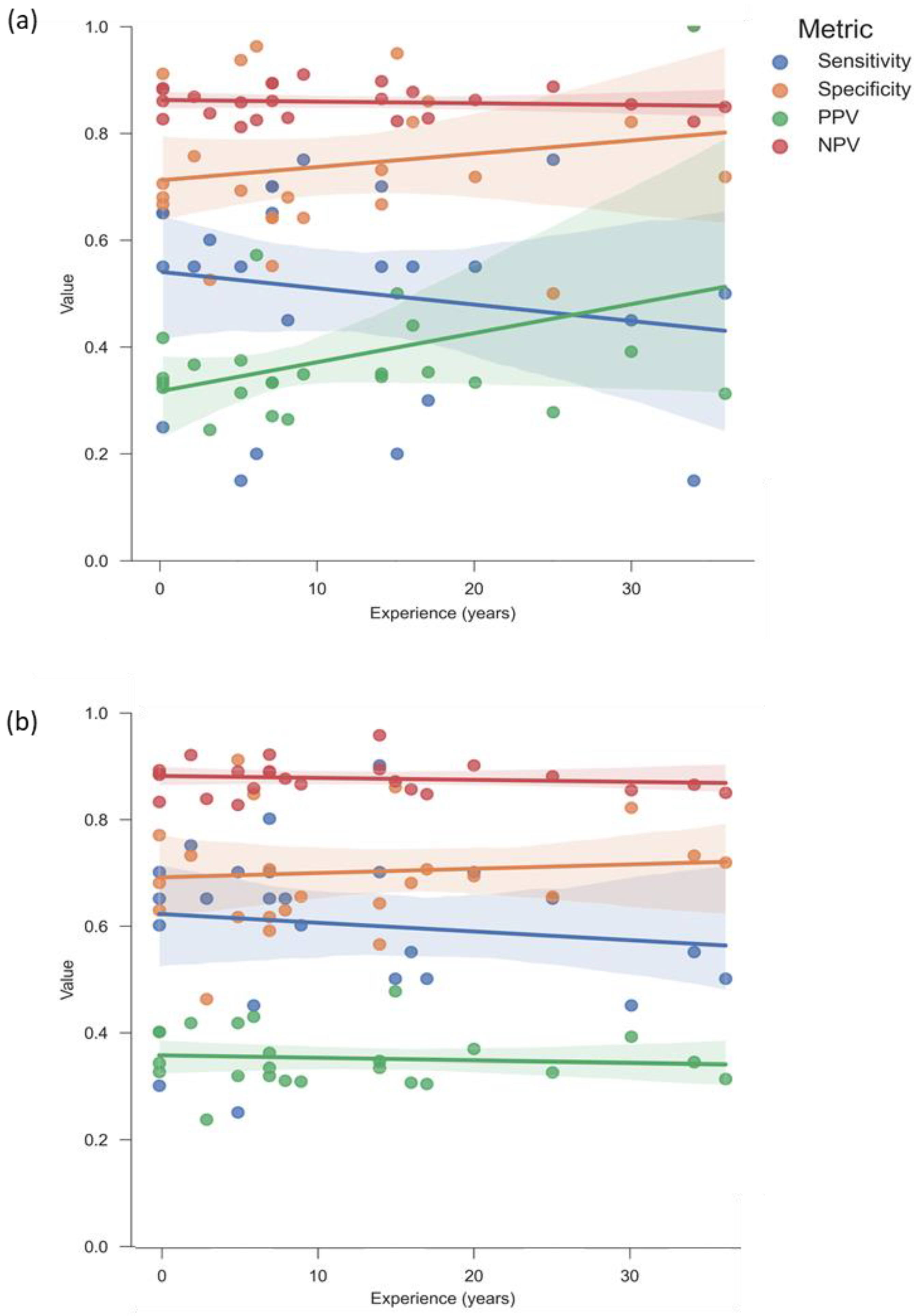
3.3. Clinicians’ Performance Metrics in Predicting Nonunion Development
3.4. No Significant Difference in Prediction Metrics Across Clinician Roles
3.5. Greater Experience Does Not Correlate with Improved Intuition
3.6. Changing Initial Ratings After Receiving Postoperative Information Did Not Improve Clinicians’ Accuracy
3.7. Clinically Relevant Patient Characteristics Influence Prediction Error of Postoperative Evaluation
4. Discussion
4.1. Principal Findings
4.2. Prediction Errors of Clinician Raters Are Associated with Clinically Relevant Patient Factors
4.3. Heuristic Biases of Tibial Nonunion Diagnosis in Context
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AO | Arbeitsgemeinschaft für Osteosynthesefragen (Association for the Study of Internal Fixation) |
| BMI | Body mass index |
| CT | Computed Tomography |
| FN | False negative |
| FP | False positive |
| LEG-NUI | Lower Extremity Grading of Nonunion Index |
| NSAID | Nonsteroidal anti-inflammatory drug |
| NU | Nonunion |
| NURD | Nonunion Risk Determination Score |
| NPV | Negative predictive value |
| ORIF | Open Reduction and Internal Fixation |
| OTA | Orthopaedic Trauma Association |
| PPV | Positive predictive value |
| TEN | Titanium Elastic Nail |
References
- Moghaddam, A.; Zietzschmann, S.; Bruckner, T.; Schmidmaier, G. Treatment of Atrophic Tibia Non-Unions According to “Diamond Concept”: Results of One- and Two-Step Treatment. Injury 2015, 46 (Suppl. S4), S39–S50. [Google Scholar] [CrossRef]
- Everding, J.; Roßlenbroich, S.; Raschke, M.J. Pseudarthrosen der langen Röhrenknochen. Chirurgie 2018, 89, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, J.; Bussmann, F.; Freischmidt, H.; Reiter, G.; Gruetzner, P.A.; El Barbari, J.S. Treatment of High-Grade Chronic Osteomyelitis and Nonunions with PerOssal®: A Retrospective Analysis of Clinical Efficacy and Patient Perspectives. J. Clin. Med. 2024, 13, 7764. [Google Scholar] [CrossRef] [PubMed]
- Brinker, M.R.; Hanus, B.D.; Sen, M.; O’Connor, D.P. The Devastating Effects of Tibial Nonunion on Health-Related Quality of Life. J. Bone Jt. Surg. Am. 2013, 95, 2170–2176. [Google Scholar] [CrossRef]
- Freischmidt, H.; Guehring, T.; Thomé, P.; Armbruster, J.; Reiter, G.; Grützner, P.A.; Nolte, P.-C. Treatment of Large Femoral and Tibial Bone Defects with Plate-Assisted Bone Segment Transport. J. Orthop. Trauma 2024, 38, 285–290. [Google Scholar] [CrossRef]
- Freischmidt, H.; Reiter, G.; Grützner, P.A.; Armbruster, J. Augmentation in surgical sepsis: Chances and limitations in the treatment of osteitis with calcium hydroxyapatite containing antibiotics. Unfallchirurgie 2022, 125, 452–459. [Google Scholar] [CrossRef]
- Reeh, F.M.; Sachse, S.; Wedekind, L.; Hofmann, G.O.; Lenz, M. Nonunions and Their Operative Treatment—A DRG-Based Epidemiological Analysis for the Years 2007–2019 in Germany. Dtsch. Arztebl. Int. 2022, 119, 869–875. [Google Scholar] [CrossRef]
- Walter, N.; Hierl, K.; Brochhausen, C.; Alt, V.; Rupp, M. The Epidemiology and Direct Healthcare Costs of Aseptic Nonunions in Germany—A Descriptive Report. Bone Jt. Res. 2022, 11, 541–547. [Google Scholar] [CrossRef]
- Tian, R.; Zheng, F.; Zhao, W.; Zhang, Y.; Yuan, J.; Zhang, B.; Li, L. Prevalence and Influencing Factors of Nonunion in Patients with Tibial Fracture: Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 377. [Google Scholar] [CrossRef]
- Andrzejowski, P.; Giannoudis, P.V. The ‘Diamond Concept’ for Long Bone Non-Union Management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Kanakaris, N.K.; Tosounidis, T.H.; Giannoudis, P.V. Surgical Management of Infected Non-Unions: An Update. Injury 2015, 46 (Suppl. S5), S25–S32. [Google Scholar] [CrossRef]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Lazzarini, L.; Mader, J.T.; Calhoun, J.H. Osteomyelitis in Long Bones. J. Bone Jt. Surg. Am. 2004, 86, 2305–2318. [Google Scholar] [CrossRef]
- Beltran, M.J.; Collinge, C.A.; Gardner, M.J. Stress Modulation of Fracture Fixation Implants. J. Am. Acad. Orthop. Surg. 2016, 24, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Gigerenzer, G.; Brighton, H. Homo Heuristicus: Why Biased Minds Make Better Inferences. Top. Cogn. Sci. 2009, 1, 107–143. [Google Scholar] [CrossRef] [PubMed]
- Whelehan, D.F.; Conlon, K.C.; Ridgway, P.F. Medicine and Heuristics: Cognitive Biases and Medical Decision-Making. Ir. J. Med. Sci. 2020, 189, 1477–1484. [Google Scholar] [CrossRef]
- McDonald, C.J. Medical Heuristics: The Silent Adjudicators of Clinical Practice. Ann. Intern. Med. 1996, 124, 56–62. [Google Scholar] [CrossRef]
- Kempainen, R.R.; Migeon, M.B.; Wolf, F.M. Understanding Our Mistakes: A Primer on Errors in Clinical Reasoning. Med. Teach. 2003, 25, 177–181. [Google Scholar] [CrossRef]
- Kalf, A.J.; Spruijt-Metz, D. Variation in Diagnoses: Influence of Specialists’ Training on Selecting and Ranking Relevant Information in Geriatric Case Vignettes. Soc. Sci. Med. 1996, 42, 705–712. [Google Scholar] [CrossRef]
- Taylor, M.K.; Marsh, E.J.; Samanez-Larkin, G.R. Heuristic Decision-Making across Adulthood. Psychol. Aging 2023, 38, 508–518. [Google Scholar] [CrossRef]
- Oestern, H.J.; Tscherne, H. Pathophysiology and classification of soft tissue damage in fractures. Orthopade 1983, 12, 2–8. [Google Scholar] [PubMed]
- JASP Team. JASP, version 0.19.3; JASP Team: Amsterdam, The Netherlands, 2024. Available online: https://jasp-stats.org/ (accessed on 21 January 2025).
- McKinney, W. Data Structures for Statistical Computing in Python. SciPy 2010, 445, 51–56. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Zura, R.; Watson, J.T.; Einhorn, T.; Mehta, S.; Rocca, G.J.D.; Xiong, Z.; Wang, Z.; Jones, J.; Steen, R.G. An Inception Cohort Analysis to Predict Nonunion in Tibia and 17 Other Fracture Locations. Injury 2017, 48, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, J.; Gee, A.O.; Kuntz, A.F.; Esterhai, J.L. Compartment Syndrome in Tibial Fractures. J. Orthop. Trauma 2009, 23, 514. [Google Scholar] [CrossRef]
- Joo, Y.-B.; Kim, Y.-M.; Park, Y.-C.; Chae, S.-H.; Kim, D.-H. Evaluating Meniscus, Ligament and Soft Tissue Injury Using MRI in Tibial Plateau Fractures: A Tscherne Classification Approach. Medicina 2024, 60, 2073. [Google Scholar] [CrossRef]
- Dickson, K.; Katzman, S.; Delgado, E.; Contreras, D. Delayed Unions and Nonunions of Open Tibial Fractures: Correlation with Arteriography Results. Clin. Orthop. Relat. Res. 1994, 302, 189–193. [Google Scholar] [CrossRef]
- Burrus, M.T.; Werner, B.C.; Yarboro, S.R. Obesity Is Associated with Increased Postoperative Complications After Operative Management of Tibial Shaft Fractures. Injury 2016, 47, 465–470. [Google Scholar] [CrossRef]
- Armstrong, B.A.; Dutescu, I.A.; Tung, A.; Carter, D.N.; Trbovich, P.L.; Wong, S.; Saposnik, G.; Grantcharov, T. Cognitive Biases in Surgery: Systematic Review. Br. J. Surg. 2023, 110, 645–654. [Google Scholar] [CrossRef]
- Edelstein, A.I.; Tanenbaum, J.T.; McGinley, E.L.; Dillingham, T.R.; Pezzin, L.E. Age-Based Heuristics Bias Treatment of Displaced Femoral Neck Fractures in the Elderly. Arthroplast. Today 2024, 27, 101356. [Google Scholar] [CrossRef]
- Janssen, S.J.; Teunis, T.; Ring, D.; Parisien, R.C. Cognitive Biases in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2021, 29, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, N.B.K.; Karmakar, B.; Pathan, S.K.; Joshi, V.; Gohel, D.M.; Coulshed, D.S.; Negishi, K.; Pathan, F.K. A Systematic Review of Frailty Scores Used in Heart Failure Patients. Heart Lung Circ. 2023, 32, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, A.; Gharin, P.; Forouzannia, S.A.; Ahmadzadeh, K.; Miri, R.; Yousefifard, M. HEART versus GRACE Score in Predicting the Outcomes of Patients with Acute Coronary Syndrome; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2023, 11, e50. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; He, W.; Guo, L. HAS-BLED vs. ORBIT Scores in Anticoagulated Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 1042763. [Google Scholar] [CrossRef]
- Quarta, D.; Grassi, M.; Lattanzi, G.; Gigante, A.P.; D’Anca, A.; Potena, D. Three Predictive Scores Compared in a Retrospective Multicenter Study of Nonunion Tibial Shaft Fracture. World J. Orthop. 2024, 15, 560–569. [Google Scholar] [CrossRef]
- Brown, C.; Nazeer, R.; Gibbs, A.; Le Page, P.; Mitchell, A.R. Breaking Bias: The Role of Artificial Intelligence in Improving Clinical Decision-Making. Cureus 2023, 15, e36415. [Google Scholar] [CrossRef]





| Inclusion | Exclusion |
|---|---|
|
|
|
| Parameter Group | Parameter [Unit] |
|---|---|
| Preoperative |
|
| Postoperative |
|
| Patient Characteristics | Frequency | Percent [%] | Mean ± SD | |
|---|---|---|---|---|
| Age [years] | Total | 98 | 100 | 39.9 ± 16.5 |
| Male | 68 | 69 | 39.8 ± 16.7 | |
| Female | 30 | 31 | 40.1 ± 16.0 | |
| Treatment | Nail | 70 | 71 | |
| Plate | 27 | 28 | ||
| TEN | 1 | 1 | ||
| Open fracture | Yes | 29 | 30 | |
| No | 69 | 70 | ||
| Obesity | Yes | 7 | 7 | |
| No | 91 | 93 | ||
| Smoking | Yes | 9 | 9 | |
| No | 89 | 91 | ||
| Compartment syndrome | Yes | 9 | 9 | |
| No | 89 | 91 | ||
| Polytrauma | Yes | 6 | 6 | |
| No | 92 | 94 | ||
| Plastic soft tissue coverage | Yes | 3 | 3 | |
| no | 95 | 97 | ||
| Fibula fractured | Yes | 76 | 78 | |
| No | 22 | 22 | ||
| Soft tissue damage | 0° or n/a | 69 | 70 | |
| 1° | 18 | 18 | ||
| 2° | 7 | 7 | ||
| 3° | 4 | 4 |
| Position | Frequency [n] | Mean Experience ± SD [Years] |
|---|---|---|
| Heads of Surgical Departments | 4 | 28.8 ± 6.5 |
| Consultants | 4 | 18.8 ± 6.5 |
| Specialists | 4 | 8.8 ± 3.1 |
| Residents | 4 | 3.8 ± 1.3 |
| Interns | 4 | 0.0 ± 0.0 |
| Radiologists | 4 | 10.0 ± 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armbruster, J.; Steinhausen, E.; Hackl, S.; Reumann, M.K.; Stengel, D.; Niemeyer, F.; Reiter, G.; Gruetzner, P.A.; Freischmidt, H. The Power of Heuristics in Predicting Fracture Nonunion. J. Clin. Med. 2025, 14, 2713. https://doi.org/10.3390/jcm14082713
Armbruster J, Steinhausen E, Hackl S, Reumann MK, Stengel D, Niemeyer F, Reiter G, Gruetzner PA, Freischmidt H. The Power of Heuristics in Predicting Fracture Nonunion. Journal of Clinical Medicine. 2025; 14(8):2713. https://doi.org/10.3390/jcm14082713
Chicago/Turabian StyleArmbruster, Jonas, Eva Steinhausen, Simon Hackl, Marie K. Reumann, Dirk Stengel, Frank Niemeyer, Gregor Reiter, Paul Alfred Gruetzner, and Holger Freischmidt. 2025. "The Power of Heuristics in Predicting Fracture Nonunion" Journal of Clinical Medicine 14, no. 8: 2713. https://doi.org/10.3390/jcm14082713
APA StyleArmbruster, J., Steinhausen, E., Hackl, S., Reumann, M. K., Stengel, D., Niemeyer, F., Reiter, G., Gruetzner, P. A., & Freischmidt, H. (2025). The Power of Heuristics in Predicting Fracture Nonunion. Journal of Clinical Medicine, 14(8), 2713. https://doi.org/10.3390/jcm14082713









